Abstract
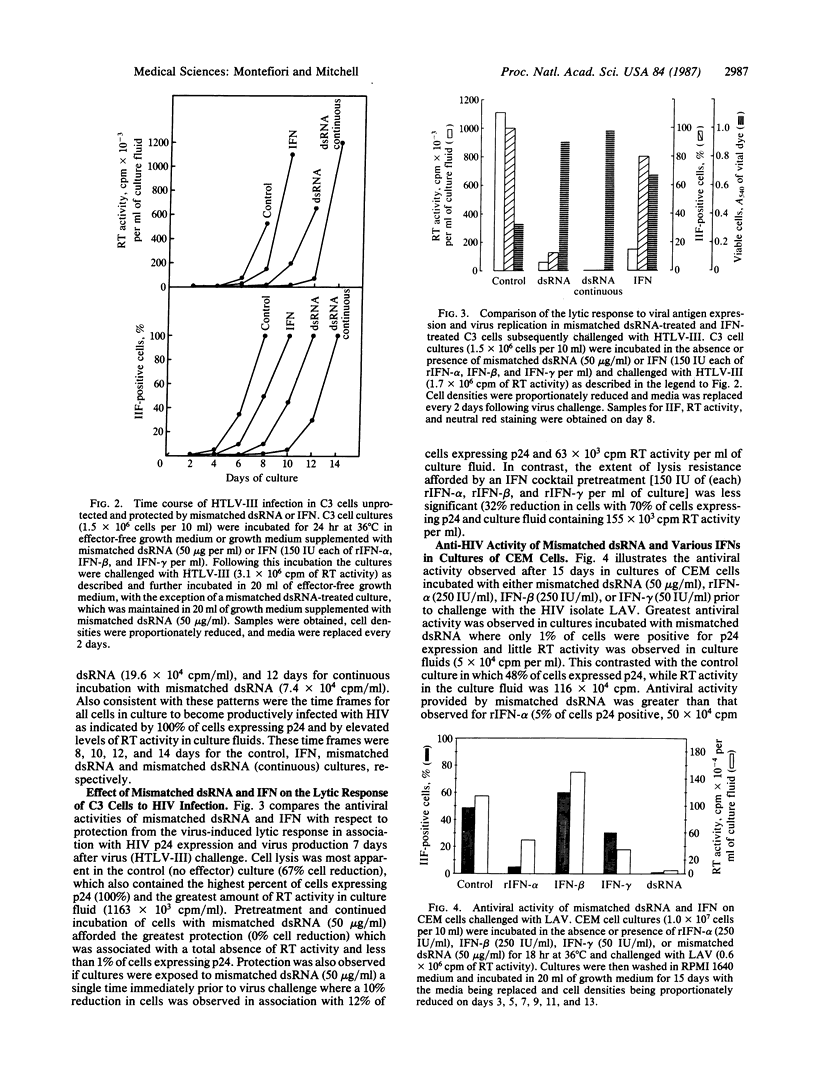
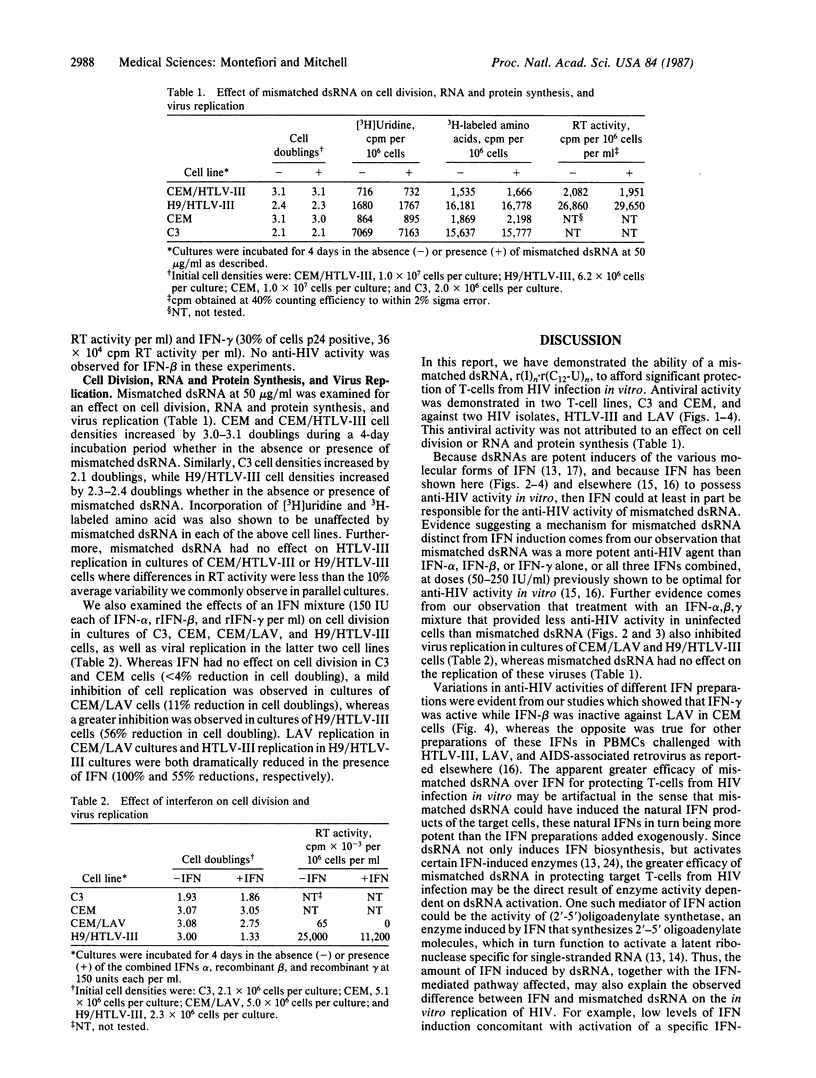
The biological response modifier r(I)n.r(C12-U)n, referred to here as mismatched double-stranded (ds) RNA, was examined for antihuman immunodeficiency virus (HIV) activity in vitro because of its known antiviral activity and ability to induce interferon (IFN) in other biological systems [Carter, W. A., Strayer, D. R., Hubbell, H. R. & Brodsky, I. (1985) J. Biol. Response Modif. 4, 495-502]. We found that cultures of the highly HIV-permissive T-cell line C3 were afforded significant protection from HIV infection when incubated in growth media supplemented with mismatched dsRNA at 10-50 micrograms/ml prior to virus challenge. Similar results were obtained at 50 micrograms of mismatched dsRNA per ml in cultures of the T-lymphoblastoid cell line CEM. Infections were monitored by indirect immunofluorescence of cells for viral p24 antigen expression, reverse transcriptase activity in culture fluids for virus production, and vital dye uptake for cytopathic effect. Antiviral activity was increased by the continued presence of mismatched dsRNA in cultures following virus challenge. A one-time exposure to mismatched dsRNA (50 micrograms/ml) provided greater antiviral activity than either a one-time exposure to recombinant IFN-alpha [250 international units (IU)/ml], IFN-beta (250 IU/ml), or IFN-gamma (50 IU/ml) in cultures of CEM cells, or a one-time exposure to a combination of all three IFNs (150 IU each per ml) in cultures of C3 cells. Mismatched dsRNA at 50 micrograms/ml had no effect on cell division, RNA and protein synthesis, or virus replication in all T-cell lines examined. A clear distinction between the activities of mismatched dsRNA and IFN was the ability of IFN to suppress the in vitro replication of HIV that occurred at IFN concentrations (150 IU each of alpha, beta, and gamma per ml) that provided less antiviral activity than mismatched dsRNA (50 micrograms/ml). The results of these in vitro studies suggest a potential therapeutic value for mismatched dsRNA in the treatment of acquired immunodeficiency syndrome (AIDS).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alizon M., Sonigo P., Barré-Sinoussi F., Chermann J. C., Tiollais P., Montagnier L., Wain-Hobson S. Molecular cloning of lymphadenopathy-associated virus. Nature. 1984 Dec 20;312(5996):757–760. doi: 10.1038/312757a0. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Bonavida B., Katz J., Gottlieb M. Mechanism of defective NK cell activity in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. I. Defective trigger on NK cells for NKCF production by target cells, and partial restoration by IL 2. J Immunol. 1986 Aug 15;137(4):1157–1163. [PubMed] [Google Scholar]
- Brodsky I., Strayer D. R., Krueger L. J., Carter W. A. Clinical studies with ampligen (mismatched double-stranded RNA). J Biol Response Mod. 1985 Dec;4(6):669–675. [PubMed] [Google Scholar]
- Carter W. A., De Clercq E. Viral infection and host defense. Science. 1974 Dec 27;186(4170):1172–1178. doi: 10.1126/science.186.4170.1172. [DOI] [PubMed] [Google Scholar]
- Carter W. A., Pitha P. M., Marshall L. W., Tazawa I., Tazawa S., Ts'o P. O. Structural requirements of the rI n -rC n complex for induction of human interferon. J Mol Biol. 1972 Oct 14;70(3):567–587. doi: 10.1016/0022-2836(72)90560-8. [DOI] [PubMed] [Google Scholar]
- Carter W. A., Strayer D. R., Hubbell H. R., Brodsky I. Preclinical studies with Ampligen (mismatched double-stranded RNA). J Biol Response Mod. 1985 Oct;4(5):495–502. [PubMed] [Google Scholar]
- De Clercq E. Chemotherapeutic approaches to the treatment of the acquired immune deficiency syndrome (AIDS). J Med Chem. 1986 Sep;29(9):1561–1569. doi: 10.1021/jm00159a001. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Forti R. L., Moldovan R. A., Mitchell W. M., Callicoat P., Schuffman S. S., Davies H. A., Smith D. M., Jr Application of an objective biological assay of human interferons to clinical specimens and a survey of a normal population. J Clin Microbiol. 1985 May;21(5):689–693. doi: 10.1128/jcm.21.5.689-693.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Hearl W. G., Johnston M. I. A misaligned double-stranded RNA, poly(I).poly(C12,U), induces accumulation of 2',5'-oligoadenylates in mouse tissues. Biochem Biophys Res Commun. 1986 Jul 16;138(1):40–46. doi: 10.1016/0006-291x(86)90243-3. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Hartshorn K. L., Rota T. R., Andrews C. A., Kaplan J. C., Schooley R. T., Hirsch M. S. Recombinant human interferon alfa-A suppresses HTLV-III replication in vitro. Lancet. 1985 Mar 16;1(8429):602–604. doi: 10.1016/s0140-6736(85)92144-0. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Masur H., Edgar L. C., Whalen G., Rook A. H., Fauci A. S. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983 Aug 25;309(8):453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Mitchell W. M., Forti R. L., Vogler L. B., Lawton A. R., Gregg C. R. Spontaneous and interferon resistant natural killer cell anergy in AIDS. AIDS Res. 1983 1984;1(3):221–229. doi: 10.1089/aid.1.1983.1.221. [DOI] [PubMed] [Google Scholar]
- Montefiori D. C., Mitchell W. M. Infection of the HTLV-II-bearing T-cell line C3 with HTLV-III/LAV is highly permissive and lytic. Virology. 1986 Dec;155(2):726–731. doi: 10.1016/0042-6822(86)90233-3. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Rook A. H., Masur H., Lane H. C., Frederick W., Kasahara T., Macher A. M., Djeu J. Y., Manischewitz J. F., Jackson L., Fauci A. S. Interleukin-2 enhances the depressed natural killer and cytomegalovirus-specific cytotoxic activities of lymphocytes from patients with the acquired immune deficiency syndrome. J Clin Invest. 1983 Jul;72(1):398–403. doi: 10.1172/JCI110981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittman S. M., Lane H. C., Higgins S. E., Folks T., Fauci A. S. Direct polyclonal activation of human B lymphocytes by the acquired immune deficiency syndrome virus. Science. 1986 Sep 5;233(4768):1084–1086. doi: 10.1126/science.3016902. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Hahn B. H., Arya S. K., Groopman J. E., Gallo R. C., Wong-Staal F. Molecular characterization of human T-cell leukemia (lymphotropic) virus type III in the acquired immune deficiency syndrome. Science. 1984 Dec 7;226(4679):1165–1171. doi: 10.1126/science.6095449. [DOI] [PubMed] [Google Scholar]
- Yamamoto J. K., Barré-Sinoussi F., Bolton V., Pedersen N. C., Gardner M. B. Human alpha- and beta-interferon but not gamma- suppress the in vitro replication of LAV, HTLV-III, and ARV-2. J Interferon Res. 1986 Apr;6(2):143–152. doi: 10.1089/jir.1986.6.143. [DOI] [PubMed] [Google Scholar]



