Abstract
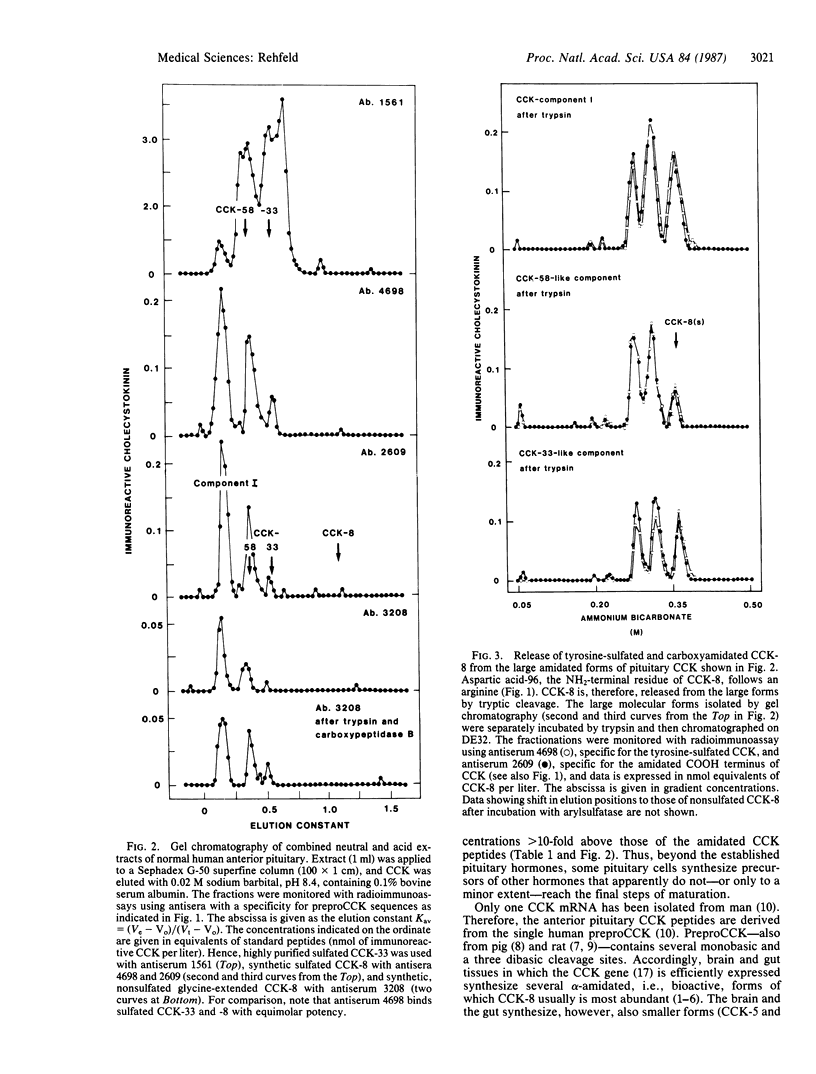
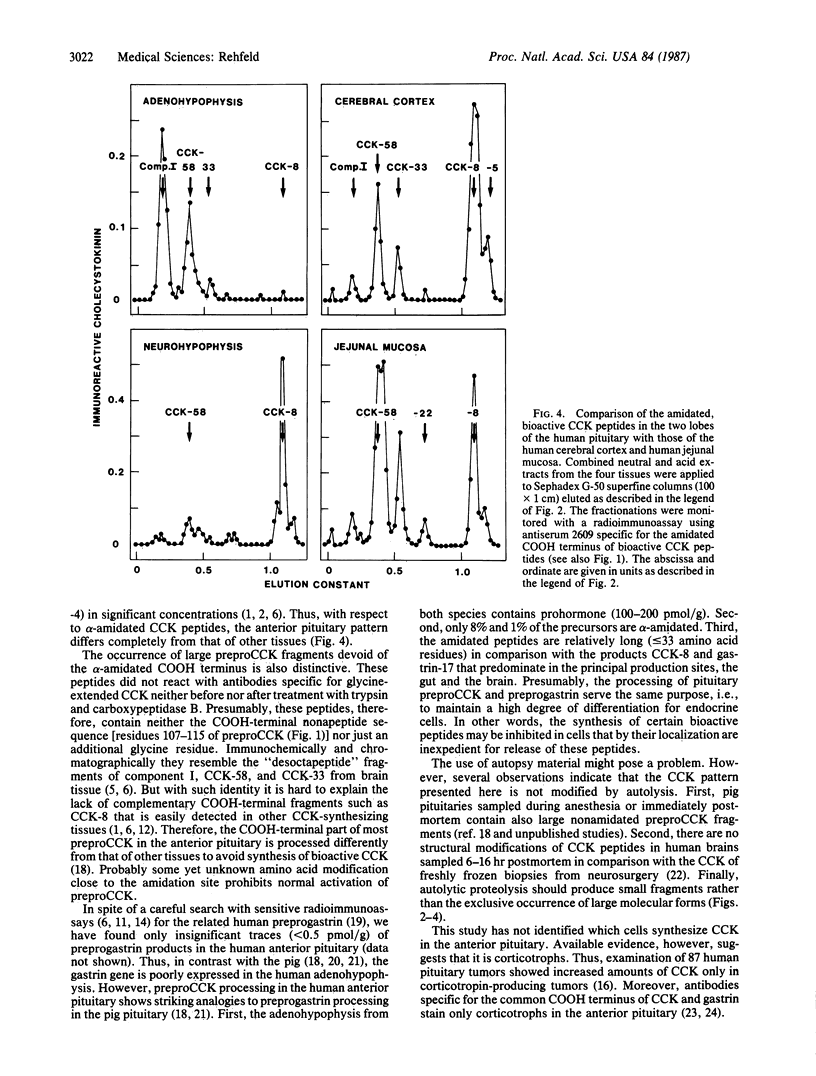
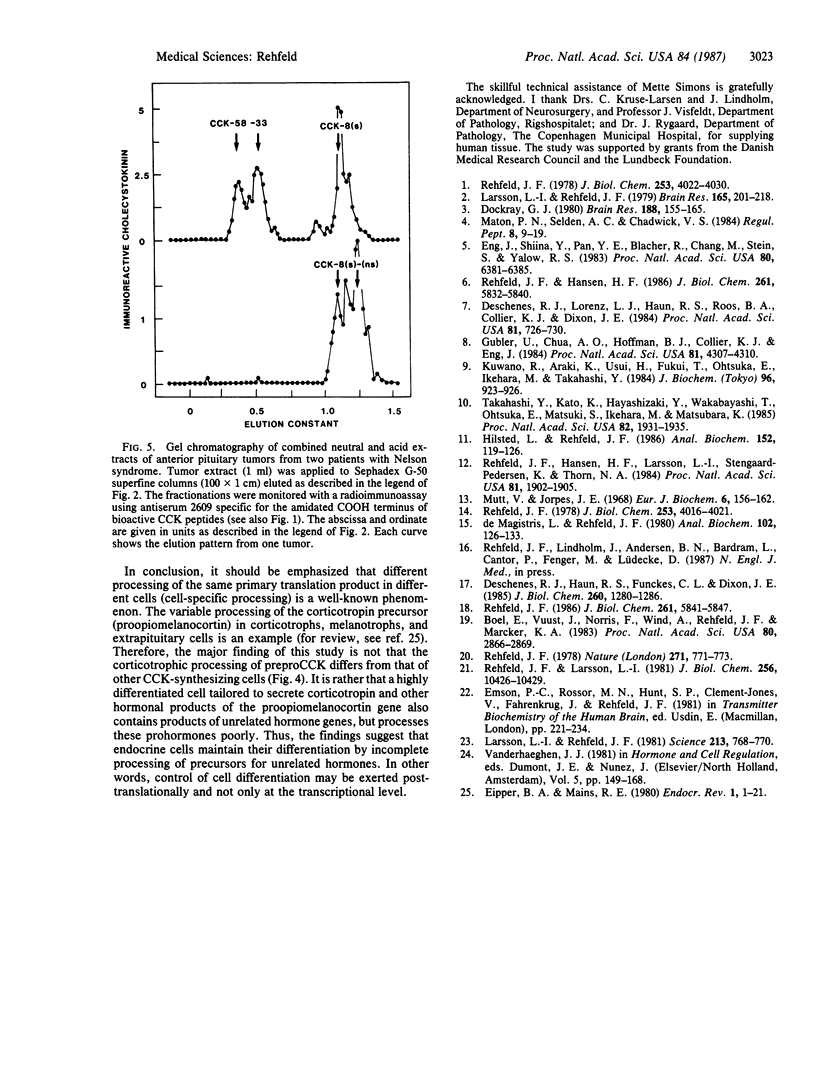
The processing of preprocholecystokinin in human pituitary extracts was investigated using gel and ion-exchange chromatography monitored by sequence-specific radioimmunoassays before and after incubation with trypsin, carboxypeptidase B, and arylsulfatase. Whereas the neural lobe contained only the bioactive alpha-carboxyamidated cholecystokinin (CCK) peptides (32 pmol/g), of which CCK-8 predominated, the anterior lobe contained substantial amounts of three large nonamidated procholecystokinin fragments (95 pmol/g; Mrs, 9000, 7000, and 5000) and small amounts of alpha-amidated CCK (8.3 pmol/g). The latter occurred only in the following large molecular forms: component I, CCK-58, and traces of CCK-33. Corticotrophic tumors processed the large forms to small CCK-8-like forms as are found in the brain and in the gut. The results show that a hormone gene, although translated, is expressed only to a limited extent as mature, active peptide outside the principal production region(s). Thus the processing of CCK to small alpha-amidated peptides in the less-differentiated tumor tissue supports the hypothesis that differentiation of endocrine cells may be sustained also at the posttranslational level.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boel E., Vuust J., Norris F., Norris K., Wind A., Rehfeld J. F., Marcker K. A. Molecular cloning of human gastrin cDNA: evidence for evolution of gastrin by gene duplication. Proc Natl Acad Sci U S A. 1983 May;80(10):2866–2869. doi: 10.1073/pnas.80.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes R. J., Haun R. S., Funckes C. L., Dixon J. E. A gene encoding rat cholecystokinin. Isolation, nucleotide sequence, and promoter activity. J Biol Chem. 1985 Jan 25;260(2):1280–1286. [PubMed] [Google Scholar]
- Deschenes R. J., Lorenz L. J., Haun R. S., Roos B. A., Collier K. J., Dixon J. E. Cloning and sequence analysis of a cDNA encoding rat preprocholecystokinin. Proc Natl Acad Sci U S A. 1984 Feb;81(3):726–730. doi: 10.1073/pnas.81.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G. J. Cholecystokinins in rat cerebral cortex: identification, purification and characterization by immunochemical methods. Brain Res. 1980 Apr 21;188(1):155–165. doi: 10.1016/0006-8993(80)90564-8. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980 Winter;1(1):1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Eng J., Shiina Y., Pan Y. C., Blacher R., Chang M., Stein S., Yalow R. S. Pig brain contains cholecystokinin octapeptide and several cholecystokinin desoctapeptides. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6381–6385. doi: 10.1073/pnas.80.20.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Chua A. O., Hoffman B. J., Collier K. J., Eng J. Cloned cDNA to cholecystokinin mRNA predicts an identical preprocholecystokinin in pig brain and gut. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4307–4310. doi: 10.1073/pnas.81.14.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilsted L., Rehfeld J. F. Measurement of precursors for alpha-amidated hormones by radioimmunoassay of glycine-extended peptides after trypsin-carboxypeptidase B cleavage. Anal Biochem. 1986 Jan;152(1):119–126. doi: 10.1016/0003-2697(86)90129-6. [DOI] [PubMed] [Google Scholar]
- Kuwano R., Araki K., Usui H., Fukui T., Ohtsuka E., Ikehara M., Takahashi Y. Molecular cloning and nucleotide sequence of cDNA coding for rat brain cholecystokinin precursor. J Biochem. 1984 Sep;96(3):923–926. doi: 10.1093/oxfordjournals.jbchem.a134911. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Rehfeld J. F. Localization and molecular heterogeneity of cholecystokinin in the central and peripheral nervous system. Brain Res. 1979 Apr 13;165(2):201–218. doi: 10.1016/0006-8993(79)90554-7. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Rehfeld J. F. Pituitary gastrins occur in corticotrophs and melanotrophs. Science. 1981 Aug 14;213(4509):768–770. doi: 10.1126/science.6266012. [DOI] [PubMed] [Google Scholar]
- Maton P. N., Selden A. C., Chadwick V. S. Differential distribution of molecular forms of cholecystokinin in human and porcine small intestinal mucosa. Regul Pept. 1984 Jan;8(1):9–19. doi: 10.1016/0167-0115(84)90024-7. [DOI] [PubMed] [Google Scholar]
- Mutt V., Jorpes J. E. Structure of porcine cholecystokinin-pancreozymin. 1. Cleavage with thrombin and with trypsin. Eur J Biochem. 1968 Oct 17;6(1):156–162. doi: 10.1111/j.1432-1033.1968.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Accumulation of nonamidated preprogastrin and preprocholecystokinin products in porcine pituitary corticotrophs. Evidence of post-translational control of cell differentiation. J Biol Chem. 1986 May 5;261(13):5841–5847. [PubMed] [Google Scholar]
- Rehfeld J. F., Hansen H. F. Characterization of preprocholecystokinin products in the porcine cerebral cortex. Evidence of different processing pathways. J Biol Chem. 1986 May 5;261(13):5832–5840. [PubMed] [Google Scholar]
- Rehfeld J. F., Hansen H. F., Larsson L. I., Stengaard-Pedersen K., Thorn N. A. Gastrin and cholecystokinin in pituitary neurons. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1902–1905. doi: 10.1073/pnas.81.6.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. I. Development of sequence-specific radioimmunoassays for porcine triacontatriapeptide cholecystokinin. J Biol Chem. 1978 Jun 10;253(11):4016–4021. [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem. 1978 Jun 10;253(11):4022–4030. [PubMed] [Google Scholar]
- Rehfeld J. F., Larsson L. I. Pituitary gastrins. Different processing in corticotrophs and melanotrophs. J Biol Chem. 1981 Oct 25;256(20):10426–10429. [PubMed] [Google Scholar]
- Rehfeld J. F. Localisation of gastrins to neuro- and adenohypophysis. Nature. 1978 Feb 23;271(5647):771–773. doi: 10.1038/271771a0. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Kato K., Hayashizaki Y., Wakabayashi T., Ohtsuka E., Matsuki S., Ikehara M., Matsubara K. Molecular cloning of the human cholecystokinin gene by use of a synthetic probe containing deoxyinosine. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1931–1935. doi: 10.1073/pnas.82.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magistris L., Rehfeld J. F. A simple enzymatic procedure for radioimmunochemical quantitation of the large molecular forms of gastrin and cholecystokinin. Anal Biochem. 1980 Feb;102(1):126–133. doi: 10.1016/0003-2697(80)90327-9. [DOI] [PubMed] [Google Scholar]