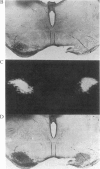
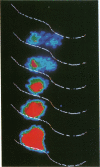
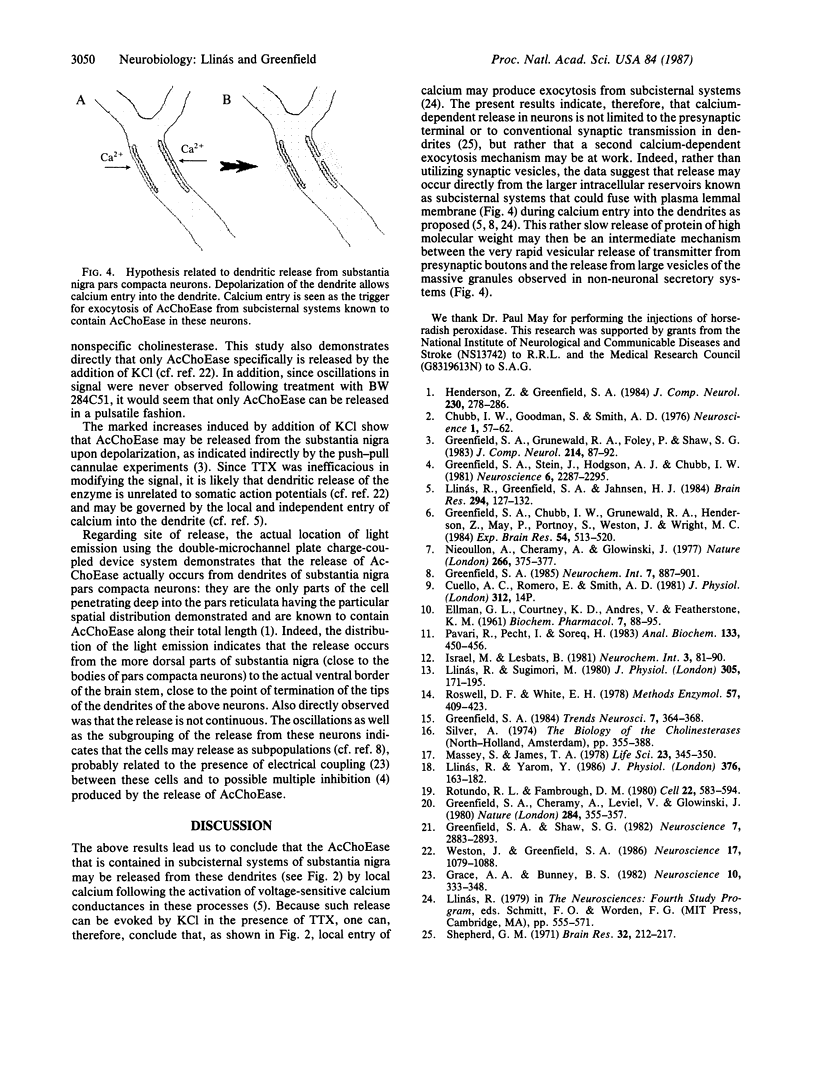
Abstract
This study presents, to our knowledge, the first on-line measurement of acetylcholinesterase (AcChoEase) release from brain tissue. It is now well established that a soluble form of the enzyme is released from central nervous system neurons, and it has been proposed on indirect grounds that such release may occur not only from presynaptic terminals but also from the dendrites of dopamine-containing nigrostriatal neurons. We have used a chemiluminescent reaction to examine the real-time release of AcChoEase from the substantia nigra in vitro in brainstem slices. The light emission was captured by two fiber optic systems, one in direct contact with the brain slice from below and the second 4-mm above the slice, allowing simultaneous imaging of the emitted light and quantitative photometry. It was determined that the light signals are not due to the spontaneous hydrolysis of acetylcholine or the presence of free choline, but are caused by the enzymatic action of AcChoEase. Using this technique, it can be directly shown that AcChoEase is spontaneously released from the soma or dendrites of nigral neurons. The release of the enzyme, which is stored in the subcisternal dendritic compartment, is resistant to blockade of voltage-dependent sodium conductances, is calcium dependent, and can be increased by addition of potassium to the bathing solution. The procedure we describe here will make it possible to study the release of endogenous AcChoEase on a time-scale close to that over which it functions.
Full text
PDF
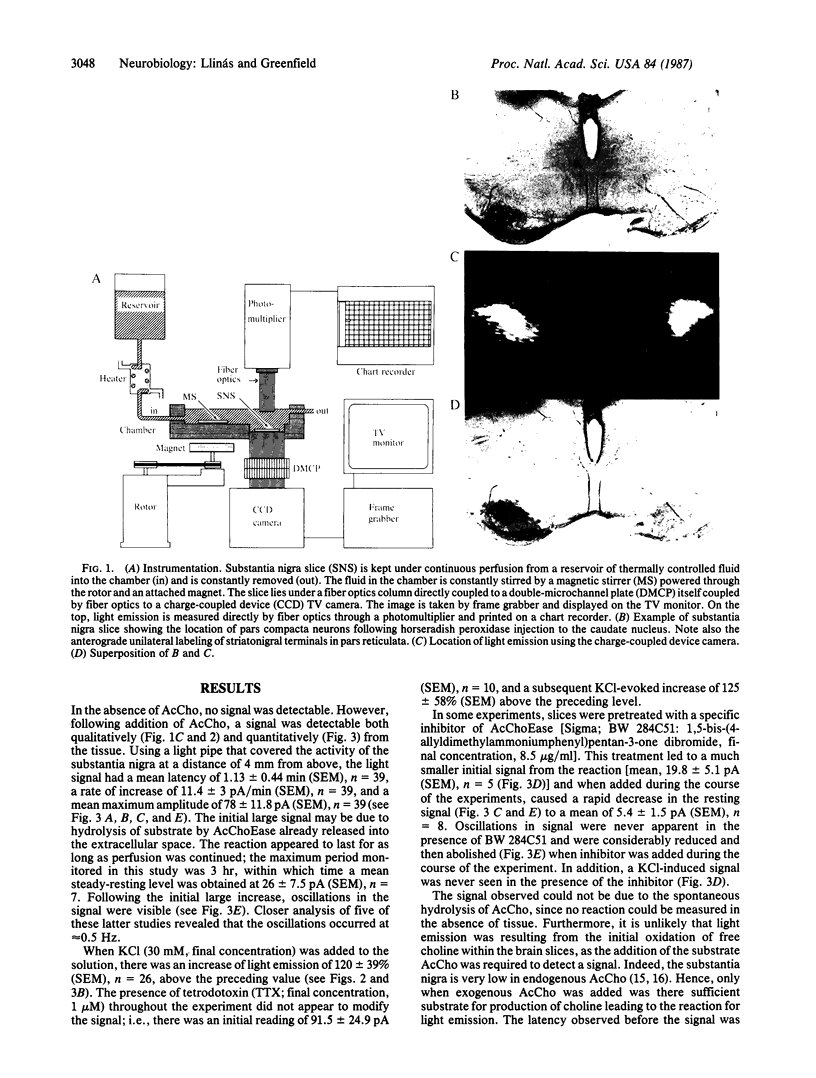
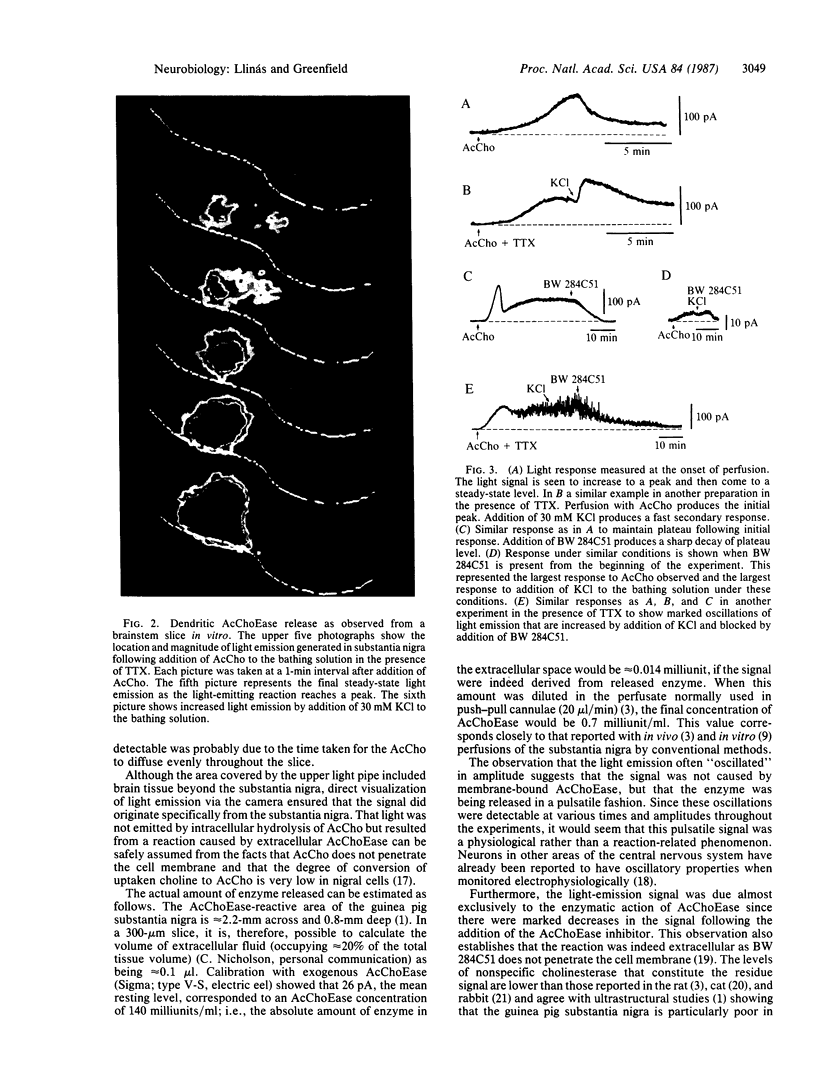
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chubb I. W., Goodman S., Smith A. D. Is acetylcholinesterase secreted from central neurons into the cerebral fluid? Neuroscience. 1976;1(1):57–62. doi: 10.1016/0306-4522(76)90048-8. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Grace A. A., Bunney B. S. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--3. Evidence for electrotonic coupling. Neuroscience. 1983 Oct;10(2):333–348. doi: 10.1016/0306-4522(83)90137-9. [DOI] [PubMed] [Google Scholar]
- Greenfield S. A., Chubb I. W., Grünewald R. A., Henderson Z., May J., Portnoy S., Weston J., Wright M. C. A non-cholinergic function for acetylcholinesterase in the substantia nigra: behavioural evidence. Exp Brain Res. 1984;54(3):513–520. doi: 10.1007/BF00235476. [DOI] [PubMed] [Google Scholar]
- Greenfield S. A., Grünewald R. A., Foley P., Shaw S. G. Origin of various enzymes released from the substantia nigra and caudate nucleus: effects of 6-hydroxydopamine lesions of the nigro-striatal pathway. J Comp Neurol. 1983 Feb 10;214(1):87–92. doi: 10.1002/cne.902140109. [DOI] [PubMed] [Google Scholar]
- Greenfield S. A., Shaw S. G. Release of acetylcholinesterase and aminopeptidase in vivo following infusion of amphetamine into the substantia nigra. Neuroscience. 1982;7(11):2883–2893. doi: 10.1016/0306-4522(82)90111-7. [DOI] [PubMed] [Google Scholar]
- Greenfield S. A., Stein J. F., Hodgson A. J., Chubb I. W. Depression of nigral pars compacta cell discharge by exogenous acetylcholinesterase. Neuroscience. 1981;6(11):2287–2295. doi: 10.1016/0306-4522(81)90018-x. [DOI] [PubMed] [Google Scholar]
- Greenfield S., Cheramy A., Leviel V., Glowinski J. In vivo release of acetylcholinesterase in cat substantia nigra and caudate nucleus. Nature. 1980 Mar 27;284(5754):355–357. doi: 10.1038/284355a0. [DOI] [PubMed] [Google Scholar]
- Henderson Z., Greenfield S. A. Ultrastructural localization of acetylcholinesterase in substantia nigra: a comparison between rat and guinea pig. J Comp Neurol. 1984 Dec 1;230(2):278–286. doi: 10.1002/cne.902300211. [DOI] [PubMed] [Google Scholar]
- Llinás R., Greenfield S. A., Jahnsen H. Electrophysiology of pars compacta cells in the in vitro substantia nigra--a possible mechanism for dendritic release. Brain Res. 1984 Feb 27;294(1):127–132. doi: 10.1016/0006-8993(84)91316-7. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol. 1986 Jul;376:163–182. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey S. C., James T. A. The uptake of 3H-choline and release of 3H-acetylcholine in the rat substantia nigra. Life Sci. 1978 Jul 24;23(4):345–350. doi: 10.1016/0024-3205(78)90019-x. [DOI] [PubMed] [Google Scholar]
- Nieoullon A., Cheramy A., Glowinski J. Release of dopamine in vivo from cat substantia nigra. Nature. 1977 Mar 24;266(5600):375–377. doi: 10.1038/266375a0. [DOI] [PubMed] [Google Scholar]
- Parvari R., Pecht I., Soreq H. A microfluorometric assay for cholinesterases, suitable for multiple kinetic determinations of picomoles of released thiocholine. Anal Biochem. 1983 Sep;133(2):450–456. doi: 10.1016/0003-2697(83)90107-0. [DOI] [PubMed] [Google Scholar]
- Rotundo R. L., Fambrough D. M. Synthesis, transport and fate of acetylcholinesterase in cultured chick embryos muscle cells. Cell. 1980 Nov;22(2 Pt 2):583–594. doi: 10.1016/0092-8674(80)90368-2. [DOI] [PubMed] [Google Scholar]
- Shepherd G. M. Physiological evidence for dendrodendritic synaptic interactions in the rabbit's olfactory glomerulus. Brain Res. 1971 Sep 10;32(1):212–217. doi: 10.1016/0006-8993(71)90168-5. [DOI] [PubMed] [Google Scholar]
- Weston J., Greenfield S. A. Release of acetylcholinesterase in the rat nigrostriatal pathway: relation to receptor activation and firing rate. Neuroscience. 1986 Apr;17(4):1079–1088. doi: 10.1016/0306-4522(86)90078-3. [DOI] [PubMed] [Google Scholar]