Abstract
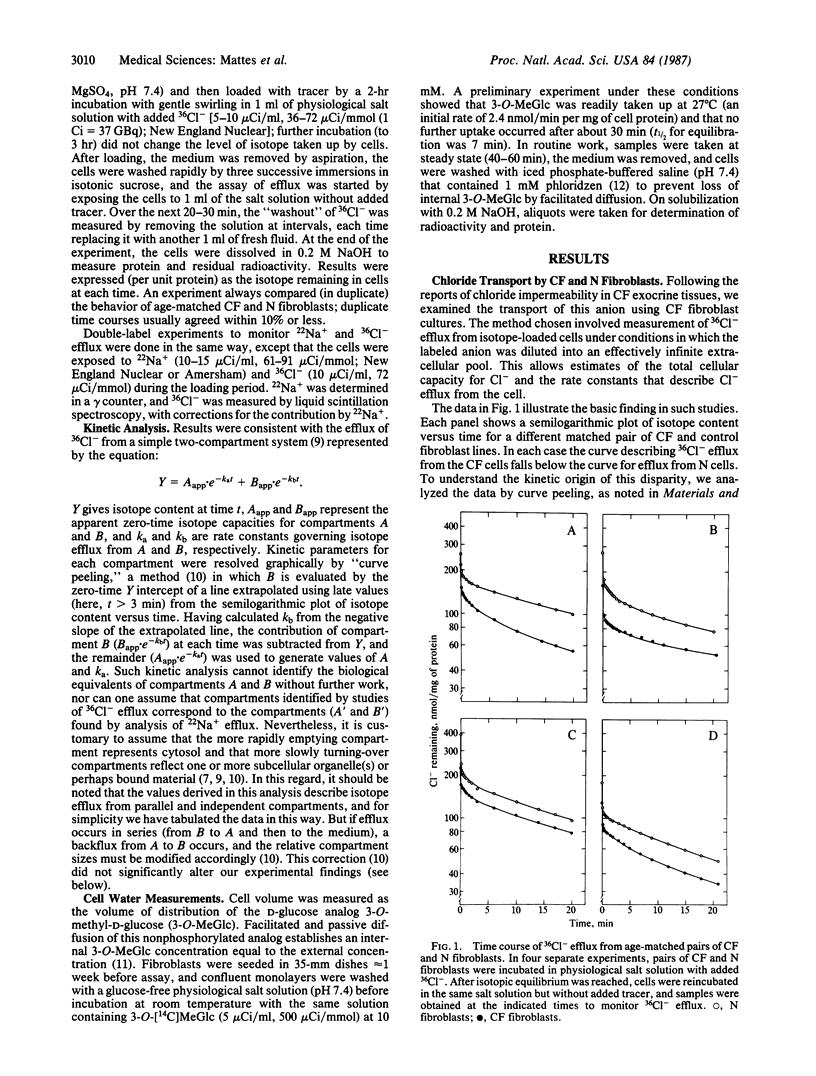
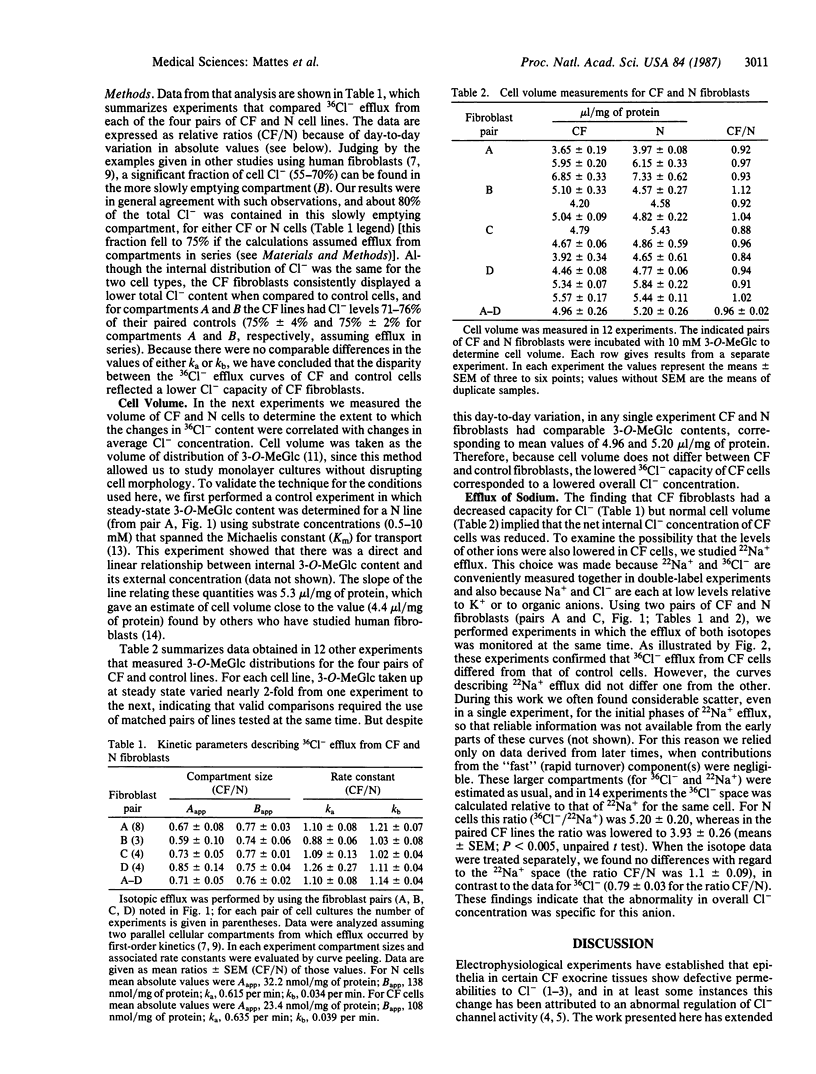
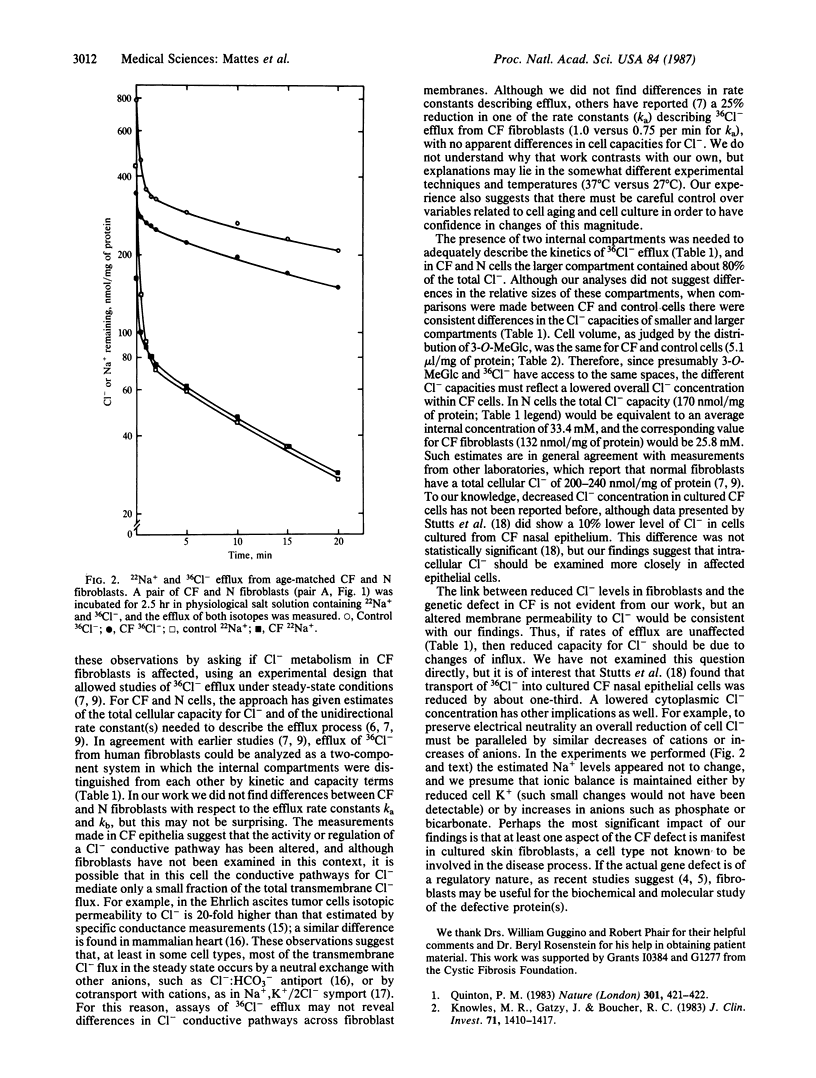
An abnormal regulation of chloride permeability has been described for epithelial cells from patients with cystic fibrosis (CF). To learn more about the biochemical basis of this inherited disease, we have studied chloride metabolism in cultured CF fibroblasts by comparing the efflux of 36Cl- from matched pairs of CF and normal fibroblasts. The rate constants describing 36Cl- efflux did not differ between the two cell types, but in each of the four pairs tested the amount of 36Cl- contained within CF cells was consistently reduced, by 25-30%, relative to normal cells. Comparisons of cell water content and 22Na+ efflux showed no differences between the two cell types, suggesting that overall intracellular chloride concentration is lower than normal in CF fibroblasts. Such data suggest that the CF gene defect is expressed in skin fibroblasts and that this defect may alter the regulation of intracellular Cl- concentration, perhaps through changes in Cl- permeability.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis M. H., Pato C. N., Gruenstein E. Analysis of neurotoxin and mitogen-stimulated sodium transport in human fibroblasts. J Biol Chem. 1982 Apr 25;257(8):4356–4361. [PubMed] [Google Scholar]
- Frizzell R. A., Rechkemmer G., Shoemaker R. L. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science. 1986 Aug 1;233(4763):558–560. doi: 10.1126/science.2425436. [DOI] [PubMed] [Google Scholar]
- Geck P., Pietrzyk C., Burckhardt B. C., Pfeiffer B., Heinz E. Electrically silent cotransport on Na+, K+ and Cl- in Ehrlich cells. Biochim Biophys Acta. 1980 Aug 4;600(2):432–447. doi: 10.1016/0005-2736(80)90446-0. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K. Anion exchange and anion-cation co-transport systems in mammalian cells. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):519–535. doi: 10.1098/rstb.1982.0149. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Pariza M. W., Becker J. E., Potter V. R. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem. 1975 Oct;68(2):537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- Knowles M. R., Stutts M. J., Spock A., Fischer N., Gatzy J. T., Boucher R. C. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983 Sep 9;221(4615):1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- Knowles M., Gatzy J., Boucher R. Relative ion permeability of normal and cystic fibrosis nasal epithelium. J Clin Invest. 1983 May;71(5):1410–1417. doi: 10.1172/JCI110894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E., Polimeni P. I., Macchia D. D. Chloride distribution and exchange in vertebrate heart and skeletal muscle. Ann N Y Acad Sci. 1980;341:524–533. doi: 10.1111/j.1749-6632.1980.tb47196.x. [DOI] [PubMed] [Google Scholar]
- Pato C. N., Davis M. H., Doughty M. J., Bryant S. H., Gruenstein E. Increased membrane permeability to chloride in Duchenne muscular dystrophy fibroblasts and its relationship to muscle function. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4732–4736. doi: 10.1073/pnas.80.15.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton P. M. Chloride impermeability in cystic fibrosis. Nature. 1983 Feb 3;301(5899):421–422. doi: 10.1038/301421a0. [DOI] [PubMed] [Google Scholar]
- Rugolo M., Romeo G., Lenaz G. Kinetic analysis of chloride efflux from normal and cystic fibrosis fibroblasts. Biochem Biophys Res Commun. 1986 Jan 14;134(1):233–239. doi: 10.1016/0006-291x(86)90552-8. [DOI] [PubMed] [Google Scholar]
- Shapiro B. L., Lam L. F., Fast L. H. Premature senescence in cultured skin fibroblasts from subjects with cystic fibrosis. Science. 1979 Mar 23;203(4386):1251–1253. doi: 10.1126/science.424752. [DOI] [PubMed] [Google Scholar]
- Stutts M. J., Cotton C. U., Yankaskas J. R., Cheng E., Knowles M. R., Gatzy J. T., Boucher R. C. Chloride uptake into cultured airway epithelial cells from cystic fibrosis patients and normal individuals. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6677–6681. doi: 10.1073/pnas.82.19.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villereal M. L., Cook J. S. Regulation of active amino acid transport by growth-related changes in membrane potential in a human fibroblast. J Biol Chem. 1978 Nov 25;253(22):8257–8262. [PubMed] [Google Scholar]
- Welsh M. J., Liedtke C. M. Chloride and potassium channels in cystic fibrosis airway epithelia. 1986 Jul 31-Aug 6Nature. 322(6078):467–470. doi: 10.1038/322467a0. [DOI] [PubMed] [Google Scholar]


