Abstract
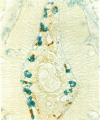
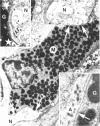
Inflammatory or allergic conditions, as well as situations where healing and repair processes occur, are characterized by the presence of increased numbers of mast cells. Previous work on the effect of neuropeptides on mast cell mediator release showed that only substance P caused such release from intestinal mucosal mast cells [Shanahan, F., Denburg, J. A., Fox, J., Bienenstock, J. & Befus, A. D. (1985) J. Immunol. 135, 1331-1337]. Accordingly, we investigated the microanatomical relationship between mast cells and enteric nerves in normal rat intestine and parasite-infected rat intestine, in which mucosal mast cell hyperplasia occurs. Combined immunohistochemistry for neuron-specific enolase and staining with alcian blue at pH 0.5 was employed on paraffin-embedded sections of normal and Nippostrongylus brasiliensis-infected rat jejunum. Sixty-seven percent of intestinal mucosal mast cells were touching subepithelial nerves, and an additional 20% were within 2 micron of nerves. Assessment of the proportion of the lamina propria occupied by mast cells (12.5%), the average mast cell area (121 +/- 28 microns 2), and the density of enteric nerves (one per 788 +/- 151 microns 2) suggested that the association was 5 times greater than would be expected by chance alone (P less than 0.0001). In consecutive sections, the nerves in contact with mast cells were also shown to contain substance P and/or calcitonin-gene-related peptide. Electron microscopy confirmed this association: 8% of the mast cells in infected rats exhibited membrane-membrane contact with unmyelinated axons containing 70- to 170-nm dense-core vesicles, and an additional 31% were situated less than 250 nm from nerves. Other mast cells appeared to embrace nerve bundles through the projection of lamellopodia. These data provide systematic quantitative evidence that a structural foundation for communication between the immune and nervous systems exists in the rat gastrointestinal tract.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloe L., Levi-Montalcini R. Mast cells increase in tissues of neonatal rats injected with the nerve growth factor. Brain Res. 1977 Sep 16;133(2):358–366. doi: 10.1016/0006-8993(77)90772-7. [DOI] [PubMed] [Google Scholar]
- Barthó L., Holzer P. Search for a physiological role of substance P in gastrointestinal motility. Neuroscience. 1985 Sep;16(1):1–32. doi: 10.1016/0306-4522(85)90043-0. [DOI] [PubMed] [Google Scholar]
- Befus A. D., Johnston N., Bienenstock J. Nippostrongylus brasiliensis: mast cells and histamine levels in tissues of infected and normal rats. Exp Parasitol. 1979 Aug;48(1):1–8. doi: 10.1016/0014-4894(79)90048-1. [DOI] [PubMed] [Google Scholar]
- Befus A. D., Spencer J. A., McDermott M. R., McLaughlin B., Bienenstock J. Isolation and characteristics of small intestinal lamina propria cells from normal and nematode (Nippostrongylus brasiliensis)-infected rats. Int Arch Allergy Appl Immunol. 1984;75(4):345–350. doi: 10.1159/000233645. [DOI] [PubMed] [Google Scholar]
- Bienenstock J., Befus A. D., Pearce F., Denburg J., Goodacre R. Mast cell heterogeneity: derivation and function, with emphasis on the intestine. J Allergy Clin Immunol. 1982 Dec;70(6):407–412. doi: 10.1016/0091-6749(82)90001-x. [DOI] [PubMed] [Google Scholar]
- Bishop A. E., Polak J. M., Facer P., Ferri G. L., Marangos P. J., Pearse A. G. Neuron specific enolase: a common marker for the endocrine cells and innervation of the gut and pancreas. Gastroenterology. 1982 Oct;83(4):902–915. [PubMed] [Google Scholar]
- Bruni A., Bigon E., Boarato E., Mietto L., Leon A., Toffano G. Interaction between nerve growth factor and lysophosphatidylserine on rat peritoneal mast cells. FEBS Lett. 1982 Feb 22;138(2):190–192. doi: 10.1016/0014-5793(82)80438-9. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Ultrastructural identification of neurotransmitters. Scand J Gastroenterol Suppl. 1981;70:1–9. [PubMed] [Google Scholar]
- Clague J. R., Sternini C., Brecha N. C. Localization of calcitonin gene-related peptide-like immunoreactivity in neurons of the rat gastrointestinal tract. Neurosci Lett. 1985 May 1;56(1):63–68. doi: 10.1016/0304-3940(85)90441-0. [DOI] [PubMed] [Google Scholar]
- Cooke H. J. Neurobiology of the intestinal mucosa. Gastroenterology. 1986 Apr;90(4):1057–1081. doi: 10.1016/0016-5085(86)90889-9. [DOI] [PubMed] [Google Scholar]
- Dzielak D. J., Thureson-Klein A., Klein R. L. Local modulation of neurotransmitter release in bovine splenic vein. Blood Vessels. 1983;20(3):122–134. doi: 10.1159/000158466. [DOI] [PubMed] [Google Scholar]
- Fehér E., Burnstock G., Varndell I. M., Polak J. M. Calcitonin gene-related peptide-immunoreactive nerve fibres in the small intestine of the guinea-pig: electron-microscopic immunocytochemistry. Cell Tissue Res. 1986;245(2):353–358. doi: 10.1007/BF00213942. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M. Types of nerves in the enteric nervous system. Neuroscience. 1980;5(1):1–20. doi: 10.1016/0306-4522(80)90067-6. [DOI] [PubMed] [Google Scholar]
- GRAHAM R. C., Jr, LUNDHOLM U., KARNOVSKY M. J. CYTOCHEMICAL DEMONSTRATION OF PEROXIDASE ACTIVITY WITH 3-AMINO-9-ETHYLCARBAZOLE. J Histochem Cytochem. 1965 Feb;13:150–152. doi: 10.1177/13.2.150. [DOI] [PubMed] [Google Scholar]
- Gabella G. Innervation of the gastrointestinal tract. Int Rev Cytol. 1979;59:129–193. doi: 10.1016/s0074-7696(08)61662-9. [DOI] [PubMed] [Google Scholar]
- Gabella G. Synapses of adrenergic fibres. Experientia. 1971 Mar 15;27(3):280–281. doi: 10.1007/BF02138146. [DOI] [PubMed] [Google Scholar]
- Heine H., Förster F. J. Histophysiology of mast cells in skin and other organs. Arch Dermatol Res. 1975 Oct 29;253(3):225–228. doi: 10.1007/BF00561148. [DOI] [PubMed] [Google Scholar]
- Hägermark O., Hökfelt T., Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1978 Oct;71(4):233–235. doi: 10.1111/1523-1747.ep12515092. [DOI] [PubMed] [Google Scholar]
- Isaacson P. Mast cells in benign nerve sheath tumours. J Pathol. 1976 Aug;119(4):193–196. doi: 10.1002/path.1711190402. [DOI] [PubMed] [Google Scholar]
- Kiernan J. A. A pharmacological and histological investigation of the involvement of mast cells in cutaneous axon reflex vasodilatation. Q J Exp Physiol Cogn Med Sci. 1975 Apr;60(2):123–130. doi: 10.1113/expphysiol.1975.sp002298. [DOI] [PubMed] [Google Scholar]
- Kiernan J. A. A study of chemically induced acute inflammation in the skin of the rat. Q J Exp Physiol Cogn Med Sci. 1977 Apr;62(2):151–161. doi: 10.1113/expphysiol.1977.sp002385. [DOI] [PubMed] [Google Scholar]
- LEVER J. D., GRAHAM J. D., IRVINE G., CHICK W. J. THE VESICULATED AXONS IN RELATION TO ARTERIOLAR SMOOTH MUSCLE IN THE PANCREAS. A FINE STUCTURAL AND QUANTITATIVE STUDY. J Anat. 1965 Apr;99:299–313. [PMC free article] [PubMed] [Google Scholar]
- Lander A. D., Fujii D. K., Gospodarowicz D., Reichardt L. F. Characterization of a factor that promotes neurite outgrowth: evidence linking activity to a heparan sulfate proteoglycan. J Cell Biol. 1982 Sep;94(3):574–585. doi: 10.1083/jcb.94.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander A. D., Fujii D. K., Reichardt L. F. Laminin is associated with the "neurite outgrowth-promoting factors" found in conditioned media. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2183–2187. doi: 10.1073/pnas.82.7.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff A. R., Stimler N. P., Munoz N. M., Shioya T., Tallet J., Dame C. Augmentation of respiratory mast cell secretion of histamine caused by vagus nerve stimulation during antigen challenge. J Immunol. 1986 Feb 1;136(3):1066–1073. [PubMed] [Google Scholar]
- Lembeck F., Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Dahlström A., Bylock A., Ahlman H., Pettersson G., Larsson I., Hansson H. A., Kewenter J. Ultrastructural evidence for an innervation of epithelial enterochromaffine cells in the guinea pig duodenum. Acta Physiol Scand. 1978 Sep;104(1):3–12. doi: 10.1111/j.1748-1716.1978.tb06245.x. [DOI] [PubMed] [Google Scholar]
- Matthew W. D., Greenspan R. J., Lander A. D., Reichardt L. F. Immunopurification and characterization of a neuronal heparan sulfate proteoglycan. J Neurosci. 1985 Jul;5(7):1842–1850. doi: 10.1523/JNEUROSCI.05-07-01842.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton C. R., Chahl L. A. Pharmacology of the neurogenic oedema response to electrical stimulation of the saphenous nerve in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1980 Nov;314(3):271–276. doi: 10.1007/BF00498549. [DOI] [PubMed] [Google Scholar]
- Newson B., Dahlström A., Enerbäck L., Ahlman H. Suggestive evidence for a direct innervation of mucosal mast cells. Neuroscience. 1983 Oct;10(2):565–570. doi: 10.1016/0306-4522(83)90153-7. [DOI] [PubMed] [Google Scholar]
- Olsson Y. Mast cells in the nervous system. Int Rev Cytol. 1968;24:27–70. doi: 10.1016/s0074-7696(08)61396-0. [DOI] [PubMed] [Google Scholar]
- Pearce F. L., Befus A. D., Gauldie J., Bienenstock J. Mucosal mast cells. II. Effects of anti-allergic compounds on histamine secretion by isolated intestinal mast cells. J Immunol. 1982 Jun;128(6):2481–2486. [PubMed] [Google Scholar]
- Perdue M. H., Chung M., Gall D. G. Effect of intestinal anaphylaxis on gut function in the rat. Gastroenterology. 1984 Mar;86(3):391–397. [PubMed] [Google Scholar]
- Probert L., De Mey J., Polak J. M. Ultrastructural localization of four different neuropeptides within separate populations of p-type nerves in the guinea pig colon. Gastroenterology. 1983 Nov;85(5):1094–1104. [PubMed] [Google Scholar]
- Shanahan F., Denburg J. A., Fox J., Bienenstock J., Befus D. Mast cell heterogeneity: effects of neuroenteric peptides on histamine release. J Immunol. 1985 Aug;135(2):1331–1337. [PubMed] [Google Scholar]
- Shanahan F., Lee T. D., Bienenstock J., Befus A. D. The influence of endorphins on peritoneal and mucosal mast cell secretion. J Allergy Clin Immunol. 1984 Oct;74(4 Pt 1):499–504. doi: 10.1016/0091-6749(84)90385-3. [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Savitt J. M., Jacobowitz D. M. Suggestive evidence for a functional unit between mast cells and substance P fibers in the rat diaphragm and mesentery. Histochemistry. 1985;82(1):5–8. doi: 10.1007/BF00502084. [DOI] [PubMed] [Google Scholar]
- Stach W. Uber die Nervengeflechte der Duodenalzotten. Licht- und elektronenmikroskopische Untersuchungen. Acta Anat (Basel) 1973;85(2):216–231. [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Lee T. D., Seldin D. C., Austen K. F., Befus A. D., Bienenstock J. Intestinal mucosal mast cells from rats infected with Nippostrongylus brasiliensis contain protease-resistant chondroitin sulfate di-B proteoglycans. J Immunol. 1986 Jul 1;137(1):291–295. [PubMed] [Google Scholar]
- Wade P. R., Westfall J. A. Ultrastructure of enterochromaffin cells and associated neural and vascular elements in the mouse duodenum. Cell Tissue Res. 1985;241(3):557–563. doi: 10.1007/BF00214576. [DOI] [PubMed] [Google Scholar]
- Wiesner-Menzel L., Schulz B., Vakilzadeh F., Czarnetzki B. M. Electron microscopical evidence for a direct contact between nerve fibres and mast cells. Acta Derm Venereol. 1981;61(6):465–469. doi: 10.2340/0001555561465469. [DOI] [PubMed] [Google Scholar]
- Wingren U., Enerbäck L. Mucosal mast cells of the rat intestine: a re-evaluation of fixation and staining properties, with special reference to protein blocking and solubility of the granular glycosaminoglycan. Histochem J. 1983 Jun;15(6):571–582. doi: 10.1007/BF01954148. [DOI] [PubMed] [Google Scholar]