Abstract
Background
Curcumin, the active ingredient from turmeric rhizomes, has been shown to have a wide range of pharmacological properties including antioxidant and anti-inflammatory effects. Curcumin has been reviewed for its multiple molecular action on inhibiting tumor angiogenesis via its mechanisms of cyclooxygenase (COX)-2, and vascular endothelial growth factor (VEGF) inhibition. In this present study, we aimed to assess the effects of curcumin on preventing diabetes-induced vascular dysfunction in association with COX-2, nuclear factor-κB (NF-κB) expression, and prostanoid production.
Methods
Twelve-week-old male Wistar rats were separated into five groups: 1) diabetes with 0.9% normal saline (DM-NSS; n =10), 2) diabetes treated with curcumin 30 mg/kg (n =10), 3) diabetes treated with curcumin 300 mg/kg (n =10), 4) the control with 0.9% normal saline (n =10), and 5) the control treated with 300 mg/kg (n =10). Daily oral feeding of curcumin was started at 6 weeks after the streptozotocin injection. Levels of 6-keto prostaglandin (PG) F1αand thromboxane (TX) B2 were determined from mesenteric perfusates using enzyme immunoassay kits. Protein kinase C (PKC)-β II and COX-2 with NF-κB levels were analyzed in the mesenteric arteries by immunofluorescent staining and immunohistochemistry, respectively.
Results
The ratio of 6-keto-PGF1αand TXB2 was significantly decreased in DM-NSS compared with the control (P < 0.05). Double-immunofluorescent staining with specific antibodies for PKC-βII and α-smooth muscle actins showed that the diabetic mesenteric arteries contained increased of PKC-βII within the vascular wall. Also, COX-2 expression and activated NF-κB in the small mesenteric artery of diabetes mellitus rats were markedly increased when compared with the control. Interestingly, curcumin could inhibit the upregulation of all of these biomarkers.
Conclusion
These findings show that curcumin can attenuate diabetes-induced vascular dysfunction in association with its potential for COX-2 and NF-κB suppression, PKC inhibition, and improving the ratio of prostanoid products PGI2/TXA2.
Keywords: diabetes, endothelial dysfunction, COX-2, prostanoids
Introduction
Diabetes mellitus (DM) is a common metabolic disease with a high and growing prevalence affecting 4% of the population worldwide: 171 million people in the year 2000 and an expected 366 million in 2030.1 Diabetic vascular diseases represent a major cause of mortality and morbidity in diabetic patients. Both micro- and macrovascular complications are the burden of the disease, not only in terms of individual health and wellbeing, but also in terms of the impact on the economic status of a patient’s family and their country.2 It is suggested that hyperglycemia induces an intracellular elevation of reactive oxygen species (ROS). The cumulative ROS can consequently cause long-term changes in the structure and functions of macromolecules, including protein, lipids, and DNA. With this rationale, the dysfunction of the endothelial cells has been documented as a common finding in diabetic patients with its underlining causes of oxidative stress. The potential contribution of increased ROS to the development of endothelial dysfunction in diabetes has received considerable interest, since it interferes with the production of nitric oxide (NO), a key factor in multiple processes of vascular functional homeostasis. It has been pointed out that one of the major pathways that increases ROS production in endothelial cells is the diacylglycerol (DAG)–protein-kinase C (PKC) pathway. Moreover, high-glucose activated PKC upregulation has been reported for its significant role in inducing diabetic endothelial dysfunction.3–6 It has been demonstrated that oxidative stress will be produced more and more via this PKC upregulation, which leads to the activations of nuclear factor-κB (NF-κB) and cyclo-oxygenase (COX)-2 expressions.7–11 Previous studies have shown that in vitro incubation of rabbit arteries with a high glucose concentration increases vasoconstrictor prostanoids. These effects were prevented by both COX inhibitors and prostaglandin (PG)H2/ thromboxane (TX)A2 receptor antagonist, thereby restoring endothelium-dependent relaxation.8 Recent biochemical studies have proposed a possible role for enhanced COX-2 expression in high glucose-induced alterations in vasoconstrictor prostanoid production in cultured endothelial cells.9 Also, it has been demonstrated that upregulation of COX isoforms is associated with a significant elevation of vascular prostaglandin synthesis.10 However, there have been only a limited number of studies investigating the consequences of alterations in microvascular prostanoid synthesis.11
Curcumin, which has been shown to have a wide range of pharmacological properties including antioxidant and anti-inflammatory effects, has been reviewed for its multiple molecular targets on inhibiting PKC, COX-2, and NF-κB expressions.12–14 The antioxidant effect of curcumin has been found to be at least 10 times greater than that of vitamin E. It has been demonstrated that the antioxidant activity of curcumin could be mediated through antioxidant enzymatic systems including superoxide dismutase (SOD), catalase, and glutathione peroxidase.15,16
Even though the beneficial effect of curcumin treatment on hypoglycemia in streptozotocin (STZ)-induced diabetes rats has been reported,17 its molecular mechanisms have not been clarified yet. The mechanism by which curcumin improves this situation is probably due to its hypocholesterolemic influence, antioxidant nature, and increase in plasma insulin levels.18–20
The anti-inflammatory effects of curcumin have been shown to mediate through the suppression of both COX-2 and lipoxygenase proteins as well as the downregulation of NF-κB. The anti-inflammatory pathways of curcumin have been discovered mostly from the tumor cell study, however, the effects of curcumin on a diabetic animal model remain unknown.20–22
Therefore, in the present study, we aimed to assess the effects of plant-derived antioxidant, curcumin, on diabetesinduced endothelial dysfunction in association with its mechanism on NF-κB, COX-2, and prostanoid ratio.
Method
Twelve-week-old male Wistar rats were housed in a temperature- and light-controlled environment, and were fed standard feed and drank tap water ad libitum. The present study was conducted in accordance with the guidelines for animal experimentation established by the National Research Council of Thailand and approved by the Institutional Animal Care and Use Committee of Chulalongkorn University.
Induction of experimental diabetes
The rats were randomly divided into nondiabetic and diabetic groups. Diabetic rats were induced by a single intravenous injection of STZ (55 mg/kg) (Sigma-Aldrich, St. Louis, MO). STZ was freshly prepared by dissolving in citrate buffer pH 4.5 (Sigma-Aldrich) and immediately injected into the tail vein after 8 hours of fasting. The control rats received instead a citrate buffer of the same volume. STZ-induced diabetic rats were included and retained for the experiments if their blood glucose was greater than 200 mg/dL. Blood glucose was measured using a glucometer (ACCU-CHEK, ADVANTAGE; Roche Diagnostics, Mannheim, Germany). Animals were separated into five groups: 1) diabetes treated with 0.9% normal saline group (DM-NSS; n =10), 2) diabetes treated with curcumin (Cayman Chemical, Ann Arbor, MI) 30 mg/kg dissolved in corn oil (DM-CUR30; n =10), 3) diabetes treated with curcumin 300 mg/kg (DM-CUR300; n =10), 4) control treated with 0.9% normal saline group (CON-NSS; n =10), and 5) control treated with 300 mg/kg bodyweight (CONCUR300; n =10). It is noted that the daily oral feeding of curcumin was started at 6 weeks after the STZ injection since it was shown in our previous study that endothelial dysfunction in STZ-rats occurred 6 weeks after STZ injection.23 The feeding of curcumin was continued for 8 weeks.
Measurement of metabolic parameters
At the end of each experiment, a blood sample from each rat was collected for further plasma glucose and glycosylated hemoglobin (HbA1c) determination, using the enzymatic method and the turbidimetric immunoinhibition method, respectively (Bangkok RIA Laboratory Co. Ltd, Bangkok, Thailand).
Determination of prostanoid levels
PGI2 is rapidly hydrolyzed nonenzymatically from 6-keto- PGF1α. Therefore, in most studies, 6-keto-PGF1αis widely used as an indicator for determining PGI2 production. TXB2, which is the hydrolysis product of TXA2, is commonly used as an indicator of TXA2 production as well.
Under pentobarbital sodium anesthesia, the abdominal cavity was opened via the midline position. The mesentery was exteriorized. The ileocecal portion of the mesentery was carefully spread on a plexiglass chamber and continuously perfused by 1 mL/min Kreb-Ringer buffer solution (37°C, pH 7.4 composition in mmol/L; 135.7 NaCl, 4.7 KCl, 2.52 CaCl2, 1.18 KH2PO4, 1.64 MgSO4.7H2O, and 7.14 NaHCO3) for 15-minute equilibration. After 15 minutes, 1 mL of the mesenteric perfusate was collected immediately for the measurements of existing 6-keto-PGF1αand TXB2 (basal level). The levels of 6-keto-PGF1αand TXB2 were determined by using enzyme immunoassay kits. According to the manufacturer’s protocols (Cayman Chemical), the quantitative levels of 6-keto-PGF1αand TXB2 could be assessed by the standard calibration curves of both substances.
Immunofluorescent staining for PKC-βII
Under pentobarbital sodium anesthesia, the rat mesentery was exposed. A single unbranched small mesenteric artery with a diameter of approximately 100 μm was selected for study. The selected microvessel was then dissected and cleared of connective tissue and briefly rinsed in icecold phosphate-buffered saline (PBS). After collection, the mesenteric arteries were then immediately fixed in 4% paraformaldehyde for 24 hours and were embedded in paraffin. These specimens were then deparaffinized in xylene, rehydrated in graded ethanol and distilled water, and the antigen unmasked with sodium citrate (10 mmol/L, pH 6.0) (Dako, Glostrup, Denmark), followed by a microwave heat source on high power for 3 minutes and then with low power for 10 minutes. After the PBS wash, the nonspecific background was blocked with 3% normal horse serum at room temperature for 20 minutes. Incubation with anti-PKC-βII (1:100 dilutions) (SC-210; Santa Cruz Biotechnology, Santa Cruz, CA) was performed at room temperature for 60 minutes. Anti-α-smooth muscle actin (1:200 dilutions) (Dako) was also used to colocalize microvascular smooth muscle. Sections were then washed in PBS and incubated with the secondary antibody swine anti-rabbit IgG-TRITC (1:50 dilution) (R0156; Dako) for PKC-βII and rabbit anti-mouse IgG-FITC (1:50 dilution) (R0261; Dako) for smooth muscle actin at room temperature for 30 minutes. The arteries received two 3-minute washes in PBS and were covered with mounting medium containing DAPI (4,6-diamidino-2-phenylindole) (Vector Laboratories, Burlingame, CA) for fluorescent counterstaining of nuclei. Labeling of the arteries with secondary antibody alone was used as negative controls. Images were obtained using laser scanning confocal microscopy (E800; Nikon, Tokyo, Japan) to establish the localization of PKC-βII and α-smooth muscle actin in the small mesenteric arteries.
Immunohistochemistry for COX-2
After the collection, the mesenteric arteries were fixed by the same protocol. Paraffin-embedded sections of the mesenteric arteries were sequentially exposed to the solutions as described previously. COX-2 was immunohistochemically detected by incubation with rabbit anti-COX-2 (1:500 dilutions) (RP111; Diagnostic Biosystems, Pleasanton, CA) followed by the appropriate secondary antibody, anti-rabbit IgG horseradish peroxidase.
Immunohistochemistry for NF-κB
Activated NF-κB was detected by incubation with anti- NF-κB p65 rabbit polyclonal antibody (1:150 dilution) (SC-109; Santa Cruz Biotechnology), which was recognized as an epitope accessible only when NF-κB is bound to DNA. This was followed by incubation with a horseradish peroxidase- conjugated secondary antibody. The data were expressed as a positive signal in the vasculature. The stained sections were examined under a microscopy system (Optiphot 2; Nikon) equipped with Nikon Digital Sight DS-Fi1, DS-L2, and 20×objective lens (CF Plan Fluor; Nikon).
Quantitative analysis for the amount of COX-2 and NF-κB p65 was measured using Global Lab Image/2 software (Data Translation, Marlborough, MA). Using the histogram tool of the software, both the lowest and highest intensity values in the brown immunoreactive area were determined, and then the total number of pixels which had the intensity within those threshold values was obtained for small regions of interest (ROI) (45 ×45 pixels). Means ±standard error of mean (SEM) of the total number of pixels of immunoreactions of COX-2 or NF-κB p65 were calculated by averaging the small ROI of each video frame.
where, xi is the total number of brown-precipitate pixels in each ROI, and n is the total number of ROI in each video frame.
Statistical analysis
All data were expressed as mean ±SEM. For comparison among groups of animals, one-way analysis of variance (ANOVA) was used. Also Tukey’s post-hoc test was used when comparing the difference of the means between the diabetic rats and the controls, and between the diabeticuntreated and diabetic-treated animals. The statistical probabilities, P < 0.001, P <, 0.01, and P <, 0.05, were considered statistically significant. The data were analyzed with the SPSS program for Windows (version 16.0; SPSS Inc., Chicago, IL).
Results
In Table 1, bodyweight, mean arterial blood pressure (mABP), plasma glucose, and HbA1c values are shown for each group. The blood glucose and percentage of HbA1c of all diabetic groups (DM-NSS, DM-CUR30, and DMCUR300) were significantly higher than those of the control (P < 0.01). Interestingly, among DM-NSS, DM-CUR30, and DM-CUR300, there was a significant difference in percentage of HbA1c (P <0.05).
Table 1.
Bodyweight and mean arterial blood pressure, blood glucose, and percentage HbA1c
| Group | BW (g) | mABP (mmHg) | BG (mg/dL) | HbA1c (%) |
|---|---|---|---|---|
| CON-NSS | 398.7 ± 12.3 | 103.1 ± 3.5 | 101.8 ± 4.89 | 3.68 ± 0.17 |
| CON-CUR300 | 403.8 ± 17.0 | 106.1 ± 3.9 | 106.8 ± 0.92 | 4.08 ± 0.41 |
| DM-NSS | 285.3 ± 8.8a | 151.6 ± 9.6a | 459.0 ± 24.40a | 10.73 ± 0.32a |
| DM-CUR30 | 292.7 ± 17.8a | 128.3 ± 3.6c | 360.8 ± 35.82a | 8.20 ± 0.88a,c |
| DM-CUR300 | 325.7 ± 13.0b | 122.2 ± 8.6c | 310.00 ± 32.73a,c | 7.90 ± 0.97a,c |
Notes: P < 0.01 significant difference compared with control;
P < 0.05 significant difference compared with control;
Significant difference compared with diabetic rat (P < 0.05). Values are means ±SEM (n =10 for each group).
Abbreviations: BG, blood glucose; BW, bodyweight; CON-NSS, control with 0.9% normal saline; CON-CUR300, control treated with 300 mg/kg; DM-CUR30, diabetestreated with curcumin 30 mg/kg; DM-CUR300, diabetes-treated with curcumin 300 mg/kg; DM-NSS, diabetes with 0.9% normal saline; HbA1c, glycosylated hemoglobin; mABP, mean arterial blood pressure; SEM, standard error of the mean.
Only the DM-NSS group had a significantly increased mABP when compared with the CON-NSS group (P <0.01). Conversely, the levels of mABP of DM-CUR30 and DMCUR300 were significantly lower than those of age-matched untreated diabetic rats (P <0.05).
Supplementation of curcumin 30 and 300 mg/kg per day could lower blood glucose in the diabetic group down to 18.73% and 30.26%, respectively. This antidiabetic action of curcumin is confirmed by previous reports, which show that curcumin can mediate its hypoglycemic effect through the stimulation of the pancreas to produce and secrete insulin.24–27 Treatment of streptozotocin in our diabetes model causing beta cell destruction leads to hyperglycemia with hypoinsulinemia. However, limited B-cells regeneration may occur, allowing cellular responses to curcumin activation. However, the curcumin supplementation could not reduce blood glucose back to its normal state. The blood glucose and HbA1c of both DMCUR30 and DM-CUR300 still significantly increased when compared with CON-NSS. In other words, the antidiabetic effect of curcumin is not great enough to be used alone.
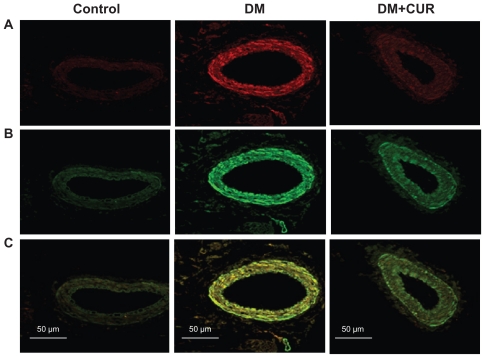
In Figure 1, immunofluorescent staining of small mesenteric arteries displays a strong signal for PKC-βII in the diabetic rats (Figure 1B). Double-immunofluorescent staining with specific antibodies for PKC-βII and α-smooth muscle actin shows that the diabetic mesenteric arteries contained PKC- βII within the vascular smooth muscle cells (Figure 1C). Interestingly, supplementation with curcumin (300 mg/kg) could reduce PKC-II expression in DM-CUR 300, with results looking similar to the control staining results. It is noted that the negative control displays a minimal detectable fluorescence when the secondary antibodies were used alone. These results suggest that mesenteric vessels in the DM-NSS have higher levels of PKC-βII both in the endothelium and the smooth muscle cells.
Figure 1.
Co-immunofluorescent staining of protein kinase C (PKC)-βII and vascular smooth muscle cells in mesenteric arteries (diameter =100–120 μm) taken from control, diabetes mellitus (DM), and DM curcumin 300 (DM-CUR 300) groups. Double-immunofluorescent staining was performed to demonstrate PKC-βII in the mesenteric arteries (red fluorescence; A), α-smooth muscle actin (green fluorescence; B), and merged image (yellow; C). Magnification: ×400.
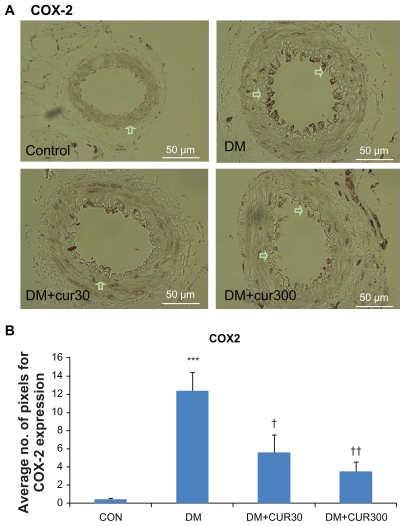
Figure 2A and 2B demonstrate that COX-2 expression in the small mesenteric artery of the DM rats was markedly increased when compared with the control. Curcumin supplementation reduced COX-2 expression in the mesenteric microvessels significantly when using low and high doses of curcumin (DM-CUR30, P < 0.05; DM-CUR300, P < 0.01); although these reduced COX-2 expressions do not seem to completely eliminate the COX-2 expression in diabetic arteries.
Figure 2.
A) Immunostaining for cyclo-oxygenase (COX)-2 in small mesenteric arteries. Sections of arterial segment show the endothelial cell layer inwards. Positive immunoreactions are observed as a brown precipitate.
Notes: CON-NSS (left upper panel), DM-NSS (right upper panel), DM-CUR30 (left lower panel), and DM-CUR300 (right lower panel) rats. Magnification: ×400. B) Expression of COX-2 in the endothelium layer in small mesenteric arteriesusing image analysis (Global Lab Image/2 software) measurements in the DMNSS, DM-CUR30, and DM-CUR300 groups. Data are expressed as mean ±SEM. ***P < 0.001 significant difference compared with control arteries; †P <0.05 significant difference compared with diabetic arteries; ††P < 0.01 significant difference compared with diabetic arteries.
Abbreviations: CON-NSS, control with 0.9% normal saline; DM-CUR30, diabetes-treated with curcumin 30 mg/kg; DM-CUR300, diabetes-treated with curcumin 300 mg/kg; DM-NSS, diabetes treated with 0.9% normal saline; SEM, standard error of the mean.
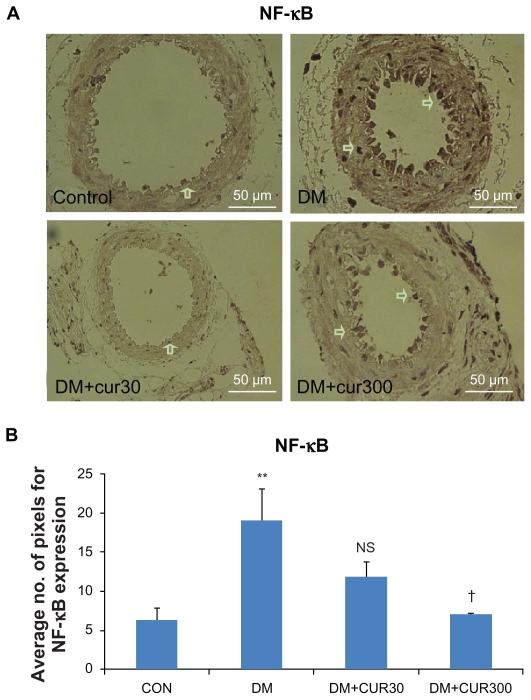
Figure 3A and 3B demonstrate that NF-κB p65 was expressed more in the diabetic small mesenteric arteries when compared with the control. DM-CUR300 seemed to attenuate this diabetes-induced NF-κB p65 upregulation significantly (P <0.05).
Figure 3.
A) The expression of nuclear factor-κB p65 (NF-κB p65) in small mesenteric arteries in the control (left upper panel), DM (right upper panel), DM-CUR30 (left lower panel) and DM-CUR300 (right lower panel) rats. Positive immunoreactions are observed as a brown precipitate. Magnification: ×400. B) Expression of NF-κB in the endothelium layer in small mesenteric arteries using image analysis (Global Lab Image/2 software) measurement in the DM-NSS, DM-CUR30, and DM-CUR300 groups.
Notes: Data are expressed as mean ±SEM. NS, no significant difference compared with diabetic arteries. **P < 0.01 significant difference compared with control arteries. †P < 0.05 significant difference compared with diabetic arteries.
Abbreviations: DM-CUR30, diabetes-treated with curcumin 30 mg/kg; DM-CUR300, diabetes-treated with curcumin 300 mg/kg; DM-NSS, diabetes treated with 0.9% normal saline; NF-κB, nuclear factor-κB; SEM, standard error of the mean.
Table 2 indicates that 6-keto-PGF1αrelease was significantly decreased in the DM group (407.6 ±37 pg/mL) when compared with the control (536.9 ±53.3 pg/mL) (P < 0.05). There was a signif icantly increased TXB2 production in the DM group (58.4 ±5.8 pg/mL) when compared with their respective controls (29.4 ±4.3 pg/mL) (P <0.05). In addition, there was a significantly decreased 6-keto- PGF1α/TXB2 ratio in the DM group (7.0) when compared with their respective controls (18.4) (P < 0.05). It is to be noted that in the prostanoids ratio study, only the high-dose supplementation of curcumin (DM-CUR300) was conducted. This high dose could be a more effective dose of curcumin supplementation to reduce the diabetes-induced vascular NF-κB p65 and COX-2 upregulation.
Table 2.
Means ±SEM of basal levels of 6-keto-PGF1α and TXB2 together with the 6-keto-PGF1α/TXB2 ratios in the mesenteric arteriolar bed of CON-NSS, DM-NSS, and DM-CUR300 groups
| Group | 6-keto-PGF1α level (pg/mL) | TXB2 level (pg/mL) | 6-keto-PGF1α/TXB2 ratio |
|---|---|---|---|
| CON-NSS | 536.9 ± 53.3 | 29.4 ± 4.3 | 18.4 ± 0.6 |
| DM-NSS | 407.6 ± 37.1a | 58.4 ± 5.8a | 7.0 ± 0.06a |
| DM-CUR 300 | 502.3 ± 27.6b | 47.4 ± 7.2b | 10.7 ± 1.0b |
Note: P < 0.05 significant difference compared with control arterioles
No significant difference compared with control arterioles. Values are means ±SEM (n = 10 for each group).
Abbreviations: CON-NSS, control with 0.9% normal saline; DM-CUR300, diabetes treated with curcumin 300 mg/kg; DM-NSS, diabetes treated with 0.9% normal saline; PGF1α, prostaglandin F1α; SEM, standard error of the mean; TXB2, thromboxane B2.
Discussion
In the present study, we have shown that the effects of curcumin supplementation on attenuating diabetes-induced endothelial dysfunction are closely associated with its antioxidant and anti-inflammatory properties. Two doses of curcumin were used in this study. The rationale of these low and high doses was based on the literature reviews that each antioxidant and anti-inflammatory action was indicated by different optimal doses.20 A high dose of curcumin supplementation seems to prevent the dysregulation for the multiple signaling pathways induced by diabetes. As such, the idea that curcumin may help prevent diabetic vascular complications was confirmed by the present finding that supplementation with a high dose of curcumin, 300 mg/kg per day, appears to improve the diabetic endothelial functions which was shown by the decrease in mABP and the suppression of COX-2 and NF-κB at the mesenteric arterial wall.
At present, the mechanisms of increased oxidative stress in diabetes are demonstrated on a multifactor basis.6–9 The pathway of DAG-PKC is well documented as one mechanism of increasing oxidative stress since such activation leads to altered NADPH (nicotinamide adenine dinucleotide phosphate) oxidase. From our previous report, it is shown that curcumin can decrease superoxide at the mesenteric vascular wall as demonstrated by the oxygen radical-sensitive fluorescent probe, hydroethidine.28 It was shown that supplementation with a high dose of curcumin could decrease malondialdehyde, the common indicator of oxidative stress, by almost 50% in the STZ-rat model.29,30
The interaction between NO and O2•−occurred at an extremely rapid rate, three times faster than the reaction rate between O2•−and SOD.31 Therefore, this hyperglycemiainduced O2•−may quench NO and directly contribute to the dysfunction of several consequently endothelium-dependent processes. In addition, it is well known that diabetes-induced ROS play a key role in enhancing inflammation through the activation of stress kinases and redox sensitive transcription factors such as NF-κB. NF-κB, a redox-sensitive factor and a key regulator of antioxidant enzymes, can initiate transcription of many genes involved in inflammatory and immune response. In unstimulated cells, NF-κB is a heterodimeric complex that is sequestered in the cytoplasm by interacting with the inhibitory I-κB family. When these cells are stimulated, I-κB is phosphorylated with subsequent release of NF-κB resulting in the translocation of NF-κB from the cytoplasm to the nucleus where it can initiate the expression of various target genes.31,32
In this study, the results showed that the expression of NF-kB p65 in the small mesenteric arterial wall was increased significantly in the DM group. The results of quantitative comparison using image analysis (Global Lab II software) indicated that only the high doses of curcumin supplementation (300 mg/kg bodyweight) could decrease this upregulated NF-κB p65 expression significantly (P < 0.05) (Figure 3B).
It was reported that NF-κB was suppressed by curcumin through inhibiting the activity of I-κB kinase (IKK).33 Oxidative stress activates NF-κB-mediated transcription of proinflammatory mediators either through the activation of its activating inhibitor, IKK, or the enhanced recruitment or activation of transcriptional co-activators. In cancer-related inflammation, although numerous different pathways are activated during the inflammatory response, NF-κB is thought to be of the most importance.34
Activation of peroxisome proliferator-activated receptors gamma (PPARγ) by curcumin resulted in inhibition of NF-κB trans-activating activity and increased expression of PPARγ at both the transcriptional and translational levels in activated hepatic stellate cells.35 In principle, curcumin has been widely demonstrated to have potent antioxidant activities. It was reported that curcumin could increase antioxidant glutathione levels by induction of glutamate cysteine ligase and act as an anti-inflammatory agent through inhibition of NF-κB signaling.36
This is the first report showing that curcumin, a polyphenol, has beneficial effects on significantly decreased COX-2 expression in the diabetic endothelial layer. Although in diabetes, the changes in vascular prostanoid production are clearly indicated in particular in association with diabetic vasculopathy, the role of COX-2 in diabetes is not very clear.37,38 an in vivo relevance to diabetic complications was suggested by the observation of high levels of COX-2 mRNA presented in monocytes from type 1 or type 2 diabetic patients, but not from normal volunteers.39 Moreover, it has been shown that this high glucose-induced COX-2 mRNA expression was implicated by the involvement of multiple pathways, including the p38 MAPK, PKC, and NF-κB. As shown in the present findings, high glucose enhanced the increase of PKC, COX-2, and NF-κB, particularly at the endothelial lining of mesenteric arteries. Moreover, the results of quantitative comparison using image analysis (Global Lab II software) showed that the high doses of curcumin supplementation could significantly decrease COX-2 (P < 0.05) (Figure 2B).
In our study, the unstimulated (basal) level of 6-keto- PGF1α (stable metabolite of PGI2) in the diabetic rats was markedly reduced compared with those of the control rats. While curcumin enhances 6-keto-PGF1α production up to almost basal level of control rats (Table 2), the basal levels of TXB2 (stable metabolite of TXA2) in diabetic rats were significantly elevated as compared with those of the control rats. These findings may have implications for the existing role of diabetes-induced COX-2 expression, since the prostaglandin which may be responsiblefor an enhanced production of TXA2 while reducing the production of PGI2. In addition, we also found a marked COX-2 immunostaining in the mesenteric arteries of diabetic animals, which was localized in the endothelial layer of the arterial wall (Figure 2A). These results are in accordance with other studies obtained from both diabetic animal models40 and human diabetes.41
Interestingly, supplementation with curcumin could improve the ratio of prostanoid change to higher PGI2 than TXA2 level (Table 2). According to prostanoid levels, slight immunostaining for COX-2 was detected in the arteries of diabetic rats supplemented with curcumin (Figure 2A). Thus, curcumin has been found to be effective in inhibiting TXA2 synthesis in inflammation by modulating the COX-2-pathway.
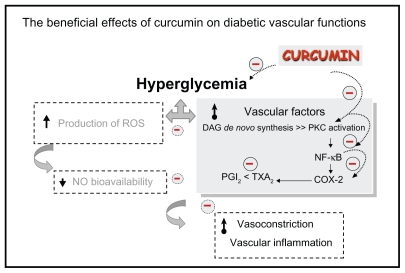
In conclusion, our findings provide in vivo evidence that curcumin supplementation (300 mg/kg) significantly improved diabetes-induced endothelial dysfunction related to its potential for COX-2 and NF-κB suppression and PKC inhibition, and resulted in an improved ratio of prostanoid products (as summarized in Figure 4).
Figure 4.
The beneficial effects of curcumin on diabetic vascular functions. Curcumin supplementation (300 mg/kg bodyweight) improved diabetes-induced vascular dysfunction associated with its potential to reduce blood sugar, COX-2 and NF-κB suppression, PKC inhibition, and improve the ratio of prostanoid products PGI2 and TXA2.
Abbreviations: COX-2, cyclooxygenase-2; DAG, diacylglycerol; NF-κB, nuclear factor-κB; PGI2, prostaglandin I2; PKC, protein kinase C; ROS, reactive oxygen species; TXA2, thromboxane A2.
Indeed, it is important to note that ruboxistaurin (LY333531) mesylate, a PKC-isozyme-selective inhibitor, is in Phase III clinical trials in patients with type 1 and type 2 DM to determine its efficacy in preventing the development of diabetic microvascular complications.42 In addition, clinical trials using up to 8000 mg curcumin per day for 3 months have shown no toxicity.43 Therefore, it is possible that curcumin supplementation might be beneficial for diabetic patients by improving microvascular functions and preventing the consequences of cardiovascular complications. Therefore, curcumin might be considered as a phytoceutical agent to be used for the treatment of diabetic vascular complications in diabetes patients in the future.
Acknowledgements
This study was supported by Ratchadaphiseksomphot Fund, Faculty of Medicine, the 90th Anniversary of Chulalongkorn University Fund, Graduate School, Chulalongkorn University.
Footnotes
Disclosure
The authors declare that they have no competing interests related to this study.
Authors’ contributions
SR participated together with co-authors in the design of this study. The experiments were carried out by SR, SP, and NT. PR was the immunotechnique assistant. SR interpreted and analyzed the data. SR prepared the initial draft of the manuscript, and SP read and revised the manuscript. All authors read and approved the manuscript.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Mortality and country Fact Sheet 2006. [Accessed Apr 2006]. Available from: http://www.who.int/entity/healthinfo/statistic.
- 3.VanderJagt DJ, Harrison JM, Ratliff DM, Hunsaker LA, Vander Jagt DL. Oxidative stress indices IDDM subjects with and without long-term diabetic complications. Clin Biochem. 2001;34:265–270. doi: 10.1016/s0009-9120(01)00204-1. [DOI] [PubMed] [Google Scholar]
- 4.Bunnag SC. Implications of microcirculation-research based information on prevention and treatment of diabetes mellitus type 2: a perspective. Clin Hemorheol Microcirc. 2006;34:43–50. [PubMed] [Google Scholar]
- 5.Chakravarthy U, Hayes RG, Stitt AW, McAuley E, Archer DB. Constitutive nitric oxide synthase expression in retinal vascular endothelial cells is suppressed by high glucose and advanced glycation end products. Diabetes. 1998;47:945–952. doi: 10.2337/diabetes.47.6.945. [DOI] [PubMed] [Google Scholar]
- 6.Muniyappa R, Srinivas PR, Ram JL, Walsh MF, Sowers JR. Calcium and protein kinase C mediate high-glucose-induced inhibition of inducible nitric oxide synthase in vascular smooth muscle cells. Hypertension. 1998;31:289–295. doi: 10.1161/01.hyp.31.1.289. [DOI] [PubMed] [Google Scholar]
- 7.Sharpe PC, Liu WH, Yue KK, et al. Glucose-induced oxidative stress in vascular contractile cells: comparison of aortic smooth muscle cells and retinal pericytes. Diabetes. 1998;47:801–809. doi: 10.2337/diabetes.47.5.801. [DOI] [PubMed] [Google Scholar]
- 8.Tesfamariam B, Brown ML, Deykin D, Cohen RA. Elevated glucose promotes generation of endothelium-derived vasoconstrictor prostanoids in rabbit aorta. J Clin Invest. 1990;85:929–932. doi: 10.1172/JCI114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosentino F, Eto M, de Paolis P, van der Loo B, Bachschmid M, Ullrich V, et al. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation. 2003;107:1017–1023. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- 10.Quilley J, Chen YJ. Role of COX-2 in the enhanced vasoconstrictor effect of arachidonic acid in the diabetic rat kidney. Hypertension. 2003;42:837–843. doi: 10.1161/01.HYP.0000085650.29823.F2. [DOI] [PubMed] [Google Scholar]
- 11.Davidge ST. Prostaglandin H synthase and vascular function. Circ Res. 2001;89:650–660. doi: 10.1161/hh2001.098351. [DOI] [PubMed] [Google Scholar]
- 12.Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK. Turmeric and curcumin: biological actions and medicinal applications. Curr Sci. 2004;87:44–53. [Google Scholar]
- 13.Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. In: Aggarwal BB, Young-Joon S, Shishodia S, editors. Targets and Therapeutic Uses of Curcumin in Health and Disease. New York, NY: Springer; 2007. [Google Scholar]
- 14.Farhangkhoee H, Khan ZA, Chen S, Chakrabarti S. Differential effects of curcumin on vasoactive factors in the diabetic rat heart. Nutr Metab (Lond) 2006;3:27. doi: 10.1186/1743-7075-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adam BK, Cai J, Armstrong J, et al. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs. 2005;16:263–275. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Khopde SM, Priyadarsini KI, Venkatesan N, Rao MNA. Free radicail scavenging ability and antioxidant efficiency of curcumin and its substituted analogue. Biophys Chem. 1999;80:85–91. doi: 10.1016/s0301-4622(99)00070-8. [DOI] [PubMed] [Google Scholar]
- 17.Mahesh T, Sri Balasubashini MM, Menon VP. Photo-irradiated curcumin supplementation in streptozotocin-induced diabetic rats: effect on lipid peroxidation. Therapie. 2004;59:639–644. doi: 10.2515/therapie:2004110. [DOI] [PubMed] [Google Scholar]
- 18.Meghana K, Sanjeev G, Ramesh B. Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: a prophylactic and protective role. Eur J Pharmacol. 2007;577:183–191. doi: 10.1016/j.ejphar.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Murugan P, Pari L. Effect of tetrahydrocurcumin on lipid peroxidation and lipids in streptozotocin-nicotinamide-induced diabetic rats. Basic Clin Pharmacol Toxicol. 2006;99:122–127. doi: 10.1111/j.1742-7843.2006.pto_447.x. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41(1):40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plummer SM, Hollooway KA, Manson MM, et al. Inhibition of cyclooxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 22.Chun KS, Keum YS, Han SS, Song YS, Kim SH, Surh YJ. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF-kappaB activation. Carcinogenesis. 2003;24:1515–1534. doi: 10.1093/carcin/bgg107. [DOI] [PubMed] [Google Scholar]
- 23.Sridulyakul P, Chakraphan D, Patumraj S. Vitamin C supplementation could reverse diabetes-induced endothelial cell dysfunction in mesenteric microcirculation in STZ-rats. Clin Hemorheol Microcirc. 2006;34:315–321. [PubMed] [Google Scholar]
- 24.Halim E, Hussain MA. Hypoglycemic, hypolipidemic and antioxidant properties of combination of curcumin from Curcuma Longa, Linn, and partially purified product from Abroba Augusta, Linn, in streptozotocin induced diabetes. Indian J Clinical Biochem. 2002;17:33–43. doi: 10.1007/BF02867969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma S, Kulkarni SK, Chopra K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2006;33:940–945. doi: 10.1111/j.1440-1681.2006.04468.x. [DOI] [PubMed] [Google Scholar]
- 26.Patumraj S, Wongeakin N, Jariyapongskul A, Futrakul N, Bunnag S. Combined effects of curcumin and vitamin C to protect endothelial dysfunction in the iris tissue of STZ-induced diabetic rats. Clin Hemorheol Microcirc. 2006;35:481–489. [PubMed] [Google Scholar]
- 27.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Bio. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 28.Rungseesantivanon S, Thengchaisri N, Ruangvejvorachai P, Patumraj S. Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complement Altern Med. 2010;10:57. doi: 10.1186/1472-6882-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wongeakin N, Sridulyakul P, Jariyapongskul A, Suksamrarn A, Patumraj S. Effects of curcumin and tetrahydrocurcumin on diabetes induced endothelial dysfunction. Afr J Biochem Res. 2009;3:259–265. [Google Scholar]
- 30.Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol. 2000;20(6):1430–1442. doi: 10.1161/01.atv.20.6.1430. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107(2):135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilmore TD. The Rel/NF-κB signal transduction pathway: introduction. Oncogene. 1999;18(49):6842–6844. doi: 10.1038/sj.onc.1203237. [DOI] [PubMed] [Google Scholar]
- 33.Pan MH, Lin-Shiau SY, Lin JK. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IκB kinase and NFκB activation in macrophages. Biochem Pharmacol. 2000;60:1665–1676. doi: 10.1016/s0006-2952(00)00489-5. [DOI] [PubMed] [Google Scholar]
- 34.Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol. 2004;14:433–439. doi: 10.1016/j.semcancer.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Xu J, Fu Y, Chen A. Activation of peroxisome proliferator-activated receptor-γ contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G20–G30. doi: 10.1152/ajpgi.00474.2002. [DOI] [PubMed] [Google Scholar]
- 36.Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-κB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal. 2005;7:32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- 37.Cohen RA. The role of nitric oxide and other endothelium-derived vasoactive substances in vascular disease. Prog Cardiovasc Dis. 1995;38:105–108. doi: 10.1016/s0033-0620(05)80002-7. [DOI] [PubMed] [Google Scholar]
- 38.Shanmugam N, Kim YS, Lanting L, Natarajan R. Regulation of cyclooxygenase- 2 expression in monocytes by ligation of the receptor for advanced glycation end products. J Biol Chem. 2003;278:34834–34844. doi: 10.1074/jbc.M302828200. [DOI] [PubMed] [Google Scholar]
- 39.Shanmugam N, Gaw Gonzalo IT, Natarajan R. Molecular mechanisms of high glucose-induced cyclooxygenase-2 expression in monocytes. Diabetes. 2004;53:795–802. doi: 10.2337/diabetes.53.3.795. [DOI] [PubMed] [Google Scholar]
- 40.Bagi Z, Erdei N, Toth A, et al. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2- derived constrictor prostaglandins. Arter Thromb Vasc Biol. 2005;25:1610–1616. doi: 10.1161/01.ATV.0000172688.26838.9f. [DOI] [PubMed] [Google Scholar]
- 41.Szerafin T, Erdei N, Fulop T, et al. Increased cyclooxygenase-2 expression and prostaglandin-mediated dilation in coronary arterioles of patients with diabetes mellitus. Circ Res. 2006;99:e12–e17. doi: 10.1161/01.RES.0000241051.83067.62. [DOI] [PubMed] [Google Scholar]
- 42.Wei Z, Xiao-Li W, Kathryn GL, Hon-Chi L. Inhibition of protein kinase C protects against diabetes-induced impairment in arachidonic acid dilation of small coronary arteries. J Pharmacol Exp Ther. 2006;319:199–207. doi: 10.1124/jpet.106.106666. [DOI] [PubMed] [Google Scholar]
- 43.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) J Altern Complement Med. 2003;9:161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]