Abstract
The incidence of gestational diabetes mellitus (GDM) is on the increase and, if not diagnosed, managed and treated adequately, can have unfavorable maternal and fetal outcomes. Several studies have shown that glycemic values considered as adequate in the past when monitoring GDM failed to contain these adverse outcomes and randomized trials are needed to ascertain whether these targets should be lowered. Dietary restrictions remain the mainstay of GDM management and suitable physical exercise can help too. The use of rapid-acting insulin analogues (lispro and aspart) are novel treatments for improving metabolic control by reducing postprandial glycemia, while long-acting insulin analogues need to be evaluated by further studies for safety in clinical use before they can be prescribed. Numerous studies have found glyburide and metformin safe in women with GDM but more randomized controlled trials are needed, with a long-term follow-up of mother and child, to confirm these results.
Keywords: gestational diabetes, glucose management, obstetric management, maternal complications, fetal complications
Introduction
Gestational diabetes mellitus (GDM) is classically defined as “Carbohydrate intolerance resulting in hyperglycemia of variable severity with onset or first recognition during pregnancy”.1 It does not rule out a prior unidentified glucose intolerance, and in fact several studies have found 10% to 15% of cases of undiagnosed type 2 diabetes mellitus among GDM patients.2 The disorder affects 5% to 7% of all pregnancies and its frequency is rising the world over.3
Insulin resistance increases in normal pregnancy due to progressively rising levels of feto-placental hormones such as progesterone, cortisol, growth hormone, prolactin and human placental lactogen.4 The pancreas normally compensates by increasing insulin secretion, but when it fails to do so, or when insulin secretion declines due to a beta-cell function impairment,5,6 then GDM develops. Maternal hyperglycemia, which is typical of GDM, causes a greater transfer of glucose to the fetus, causing fetal hyperinsulinemia7 and an overgrowth of insulin-sensitive (mainly adipose) tissues, with consequent excessive, unbalanced fetal growth, causing more trauma at birth, shoulder dystocia and perinatal deaths. Hyperinsulinemia can also cause numerous neonatal metabolic complications, such as hypoglycemia, hyperbilirubinemia, hypocalcemia, hypomagnesemia, polycythemia, respiratory distress syndrome, and a greater long-term risk of diabetes mellitus and obesity in the child.8,9 GDM is related to maternal complications too, such as hypertension, pre-eclampsia, greater need for cesarean delivery,8,9 and a greater risk of developing diabetes mellitus later on. It is worth emphasizing that rising levels of obesity worldwide have prompted an increase in the numbers of obese women who become pregnant, and who develop GDM. Pregnancy complicated by obesity is characterized by higher adverse maternal and fetal outcome rates, especially in GDM patients.10
Given the pathophysiology of GDM, most women with GDM subsequently develop type 2 diabetes. A systematic literature review found a cumulative incidence of conversion from GDM to type 2 diabetes that varied widely (from 2.6% to 70%), largely due to differences in the duration of follow-up and cohort retention rates. After adjusting for such differences, a rapid rise in the cumulative incidence of type 2 diabetes emerged in the first 5 years after delivery.11
Managing GDM: clinical evidence
The potential benefits of medical and obstetric GDM management are fewer maternal and fetal complications during and after pregnancy,12 but the clinical entity of GDM13 and the utility (in terms of costs and maternal and fetal benefits) of screening for GDM have recently been questioned.14,15 Since it is sometimes difficult to distinguish GDM from prior undiagnosed type 2 diabetes, it is also not easy to assess the risks and benefits of treating GDM. In a recent review on the treatment of GDM and glucose intolerance in pregnancy, Tufnell et al14 concluded that there are insufficient data for any conclusion on the effects of treatment for these conditions on perinatal outcome. A systematic review for the US Preventive Services Task Force said that, “limited evidence suggests that GDM treatment after 24 weeks improves some maternal and neonatal outcomes. Evidence is even more sparse for screening before 24 weeks of gestation.”15
On the other hand, Langer et al16 recently recorded composite adverse fetal outcomes (stillbirth, macrosomia, large for gestational age, hypoglycemia, erythrocytosis, hyperbilirubinemia) in 59% of untreated and 18% of treated GDM women, and in 11% of normal controls. The Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS)17 unequivocally demonstrated that treating GDM (as defined by World Health Organization [WHO] criteria) improves maternal and fetal outcome. The rate of severe perinatal outcomes in newborn was significantly lower in the treated group than in the control group (1% vs 4%, p = 0.01), and quality of life was improved in the treated group. Cost analysis showed that the treatment was cost-effective. 18 Finally, a cost-effectiveness study by the National Institute for Clinical Excellence (NICE) demonstrated that GDM screening, diagnosis and treatment is cost-effective, irrespective of the type of pharmacological treatment used.19 Hence, even if some controversy remains, GDM is a genuine clinical entity that warrants diagnosis and treatment.20
Glucose management (Table 1)
Table 1.
Glycemic target in pregnancy
| ADA3 4th IWC 199822 | ACOG 200152 | Parretti 200126 SBGM | Yogev 2003 27 CGMS | Siegmund 200728 CGMS | |
|---|---|---|---|---|---|
| FPG (mg/dL) | <95 | 60–90 | 62 ± 4.5 | 75 ± 12 | 77.3 ± 9.0 |
| 1 h PPPG (mg/dL) | <140 | <130–140 | 94 ± 6 | 105 ± 13 | 100.0 ± 12.6 |
| 2 h PPPG (mg/dL) | <120 | <120 | 81.4 ± 5.7 | 97 ± 11 | – |
| Mean blood glucose (mg/dL) | – | 100 | 74.7 ± 5.2 | 83.7 ± 18 | 87.2 ± 7.2 |
Abbreviations: FPG, fasting plasma glucose; 1 hPPPG, 1 hour postprandial plasma glucose; 2 hPPPG, 2 hour postprandial plasma glucose; SBGM, self blood glucose monitoring; CGMS, continuous glucose monitoring system.
The goal of glucose management in GDM is to keep glucose values as near normal as possible.
The Fifth International Workshop Conference (FIWC) on GDM21 suggests capillary whole blood glucose concentrations below 96 mg/dL (<5.3 mmol/L) before meals and either below 140 mg/dL (<7.8 mmol/L) 1 h afterwards or below 120 mg/dL (<6.7 mmol/L) 2 h afterwards. The reference plasma glucose levels suggested by the American Diabetes Association (ADA) are below 105 mg/dL (5.8 mmol/L) before meals and either below 155 mg/dL (8.6 mmol/L) 1 h afterwards, or below 130 mg/dL (7.2 mmol/L) 2 h afterwards.3
It is worth emphasizing, however, that these recommendations do not consider glycemic values higher than those normally recorded in pregnancy, they refer to glycemic levels associated with pregnancy outcome.22 This is mainly because studies on glycemia in normal pregnant women are scarce, concern few hospitalized women and glucose levels were measured only on one day in the third trimester of pregnancy.23–25
In a more recent study on normal-weight pregnant women monitoring their own blood glucose levels, Parretti et al26 found lower glycemic levels than those previously reported, both before and after meals, which gradually increased from the 28th to the 38th week of gestation.
Nowadays, continuous glucose monitoring systems (CGMS) use subcutaneous sensors impregnated with glucose oxidase to assess subcutaneous interstitial glucose levels every 10 seconds. These devices have added to our understanding of normal glucose values in pregnancy. This approach revealed that in normal pregnant women27,28 glucose levels were similar to those found by Parretti et al26 with significantly lower levels at night than during the day.27 The same authors27 also showed that obese pregnant women have significantly lower blood glucose levels at night. Langer et al subsequently found that maintaining a good glycemic control in obese women (mean plasma glucose <100 mg/dL) is only associated with a favorable fetal outcome in women treated with insulin.29 This might mean that different glycemic values are needed in obese GDM women to reduce fetal and maternal complications, though prospective studies are needed to clarify this issue before current recommendations can be changed.
Measuring glucose levels after meals is more important than pre-prandial levels in GDM patients because it correlates better with certain adverse neonatal events, eg, malformations, macrosomia, hypoglycemia, and shoulder dystocia.30,31
There is some debate as to whether glucose should be measured 1 or 2 hours after a meal. Continuous blood glucose monitoring has recently shown that glucose peaks occur about 70 ± 13 min after meals in non-diabetic pregnant women27 and after about 90 min in diabetic women, whatever their glycemic control and type of treatment.32 No differences in postprandial glycemic profile emerged between breakfast, lunch and dinner. A recent review showed that patients managed according to the glucose levels measured an hour after meals, generally had babies with a lower birth weight, less neonatal hypoglycemia, less macrosomia and fewer cesarean deliveries than patients whose glucose levels were tested 2 hours after meals.33
As for glycemic levels and neonatal complications of GDM, Langer et al34,35 found higher macrosomia rates with mean blood glucose levels higher than 105 mg/dL, whereas the risk of babies being small for their gestational age was high when the mean blood glucose levels dropped below 87 mg/dL; mean blood glucose values should therefore be kept between 87 mg/dL and 105 mg/dL in GDM patients to avoid these fetal complications.21
As for fetal deaths, Pettitt et al36 reported a higher risk of stillbirth related mainly to excessive fetal growth, and Bartha et al37 found that stillbirths were more common among women whose GDM was diagnosed early than in those diagnosed later in their pregnancy; Aberg et al confirmed these data.38 Beischer et al39 also documented a higher perinatal mortality rate in untreated than in treated GDM patients. The increase in fetal death rates thus depends on the severity of glucose intolerance and the degree of glycemic control. The ADA’s position statement3 suggests that fasting blood glucose levels higher than 105 mg/dL carries a greater risk of perinatal mortality in GDM patients. Karlson and Kielmer40 also found an association between mean blood glucose levels and perinatal mortality, which was 4% in GDM women with mean plasma glucose levels below 100 mg/dL, 15% if it was 100 mg/dL to 150 mg/dL, and 24% if it exceeded 150 mg/dL.
As for glycemic control and the other GDM-related fetal complications (hypoglycemia, hypocalcemia, hyperbilirubinemia and respiratory complications), Langer et al postulated earlier that mean blood glucose levels below 100 mg/dL (as in normal pregnant women) reduce the incidence of all these complications.41,42 A more recent paper16 clearly showed that maternal and fetal complication rates were still higher in GDM than in normal pregnant women, even when strictly treated to achieve the above-mentioned plasma glucose levels.
The thresholds used in the past to prevent maternal and fetal complications of GDM may need to be reconsidered in the light of recent findings in normal pregnant women monitoring their own blood glucose levels26 or using a CGMS,27,28 and of the results of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study.43 This multicenter, blinded study addressed the risk of adverse outcomes associated with various degrees of maternal glucose intolerance less severe than overt diabetes, showing an association between increasing levels of fasting, 1-hour, and 2-hour postprandial plasma glucose (on oral glucose tolerance testing) and birth weight above the 90th percentile and cord C-peptide above the 90th percentile, as well as a positive association between rising plasma glucose levels and each of five secondary outcomes examined (premature delivery, shoulder dystocia or birth injury, admission to the neonatal intensive care, hyperbilirubinemia and preeclampsia).
Achieving a good glycemic control is therefore very important to reduce fetal complications in GDM and it is worth emphasizing that a good glucose control needs to be achieved without any hypoglycemic episodes, since CGMS has shown that women with GDM may have hypoglycemic episodes too (contrary to common convictions), especially if they are treated with insulin, and such episodes are generally asymptomatic.44 Finally, we have to consider the daily fluctuations in glycemia, which may contribute to certain neonatal complications, as demonstrated recently.45,46 Taslimi et al found that the glycemia index correlated better with birth weight percentile than blood glucose monitored by conventional methods in pregnant women with type 1 diabetes and GDM.46
Glycemic control can also be evaluated by measuring HbA1c. Although standardized measurements have shown that HbA1c levels are significantly higher in GDM patients than in normal pregnant women,47 the association between HbA1c levels and pregnancy outcome in these patients is weak.48,49 When considering HbA1c levels in diabetic pregnant patients, we must refer to the values for normal pregnant women (which are lower than in women who are not pregnant)47,50 and standardized methods are needed to obtain valid results.51
Obstetric management
Obstetric management is just as essential as metabolic management, and fetal monitoring is very important to decide the best time and mode of delivery.
Insulin-treated GDM patients and those with a poor metabolic control and/or comorbidities need to be monitored using “stress-free tests” in the 32nd week of gestation, and umbilical blood flow should be assessed using a biophysical profile and Doppler velocimetry as recommended by the Fourth IWC on GDM,22 the American College of Obstetricians and Gynecologists (ACOG),52 and the European Association of Perinatal Medicine (EAPM).53 In GDM patients on a controlled diet, it is not clear whether strict obstetric management should entail anything more than monitoring fetal movements in the last 8 to 10 weeks of pregnancy. Garner et al54 recorded no stillbirths among GDM women under routine obstetric management with no predelivery fetal monitoring. According to the ACOG52 and the FIWC on GDM,21 fetal movement should be monitored during the last 8 to 10 weeks of pregnancy in GDM patients with good metabolic control achieved by dietary measures and with an appropriate fetal growth. Any decline in the perceived fetal movements should be reported immediately to the obstetrician. The results of a large randomized multicenter study that compared perinatal outcome between women with mild GDM randomized to dietary and/or insulin therapy and women randomized to nonspecific treatment should clarify the optimal strategy for managing GDM.55
Screening for congenital anomalies is only recommended in GDM patients with HbA1c levels higher than 7% or fasting plasma glucose levels higher than 120 mg/dL, given the high risk of undiagnosed prepregnancy diabetes and the consequently high risk of congenital malformations.21
An important point to consider when monitoring GDM is the chance to predict and treat macrosomia, the most common complication of this condition. In order to plan any treatment designed to contain excess fetal growth, we need to know when this phenomenon sets in. An ultrasound (US) study on fetal lean and fat mass in GDM and normal pregnant women at various gestational ages showed that lean mass increases in the GDM fetus from the 20th gestational week (g.w) onwards and fat mass from the 26th g.w.56 The accuracy of US estimates of fetal weight is commonly expressed as sensitivity, specificity, positive and negative predictive values, and proportions of estimates within 10% of actual birth weight.57,58 The mean absolute error in the birth weight predicted using US is in the range of 6% to 12% of the actual birth weight, with 40% to 75% of estimates coming within 10% of the actual birth weight.59 However, US fetal measurements have proved to depend on the examiner’s expertise.60 Moreover, studies comparing clinical and US measurements found neither method superior, US proving more accurate only in the low birth weight range.61 A recent study also showed that the positive predictive value of US in assessing birth weights above the 95th percentile in women with varying degrees of glucose intolerance is only 50%.62 In another recent study, Pates et al63 found that US estimates of fetal weight of 4000 g or more, based on fetal abdominal circumference, biparietal diameter, head circumference and femur length, within a week of delivery and a higher than normal amniotic fluid index in combination with clinical risk factors (maternal parity, BMI and diabetes) improved the prediction of macrosomia from 61% to 71%.
Newly-developed technologies, such as 3D US and magnetic resonance imaging, are promising in this setting, having proved highly accurate in measuring fetal weight from a volumetric assessment of the fetus.64,65 Most such studies published to date refer to only a few cases, however, and the cost-benefit ratio of these technologies needs to be determined before it can be considered routinely applicable.66
There is evidence from some studies that a fetal abdominal circumference beyond the 75th percentile and fasting plasma glucose levels beyond 105 mg/dL correlate with a high risk of the newborn being large for gestational age (LGA); in such cases, stricter glycemic targets (80 mg/dL while fasting and 100 mg/dL to 120 mg/dL, 2 h after meals) and a more frequent use of insulin for treatment may reduce the number of babies that are LGA by 50%.67,68 Hence the recommendation from FIWC on GDM21 that fetal abdominal circumference be assessed every 2 to 4 weeks, from the second trimester to provide useful indicators for the treatment of GDM.
Given the contribution of fat mass to fetal weight, measuring soft tissue thickness might seem more accurate than whole body (fat and lean tissue) measurements.69 This method is highly sensitive, but scarcely specific; whereas measuring subcutaneous fetal fat tissue has proved to be both sensitive and specific.70,71 Such methods have yet to become routine in monitoring GDM patients, and further studies are needed to establish their real utility and cost-benefit ratio.66
Diet
The goals of medical therapy in GDM patients are to establish the right diet in terms of quality and quantity of nutrients to ensure normal maternal weight gain and fetal growth, optimize glycemic control, avoid ketoacidosis and reduce glucose levels after meals, since adverse maternal and fetal outcomes have been associated with hyperglycemia after meals. To achieve these goals, it is important for GDM patients to be followed-up by a team and included in an educational program, customize weight gain and calorie intake, and establish their needs in terms of type and distribution of carbohydrates, optimal protein, fat and micronutrient intake, and amount and type of physical activity.72
Maternal weight gain during pregnancy and BMI before pregnancy affect the infant’s birth weight. Taking the original recommendation on Nutrition for Pregnancy of the Institute of Medicine (IOM) into account,73 the Fourth IWC on GDM,22 and the EAPM Guidelines53 recommend a weight gain of 12.7 kg to 18.2 kg for pregnant women with a prepregnancy BMI lower than 19.8; 11.4 kg to 15.9 kg for pregnant women with a prepregnancy BMI between 19.8 and 26; 6.8 kg to 11.4 kg for pregnant women with a prepregnancy BMI between 26.1 and 29; and 6.8 kg for pregnant women with a prepregnancy BMI higher than 29. The ideal calorie intake in GDM patients is a debatable point; while it is generally agreed that underweight and normal-weight GDM patients should have the calorie intake recommended by the IOM,73 there is no such consensus for overweight and obese GDM patients and some authors advocate severe calorie restriction in such cases.74,75 Studies by Knoop et al74 and Magee et al75 using severe calorie restriction (1,200 kcal/day or a 50% reduction in daily kcal intake) have clearly shown, however, that even if this approach can improve glycemia, it also increases ketonuria, which has been associated with an altered mental development in children born to GDM mothers.76 Conversely, a more modest calorie restriction (1,600–1,880 kcal/day or a 33% reduction in daily kcal intake) improved glycemia and triglyceride levels without causing ketonuria.77,78
So, in accordance with the ADA,3 we suggest a daily calorie intake and distribution as shown in Table 2.
Table 2.
Calorie intake and distribution in GDM women
| Calorie intake in accordance with BMI (kcal/kg actual weight) | Caloric distribution | |
|---|---|---|
| •<19.8 | 36–40 | •Breakfast 10%–15% |
| •19.8–26 | 30 | •Snack 5%–10% |
| •26.1–33 | 24 | •Lunch 20%–30% |
| •>33 | 12–18 | •Snack 5%–10% |
| +340–452 kcal/die in 2nd and 3rd trimester | •Dinner 30%–40% | |
| •Snack 5%–10% (25 g CHO + 10 g P) | ||
| Nutrient distribution | ||
| CHO 45%–50% (complex carbohydrate and fiber) | ||
| P 15%–20% | ||
| L 30%–35% (mono and polyunsaturated) | ||
Abbreviations: CHO, carbohydrates; P, proteins; L, lipids.
As for the optimal carbohydrate intake, since carbohydrates are a fundamental dietary resource for fetal brain development and at least 130 g/day of carbohydrates are needed in the normal population, pregnant women need about 33 g more, ie, 175 g of carbohydrates a day.73
It has recently been demonstrated that foods with a low glycemic index induce smaller increases in postprandial glucose levels and this might be important in GDM women, whose glucose levels are often high after meals.79,80 So, even if no randomized trials have been conducted as yet to test a carbohydrate-rich diet with a low glycemic index in GDM patients, we would recommend using such a diet, suitably tailored to individual patients, in cases of GDM.
As for the other nutrients, vitamins and minerals, GDM women need to follow the same guidelines as normal pregnant women.
Exercise in GDM
It is well known that physical activity reduces glucose and insulin resistance in diabetic patients thereby helping them to control their weight, so it may be useful as adjunctive therapy in GDM women too.
The ADA suggests that women without medical or obstetric contraindications should be encouraged to start or continue a program of moderate exercise as part of their treatment for GDM.3 The Fourth IWC statement on GDM says that, “a planned physical activity of 30 min/day is recommended.”22 However, when exercise was studied in terms of its capacity to reduce plasma glucose and delay or prevent the need for insulin therapy, the results were inconclusive (due probably to the small samples considered, the lack of any randomization of the subjects, and the poor control or inadequate reporting of the intensity of the exercise concerned). The Cochrane study concluded that there is insufficient evidence to either recommend or advise against enrolling GDM patients in exercise programs,81 although several epidemiological studies have shown a link between physical activity and a lower risk of GDM.82,83
Here again, further studies are needed to determine the optimal frequency, intensity, timing and type of exercise to recommend in GDM patients. Given the potential benefits of exercise in GDM, for uncomplicated pregnancies we can follow the recommendations of the Fourth IWC on GDM22 and the ACOG,52 provided GDM women monitor their blood glucose levels and fetal activity before and after exercising (Table 3).
Table 3.
Guidelines of the ACOG for exercise during pregnancy
| Exercise recommended in pregnancy | Exercise to be avoided in pregnancy |
|---|---|
| 1. walking | 1. skiing |
| 2. jogging/running | 2. horseback riding |
| 3. aerobic dance | 3. ice hockey |
| 4. swimming | 4. socce |
| 5. cycling | 5. basketball |
| 6. dancing | 6. scuba diving |
Intensity of exercise
| |
Duration and frequency
| |
Insulin therapy
Insulin is needed when dietary restrictions fail to achieve near-normal glucose control in GDM women. How long dietary measures alone should be attempted before starting insulin therapy depends on the patient’s glycemic control and the baby’s gestational age when GDM is diagnosed. It takes two weeks before one can say whether dietary measures alone suffices. Insulin dosage is calculated on a patient’s weight and it is best to start with 0.7 U/kg in normal-weight patients and 0.8–1.0 U/kg in the obese.84 Basal insulin is administered at bedtime, or both before breakfast and at bedtime; rapid-acting insulin (regular, lispro, aspart) is taken before meals.
Since improving postprandial hyperglycemia can reduce some negative maternal and fetal outcomes, the new insulin analogues may be beneficial in GDM patients.85,86 Regarding their safety, the first question is whether they cross the placenta and have harmful effects on the fetus; the second is whether they are really effective.
Animal studies found no embryotoxic or teratogenic effects of insulin lispro, and clinical and in vitro perfusion studies have shown that insulin lispro does not cross the placenta at the doses currently used in pregnancy.87 In GDM patients, insulin lispro has been associated with the formation of antibody resembling regular insulin and with lower levels of glucose, insulin and C-peptide after meals, and fewer hypoglycemic episodes than in patients on regular insulin.88
As for insulin aspart, animal studies have again identified no embryotoxic or teratogenic effects, while no studies have been performed so far on its ability to cross the placenta. In GDM, using aspart has proved more effective than regular insulin in reducing postprandial glucose and C-peptide concentrations.89
No data are currently available on the use of glulysine in pregnancy.
Regarding long-acting insulin analogues, animal studies on insulin glargine have disclosed no embryotoxic or teratogenic effects, while some toxic effects (early intrauterine death, congenital malformations) correlated with the hypoglycemia induced by high doses of insulin.90 No data are available as yet on the capacity of these insulin analogues to cross the placenta. In a case-control pilot study involving 22 GDM cases treated with insulin glargine, Price et al could find no differences in maternal and fetal outcomes.91
We still have no data on the use of insulin detemir in pregnancy.
Based on these studies, insulin lispro and aspart appear to be just as safe and effective as regular insulin in GDM, while achieving a better postprandial glucose concentration and fewer hypoglycemic episodes. The safety of the new long-acting insulin analogues in pregnancy needs to be further analyzed, so they are not currently recommended in diabetic pregnant women.
Oral hypoglycemic drugs
These drugs are generally not prescribed during pregnancy because they are believed to cross the placenta and might cause fetal/neonatal hyperinsulinemia and hypoglycemia. There is also concern as to their teratogenicity when used in the first trimester of pregnancy. For hypoglycemic drugs, we still need to answer the same questions as for the new insulin analogues.
Glyburide has revealed only a minimal placental transfer92 and several studies (involving almost 1261 GDM cases) showed adequate glycemic control in most patients, fewer hypoglycemic episodes than with insulin treatment, and neonatal outcomes similar to those achieved with insulin.93,94 On the other hand, a recent study on 500 GDM women reported a significantly higher frequency of preeclampsia and the need for phototherapy in glyburide-treated patients.95
Given the above, this drug might be prescribed in GDM patients if maternal glycemic control and fetal development are monitored closely.
Metformin has been shown to cross the placenta, with fetal levels becoming about half those of the mother.96
The recent randomized Metformin and Gestational Diabetes (MIG) trial assessing the safety and efficacy of metformin in 363 GDM women showed that metformin, alone or with insulin supplementation, was not associated with more perinatal complications than insulin alone, but insulin had to be associated with the metformin in 46% of cases to achieve an adequate metabolic control.97
Metformin has been associated with fewer cases of GDM among women with polycystic ovary syndrome,98 and it was responsible for no neonatal complications in terms of malformations, birth weight or neonatal hypoglycemia.99
Because it crosses the placenta and relatively few cases have been treated so far, it would be prudent to await more information before assuming it is safe to use this drug in pregnancy.
Acarbose might be used if the patient tolerates the related gastrointestinal discomfort; a randomized trial on this drug is underway and the results will tell us more about its safety profile.100
Thiazolinediones101 cross the placenta and, apart from one or two case studies, we know nothing about its safety in pregnancy. The same goes for incretin mimetics, which should not be used in pregnancy for the time being.102
Conclusions
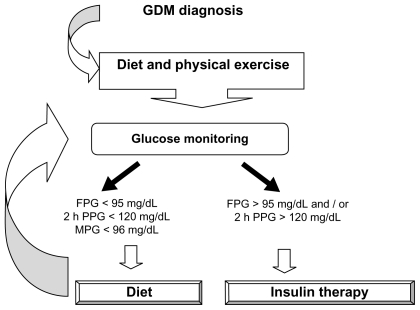
The incidence of GDM is increasing and, if not diagnosed, managed and treated adequately (Figure 1), can have unfavorable maternal and fetal outcomes. Several studies have shown that the glycemic values considered adequate in the past when monitoring GDM were unable to contain these adverse outcomes and randomized trials are needed to ascertain whether these targets should be lowered. New methods for assessing glycemic control and fetal development seem promising, but have to be tested for routine use. Dietary restrictions remain the mainstay of GDM management, and suitable physical exercise can help too. Rapid-acting insulin analogues (lispro and aspart) are novel treatments for improving metabolic control by reducing postprandial glycemia, while long-acting insulin analogues need further study on the related safety issues before they can be prescribed. Numerous studies have found glyburide and metformin safe in GDM pregnancies, but more randomized controlled trials are needed in type 2 diabetic and GDM women, with a long-term follow-up of mother and child, to confirm these results.
Figure 1.
Management of gestational diabetes.
Abbreviations: FPG, fasting plasma glucose; 2 h PP, 2 h postprandial glucose; MPG, mean plasma glucose.
Footnotes
Disclosures
The authors disclose no conflicts of interest.
References
- 1.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 2.Langer O. Type 2 diabetes in pregnancy: exposing deceptive appearances. J Matern Fetal Neonatal Med. 2008;21(3):181–189. doi: 10.1080/14767050801929497. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Gestational Diabetes Mellitus (Position Statement) Diabetes Care. 2004;27(Suppl 1):S88–S90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 4.Ryan EA, Enns L. Role of gestational hormones in the induction of insulin resistance. J Clin Endocrinol Metab. 1988;67:341–347. doi: 10.1210/jcem-67-2-341. [DOI] [PubMed] [Google Scholar]
- 5.Kuhl C. Etiology and pathogenesis of gestational diabetes. Diabetes Care. 1998;21:B19–B26. [PubMed] [Google Scholar]
- 6.Lapolla A, Dalfrà MG, Mello G, et al. Early detection of insulin sensitivity and beta cell function with simple tests indicate future derangements in late pregnancy. J Clin Endocrinol Metab. 2008;93(3):876–880. doi: 10.1210/jc.2007-1363. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen J. The Pregnant Diabetic and her Newborn. 2nd ed. Munksgaard; Copenhagen: Williams and Wilkins; Baltimore: 1977. p. 211. [Google Scholar]
- 8.Casey BM, Lucas MJ, McIntire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynaecol. 1997;90:869–873. doi: 10.1016/s0029-7844(97)00542-5. [DOI] [PubMed] [Google Scholar]
- 9.Kjos AL, Buchanan TA. Gestational diabetes mellitus. N Engl J Med. 1999;341:1749–1756. doi: 10.1056/NEJM199912023412307. [DOI] [PubMed] [Google Scholar]
- 10.Langer O. Management of obesity in GDM: old habits die hard. J Matern Fetal Neonatal Med. 2008;21(3):165–171. doi: 10.1080/14767050801929877. [DOI] [PubMed] [Google Scholar]
- 11.Kim C, Newton KM, Knoop RH. Gestational diabetes and the incidence of type 2 diabetes. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 12.Scott DA, Loveman E, McIntire L, Waugh N. Screening for gestational diabetes: a systematic review and economic evaluation. Health Technol Assess. 2002;6(11):1–172. doi: 10.3310/hta6110. [DOI] [PubMed] [Google Scholar]
- 13.Hunter DJS, Milner R. Gestational diabetes and birth trauma. Am J Obstet Gynecol. 1985;152:918–919. doi: 10.1016/s0002-9378(85)80101-0. [DOI] [PubMed] [Google Scholar]
- 14.Tuffnell DJ, West J, Walkinshaw SA. Treatments for gestational diabetes and impaired glucose tolerance in pregnancy. The Cochrane Library. 2008;(4):1–30. doi: 10.1002/14651858.CD003395. [DOI] [PubMed] [Google Scholar]
- 15.Hiller TA, Vesco KK, Pedula KL, Beil TL, Whitlock EP, Pettitt DJ. Screening for gestational diabetes mellitus: a systematic review for the US Preventive Service Task Force. Ann Intern Med. 2008;148:766–775. doi: 10.7326/0003-4819-148-10-200805200-00009. [DOI] [PubMed] [Google Scholar]
- 16.Langer O, Yogev Y, Most O, Xenachis EM. Gestational diabetes: the consequences of not treating. Am J Obstet Gynaecol. 2005;192:989–997. doi: 10.1016/j.ajog.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 17.Crowther CA, Hiller JE, Moss AJ, Jeffries WS, Robinson JS for the Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New Engl J Med. 2005;352(24):2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 18.Moss JR, Crowther CA, Hiller JE, Willson KJ, Robinson JS for the Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Costs and consequences of treatment for mild gestational diabetes mellitus-evaluation from the ACHOIS randomised trial. BMC Pregnancy Childbirth. 2007;7:27–33. doi: 10.1186/1471-2393-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute for Health and Clinical Excellence. Cost-effectiveness of screening, diagnosis and treatment for gestational diabetes. Mar, 2008. pp. 165–190. Appendix D. [Google Scholar]
- 20.Langer O. Gestational diabetes: the consequences of not treating. In: Hod M, Jovanovic L, Di Renzo GC, DeLeiva A, Langer O, editors. Textbook of Diabetes and Pregnancy. 2nd ed. Informa Healthcare; 2008. pp. 107–117. [Google Scholar]
- 21.Metzer BE, Buchanan TA, Coustan DR, et al. Summary and Recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(2):S251–S260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 22.Metzger BE, Coustan DM. Organizing Committee: Summary and Recommendations of the Fourth International Workshop Conference on Gestational Diabetes Mellitus. Diabetes Care. 1998;21 (Suppl 2):B161–167. [PubMed] [Google Scholar]
- 23.Gilmer MD, Beard RW, Brooke FM, Oakley NW. Carbohydrate metabolism in pregnancy. Part 1: diurnal plasma glucose profile in normal and diabetic women. Br Med J. 1975;3:399–402. doi: 10.1136/bmj.3.5980.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cousins L, Rigg L, Hollingsworth D, Brink G, Aurand J, Yen SS. The 24-hour excursion and diurnal rhythm glucose, insulin and C-peptide in normal pregnancy. Am J Obstet Gynecol. 1980;136:483–488. doi: 10.1016/0002-9378(80)90675-4. [DOI] [PubMed] [Google Scholar]
- 25.Phelps RL, Metger BE, Freinkel N. Carbohydrate metabolism in pregnancy. XVII. Diurnal profiles of plasma glucose, insulin, free fatty acids, triglycerides, cholesterol, and individual amino acids in late normal pregnancy. Am J Obstet Gynecol. 1981;140:730–736. [PubMed] [Google Scholar]
- 26.Parretti E, Mecacci F, Papini M, et al. Third trimester maternal glucose levels from diurnal profiles in non diabetic pregnancies: correlation with sonographic parameters of foetal growth. Diabetes Care. 2001;24:1319–1323. doi: 10.2337/diacare.24.8.1319. [DOI] [PubMed] [Google Scholar]
- 27.Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight non diabetic pregnant women. Am J Obstet Gynecol. 2004;191:949–953. doi: 10.1016/j.ajog.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 28.Siegmund T, Rad NT, Ritterath C, Siebert G, Henrich W, Buhling KJ. Longitudinal changes in the continuous glucose profile measured by the CGMSR in healthy pregnant women and determination of cut-off values. E J Obstet Gynaecol Reprod Biol. 2008;139:46–52. doi: 10.1016/j.ejogrb.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Langer O, Yogev Y, Xenakis EMJ, Brustman L. Overweight and obese in gestational diabetes: the impact on pregnancy outcome. AM J Obstet Gynaecol. 2005;192:1768–1776. doi: 10.1016/j.ajog.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 30.Jovanovic-Peterson L, Peterson CM, Reed GF, et al. Maternal postprandial glucose levels and infant birth weight: Diabetes in Early Pregnancy Study: The National Institute of Child Health and Human Development –Diabetes in Early Pregnancy Study. Am J Obstet Gynecol. 1991;164:103–111. doi: 10.1016/0002-9378(91)90637-7. [DOI] [PubMed] [Google Scholar]
- 31.De Veciana M, Major CA, Morgan MA, et al. Post prandial versus pre prandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333:1237–1241. doi: 10.1056/NEJM199511093331901. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Haroush A, Yogev Y, Chen R, Rosen B, Hod M, Langer O. The postprandial glucose profile in the diabetic pregnancies. Am J Obstet Gynecol. 2004;191:576–581. doi: 10.1016/j.ajog.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 33.Leguizamon G, Krupitzki H, Glujovsky D, Olivera Ravasi M, Reece EA. Blood glucose monitoring in gestational diabetes mellitus:1-versus 2-h blood glucose determinations. J Matern Fetal Neonatal Med. 2002;12:384–388. doi: 10.1080/jmf.12.6.384.388. [DOI] [PubMed] [Google Scholar]
- 34.Langer O, Mazze R. The relationship between large for gestational age infants and glycemic control in women with gestational diabetes. Am J Obstet Gynecol. 1988;159:1478–1483. doi: 10.1016/0002-9378(88)90578-9. [DOI] [PubMed] [Google Scholar]
- 35.Langer O. Prevention of macrosomia. Baillieres Clin Obstet Gynaecol. 1991;5:333–347. doi: 10.1016/s0950-3552(05)80101-4. [DOI] [PubMed] [Google Scholar]
- 36.Pettitt DJ, Knowless WC, Baird HR, Bennett PH. Gestational diabetes: infant and maternal complications of pregnancy in relation to third trimester glucose tolerance in Pima Indians. Diabetes Care. 1980;3:458–464. doi: 10.2337/diacare.3.3.458. [DOI] [PubMed] [Google Scholar]
- 37.Bartha JL, Martinez-Del-Fresno P, Comino-Delgado R. Gestational diabetes mellitus diagnosed during early pregnancy. Am J Obstet Gynaecol. 2000;182:346–350. doi: 10.1016/s0002-9378(00)70222-5. [DOI] [PubMed] [Google Scholar]
- 38.Aberg A, Rydhstrom H, Kallen B, Kallen K. Impaired glucose tolerance during pregnancy is associated with increased fetal mortality in preceding sibs. Acta Obstet Gynecol Scand. 1997;76:212–217. [PubMed] [Google Scholar]
- 39.Beischer NA, Wein P, Sheedy MT, Steffen B. Identification and treatment of women with hyperglycemia diagnosed during pregnancy can significantly reduce perinatal mortality rates. Aust N Z J Obstet Gynaecol. 1996;182:239–247. doi: 10.1111/j.1479-828x.1996.tb02703.x. [DOI] [PubMed] [Google Scholar]
- 40.Karlson K, Kjellmer I. The outcome of diabetic pregnancies in relation to the mother’s blood sugar level. Am J Obstet Gynaecol. 1972;112:213–220. doi: 10.1016/0002-9378(72)90118-4. [DOI] [PubMed] [Google Scholar]
- 41.Langer O, Rodriguez DA, Xenachis EMJ, McFarland MB, Berkus MD, Arrendono MD. Intensified versus conventional management of gestational diabetes. Am J Obstet Gynaecol. 1994;170:1036–1047. doi: 10.1016/s0002-9378(94)70097-4. [DOI] [PubMed] [Google Scholar]
- 42.Langer O. A spectrum of glucose thresholds may effectively prevent complications in the pregnant diabetic patients. Semin Perinatol. 2002;26:196–205. doi: 10.1053/sper.2002.33962. [DOI] [PubMed] [Google Scholar]
- 43.The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcome. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 44.Chen R, Yogev Y, Ben-Haroush A, Jovanovic L, Hod M, Philip M. Continuous glucose monitoring for the evaluation and improved control of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2003;14:256–260. doi: 10.1080/jmf.14.4.256.260. [DOI] [PubMed] [Google Scholar]
- 45.Kerssen A, deValk HW, Visser GHA. Day-to-day glucose variability during pregnancy in women with type 1 diabetes mellitus: glucose profiles measured with the continuous glucose monitoring. BJOG. 2004;11:919–924. doi: 10.1111/j.1471-0528.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 46.Taslimi MM, Navabi K, Acosta R, Helmer A, El-Sayed YY. Concealed maternal blood glucose excursions correlate with birth weight centile. J Diab Science Technol. 2008;2(3):456–460. doi: 10.1177/193229680800200315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mosca A, Paleari R, Dalfrà MG, et al. Reference intervals for haemoglobin A1c in pregnant women: data from an Italian Multicentric study. Clin Chem. 2006;52:1138–1143. doi: 10.1373/clinchem.2005.064899. [DOI] [PubMed] [Google Scholar]
- 48.Wyse LJ, Jones M, Mandel F. Relationship of glycosylated hemoglobin, fetal macrosomia and birthweight macrosomia. Am J Perinatol. 1994;11:260–262. doi: 10.1055/s-2007-994587. [DOI] [PubMed] [Google Scholar]
- 49.Weissmann-Brenner A, O’Reilly-Green C, Ferber A, Divon MY. Does the availability of maternal HbA1c results improve the accuracy of sonographic diagnosis of macrosomia? Ultrasound Obstet Gynaecol. 2004;23:466–471. doi: 10.1002/uog.1031. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;5:1200–1201. doi: 10.2337/diacare.27.5.1200. [DOI] [PubMed] [Google Scholar]
- 51.Marshall SM, Barth JH. Standardization of HbA1c measurements: a consensus statement. Diabet Med. 2000;17:5–6. doi: 10.1046/j.1464-5491.2000.00228.x. [DOI] [PubMed] [Google Scholar]
- 52.ACOG Practice Bulletin Clinical Management Guidelines for Obstetrician-Gynaecologists. No 30. Obstet Gynaecol. 2001;98(3):525–538. [PubMed] [Google Scholar]
- 53.Hod M, Carrapato M, editors. European Association of Perinatal Medicine (EAPM) Diabetes and pregnancy update and guidelines. 2000. [Google Scholar]
- 54.Garner P, Okun N, Keely E, et al. A randomised controlled trial of strict glycemic control and tertiary level obstetric care versus routine obstetric care in the management of gestational diabetes: a pilot study. Am J Obstet Gynaecol. 1997;177:190–195. doi: 10.1016/s0002-9378(97)70461-7. [DOI] [PubMed] [Google Scholar]
- 55.Landon MB, Thom E, Spong CY, et al. The National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network randomized clinical trial in progress. Diabetes Care. 2007;30(Suppl 2):S194–S199. doi: 10.2337/dc07-s215. [DOI] [PubMed] [Google Scholar]
- 56.Larciprete G, Valensise H, Vasapollo B, et al. Fetal subcutaneous tissue thickness (SCTT) in healthy and gestational diabetic pregnancies. Ultrasound Obstet Gynecol. 2003;22:591–597. doi: 10.1002/uog.926. [DOI] [PubMed] [Google Scholar]
- 57.Ben-Haroush A, Yogev Y, Hod M. Fetal weight estimation in diabetic pregnancies and suspected fetal macrosomia. J Perinat Med. 2004;32:113–121. doi: 10.1515/JPM.2004.021. [DOI] [PubMed] [Google Scholar]
- 58.Colman A, Maharaj D, Hutton J, Tuohy J. Reliability of ultrasound estimation of fetal weight in term singleton pregnancies. NZ Med J. 2006;119:U2146. [PubMed] [Google Scholar]
- 59.Benacerraf BR, Gelman R, Frigoletto FD. Sonographically estimated fetal weights: accuracy and limitation. Am J Obstet Gynecol. 1988;159:1118–1121. doi: 10.1016/0002-9378(88)90425-5. [DOI] [PubMed] [Google Scholar]
- 60.Predanic M, Cho A, Ingrid F, Pellettieri J. Ultrasonographic estimation of fetal weight. J Ultrasound Med. 2002;21:495–500. doi: 10.7863/jum.2002.21.5.495. [DOI] [PubMed] [Google Scholar]
- 61.Sherman DJ, Arieli S, Tovbin J, Siegel G, Caspi E, Bukovsky I. A comparison of clinical and ultrasonic estimation of fetal weight. Obstetrics and Gynecology. 1998;91(2):212–217. doi: 10.1016/s0029-7844(97)00654-6. [DOI] [PubMed] [Google Scholar]
- 62.Kernaghan D, Ola B, Fraser RB, Farrell T, Owen P. Fetal size and growth velocity in the prediction of the large for gestational age (LGA) infant in a glucose impaired population. Eur J Obstet Gynecol Reprod Biol. 2007 Jun;132(2:):189–192. doi: 10.1016/j.ejogrb.2006.07.012. Epub 2006 Aug 22. [DOI] [PubMed] [Google Scholar]
- 63.Pates JA, McIntire DD, Casey BM, Leveno KJ. Predicting macrosomia. J Ultrasound Medicine. 2008;27:39–43. doi: 10.7863/jum.2008.27.1.39. [DOI] [PubMed] [Google Scholar]
- 64.Uotila J, Dastidar P, Heinonen T, Rymin P, Punnonen R, Laasonen E. Magnetic resonance imaging compared to ultrasonography in fetal weight and volume estimation in diabetic and normal pregnancy. Acta Obstet Gynecol Scand. 2000;79:255–259. [PubMed] [Google Scholar]
- 65.Lee W, Deter RL, Emersole JD, Huang R, Blanckaert K, Romero R. Birth weight prediction by three-dimensional ultrasonography: fractional limb volume. J Ultrasound Med. 2001;20:1283–1292. doi: 10.7863/jum.2001.20.12.1283. [DOI] [PubMed] [Google Scholar]
- 66.Meizner I, Mashiach R. Sonography in diabetic pregnancies. In: Hod M, Jovanovic L, Di Renzo GC, DeLeiva A, Langer O, editors. Textbook of Diabetes and Pregnancy. 2nd ed. Informa Healthcare; 2008. pp. 253–258. [Google Scholar]
- 67.Schaefer-Graf UM, Kios SL, Fauzan OH, et al. A randomized trial evaluating a predominantly fetal growth-based strategy to guide management of gestational diabetes in Caucasian women. Diabetes Care. 2004;27:297–302. doi: 10.2337/diacare.27.2.297. [DOI] [PubMed] [Google Scholar]
- 68.Bonomo M, Cetin I, Pisoni MP, et al. Flexible treatment of gestational diabetes modulated on ultrasound evaluation of intrauterine growth: a controlled randomized clinical trial. Diab Metab. 2004;30:237–243. doi: 10.1016/s1262-3636(07)70114-3. [DOI] [PubMed] [Google Scholar]
- 69.Abramowicz JS, Sarosh R, Abramowicz S. Fetal cheek-to-cheek diameter in the prediction mode of delivery. Am J Obstet Gynaecol. 2005;192:1205–1213. doi: 10.1016/j.ajog.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Bernstein IM, Catalano PM. Influence of foetal fat on the ultrasound estimation of foetal weight in diabetic mothers. Obstet Gynaecol. 1992;79:561–563. [PubMed] [Google Scholar]
- 71.Parretti E, Carignani I, Cioni R, et al. Sonographic evaluation of fetal growth and body composition in women with different degrees of normal glucose metabolism. Diabetes Care. 2003;26:2741–2748. doi: 10.2337/diacare.26.10.2741. [DOI] [PubMed] [Google Scholar]
- 72.Reader D, Splett P, Gunderson E. Impact of gestational diabetes mellitus nutrition practice guidelines implemented by registered dieticians on pregnancy outcome. J Am Diet Assoc. 2006;106:1426–1433. doi: 10.1016/j.jada.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 73.Food and Nutrition Board. Part 1: weight gain Part 2: nutrient supplements. Washington DC: Institute of Medicine, National Academy of Sciences; 1990. Nutrition during pregnancy. [Google Scholar]
- 74.Knoop RH, Magee MS, Raisys V, Benetti T. Metabolic effects of hypocaloric diets in management of gestational diabetes. Diabetes. 1991;40(Suppl 2):165–171. doi: 10.2337/diab.40.2.s165. [DOI] [PubMed] [Google Scholar]
- 75.Magee MS, Knopp RH, Benedetti TJ. Metabolic effects of 1200 kcal diet in obese pregnant women with gestational diabetes. Diabetes. 1990;34:234–240. doi: 10.2337/diab.39.2.234. [DOI] [PubMed] [Google Scholar]
- 76.Rizzo T, Metger BE, Burns WJ. Correlations between antepartum maternal metabolism and child intelligence. N Engl J Med. 1991;325:911–916. doi: 10.1056/NEJM199109263251303. [DOI] [PubMed] [Google Scholar]
- 77.Rae A, Bond D, Evans S, North F, Robertman B, Walters B. A randomized controlled trial of dietary energy restriction in the management of obese women with gestational diabetes. Aust NZJ Obstet Gyn. 2000;40:416–422. doi: 10.1111/j.1479-828x.2000.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 78.Algert S, Shragg P, Hollingsworth DR. Moderate caloric restriction in obese women with gestational diabetes. Obstet Gynaecol. 1985;65:487–491. [PubMed] [Google Scholar]
- 79.Gillen l, Tapsell LC, Martin GS. The type and frequency of consumption of carbohydrate-rich foods may play a role in the clinical expression of insulin resistance during pregnancy. Nutrition and Dietetics. J Diet Assoc Australia. 2002;59(2):135–143. [Google Scholar]
- 80.Nolan J. Improved glucose tolerance in gestational diabetic women on a low fat, high unrefined carbohydrate diet. Aust N Zel J Obstet Gynaecol. 1984;24(3):174–177. doi: 10.1111/j.1479-828x.1984.tb01483.x. [DOI] [PubMed] [Google Scholar]
- 81.Ceysens G, Rouiller D, Boulvain M. Exercise for diabetic pregnant women. Cochrane Database Syst. Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dyck R, Klomp H, Tan L, Turnell RW, Boctor MA. A comparison of rates, risk factors and outcomes of gestational diabetes between aboriginal and non aboriginal women in the Saskatoon health district. Diabetes Care. 2002;25:487–493. doi: 10.2337/diacare.25.3.487. [DOI] [PubMed] [Google Scholar]
- 83.Dempsey J, Butler C, Sorensen TK, et al. A case-control study of maternal recreational physical activity and risk of gestational diabetes mellitus. Diab Res Clin Pract. 2004;66:203–215. doi: 10.1016/j.diabres.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 84.American Diabetes Association. ADA Clinical Education Series. 3rd ed. 2000. Medical management of pregnancy complicated by diabetes; pp. 126–128. [Google Scholar]
- 85.Lapolla A, Dalfrà MG, Fedele D. Insulin therapy in pregnancy complicated by diabetes: are insulin analogs a new tool? Diabetes Metab Res Rev. 2005;21:241–252. doi: 10.1002/dmrr.551. [DOI] [PubMed] [Google Scholar]
- 86.Di Cianni G, Torlone E, Lencioni E, et al. Perinatal outcomes associated with the use of glargine during pregnancy. Diabet Med. 2008;25:993–996. doi: 10.1111/j.1464-5491.2008.02485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boskovic R, Feig DS, Derewlany L, Knie B, Portnoi G, Koren G. Transfer of insulin across the human placenta: in vitro perfusion studies. Diabetes Care. 2003;26(5):1390–1394. doi: 10.2337/diacare.26.5.1390. [DOI] [PubMed] [Google Scholar]
- 88.Jovanovic J, Ilic S, Pettitt DJ, et al. Metabolic and immunologic effects of insulin lispro in gestational diabetes. Diabetes Care. 1999;22:1422–1427. doi: 10.2337/diacare.22.9.1422. [DOI] [PubMed] [Google Scholar]
- 89.Pettitt DJ, Ospina P, Howard C, Zisser H, Jovanovic L. Efficacy, safety and lack of immunogenicity of insulin aspart compared with regular human insulin for women with gestational diabetes mellitus. Diabetic Medicine. 2007;24:1129–1135. doi: 10.1111/j.1464-5491.2007.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hofmann T, Horstmann G, Stammberger I. Evaluation of the reproductive toxicity and embryotoxicity of insulin glargine (Lantus) in rats and rabbits. Int J Toxicol. 2002;21(3):181–189. doi: 10.1080/10915810290096315. [DOI] [PubMed] [Google Scholar]
- 91.Price N, Bartlett G, Gillmer MD. Use of insulin glargine during pregnancy: a case-control pilot study. BJOG. 2007;114:453–457. doi: 10.1111/j.1471-0528.2006.01216.x. [DOI] [PubMed] [Google Scholar]
- 92.Elliot BD, Langer O, Schenker S, Johnson RD, Prihoda T. Comparative placental transport of oral hypoglycemic agents in humans: a model of human placental drug transfer. Am J Obstet Gynaecol. 1994;171:653–660. doi: 10.1016/0002-9378(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 93.Langer O, Yogev Y, Xenachis EMJ, Rosenn B. Insulin and glyburide therapy: dosage, severity level of gestational diabetes, and pregnancy outcome. Am J Obstet Gynaecol. 2005;192:134–139. doi: 10.1016/j.ajog.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 94.Holt RI, Clarke P, Parry EC, Coleman MAG. The effectiveness of glibenclamide in women with gestational diabetes. Diabetes Obes Metab. 2008;10:906–911. doi: 10.1111/j.1463-1326.2007.00828.x. [DOI] [PubMed] [Google Scholar]
- 95.Jacobson GF, Ramos GA, Ching JY, Kirby RS, Ferrara A, Field DR. Comparison of glyburide and insulin for the management of gestational diabetes in a large managed care organization. Am J Obstet Gynaecol. 2005;193:118–124. doi: 10.1016/j.ajog.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 96.Hague WM, Davoren PM, McIntyre D, Norris R, Xiaonian X, Charles B. Metformin crosses the placenta: a modulator for fetal insulin resistance. BMJ. 2003 4 December; [Google Scholar]
- 97.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP for the MIG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003–2015. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 98.Glueck CJ, Wang P, Kobayashi S, Phillips H, Sieve-Smith L. Metformin therapy throughout pregnancy reduces the development of gestational diabetes in women with polycystic ovary syndrome. Fertil Steril. 2002;77(3):520–525. doi: 10.1016/s0015-0282(01)03202-2. [DOI] [PubMed] [Google Scholar]
- 99.Glueck CJ, Goldenberg N, Pranikoff J, Loftspring M, Sieve L, Wang P. Height, weight and motor-social development during the first 18 months of life in 126 infants born to 109 mothers with polycystic ovary syndrome who conceived on and continued metformin through pregnancy. Hum Reprod. 2004;19(6):1323–1330. doi: 10.1093/humrep/deh263. [DOI] [PubMed] [Google Scholar]
- 100.de Veciana M, Trail PA, Lau TK, Dulaney K. A comparison of oral acarbose and insulin in women with gestational diabetes mellitus. Obstet Gynecol. 2002;99:5S. [Google Scholar]
- 101.Chan LY, Yeung JH, Lau TK. Placental transfer of rosiglitazone in the first trimester of human pregnancy. Fert Steril. 2005;83:955–958. doi: 10.1016/j.fertnstert.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 102.Hiles RA, Bawdon RE, Petrella EM. Ex vivo human placental transfer of the peptides pramlintide and exenatide. Hum Exp Toxicol. 2003;22(12):623–628. doi: 10.1191/0960327103ht402oa. [DOI] [PubMed] [Google Scholar]