Abstract
Classical experimental models of hemorrhage are characterized by the use of anesthetics that may interfere with the typical immune responses and pathology of hemorrhage/resuscitation. Thus, therapeutic strategies successful in anesthetized animals might not be beneficial in clinical trials. In this study, we analyzed whether ethyl pyruvate could provide therapeutic benefits during resuscitation in awake (unanesthetized) hemorrhage. Our results indicate that hemorrhage in unanesthetized animals required approximately 25% higher blood withdrawal than anesthetized animals to achieve the same targeted mean arterial blood pressure. Resuscitation with Hextend reestablished circulatory volume and improved survival during resuscitation of awake rodents. Yet, over 75% of the animals resuscitated with Hextend died within the first hours after hemorrhage. Resuscitation with Hextend containing 50 mM ethyl pyruvate protected over 87% of the animals. This survival benefit did not correlate with significant changes in the metabolic markers but with an anti-inflammatory potential during resuscitation. Unlike classical hemorrhage in anesthetized animals, ethyl pyruvate reestablished mean arterial blood pressure significantly earlier than Hextend in unanesthetized rodents. Unanesthetized animals showed twofold higher serum tumor necrosis factor (TNF)-α than anesthetized animals subjected to the same blood pressure. This process was not due to the response of a single organ, but affected all the analyzed organs including the lung, heart, spleen, and liver. Although resuscitation with Hextend failed to attenuate systemic TNF-α levels, it inhibited TNF-α levels in the lung, heart, and liver but not in the spleen. Unlike Hextend, resuscitation with ethyl pyruvate prevented high serum TNF-α levels and blunted TNF-α responses in all the organs including the spleen. These studies indicate that the inflammatory responses in anesthetized animals differ from that in unanesthetized animals and that awake hemorrhage can provide advantages in the study of anti-inflammatory strategies during resuscitation. Ethyl pyruvate may attenuate systemic inflammatory responses during resuscitation and improve survival in experimental models of awake hemorrhage.
Keywords: Hemorrhage, Resuscitation, Cytokines, TNF-α, Inflammation, Ethyl pyruvate
Introduction
Conventional resuscitation fluids are designed to reestablish circulatory volume and tissue perfusion, but they fail to target inflammatory responses [1, 2]. This consideration is particularly significant because overzealous production of inflammatory cytokines contributes to lethal cardiovascular shock and multiple organ failure [3, 4]. Among these, tumor necrosis factor (TNF)-α is one of the most characteristic inflammatory and cardio-depressant factors contributing to cardiovascular shock in hemorrhage and resuscitation [3, 5, 6]. Recombinant TNF-α is capable of triggering a spectrum of hemodynamic, metabolic, and pathological symptoms similar to that found in “hemorrhagic shock”, and TNF-α neutralization can prevent cardiovascular shock. For these reasons, there is an interest in developing advanced resuscitation fluids capable of restraining TNF-α production during resuscitation. Hextend is a novel plasma volume expander containing 6% hydroxyethyl starch in Ringer’s lactate solution [7]. Hetastarch creates oncotic pressure, which is normally provided by blood proteins and permits retention of intravascular fluid. The Tactical Combat Casualty Care guidelines recommend Hextend in mass casualty, military, or rural settings for its strategic advantages in settings of limited supply or hypovolemic resuscitation. Resuscitation with Hextend appears to prevent multiple organ injury [8] and improve short-term survival as compared to saline solution [9–11]. Still, common and advanced resuscitation fluids are based on lactated solutions [12]. Lactic acid (2-hydroxypropanoic acid) is used as an inert compound and does not provide any therapeutic value, and it can exacerbate lactic acidosis during hypovolemia [13]. Thus, several investigators proposed that lactic acid could be substituted by similar molecules to provide therapeutic potentials to resuscitation fluids. Pyruvate is a structurally similar molecule acting as a potential antioxidant and anti-inflammatory that protects mammalian cells from cytotoxicity [14]. Unlike lactate, pyruvate appears to be beneficial in experimental models of stroke and hemorrhage [15]. The clinical potential of pyruvate is limited by its instability and toxicity [14]. Aqueous solutions of pyruvate spontaneously degrade via condensation to form 2,4-dihydroxy-2-methylglutarate, a mitochondrial poison [16]. Ethyl pyruvate is a stable lipophilic derivative that can prevent degradation; it is also compatible with blood products, but unlike lactate, it can provide therapeutic benefits to the resuscitation fluids [17]. Ethyl pyruvate is a stable, soluble, non-toxic compound that lacks the potential toxicity of pyruvate, and it is classified as generally recognized as safe by the Food and Drug Administration. These studies suggest that ethyl pyruvate might provide a therapeutic potential for resuscitation fluids.
Recently, we reported that ethyl pyruvate can inhibit TNF-α production from human and murine macrophages [18, 19]. In vivo, ethyl pyruvate attenuated systemic inflammation and improved survival in classical experimental models of hemorrhage [20–22] and sepsis [18, 19, 23–25]. However, a recent study indicated that a single dose of ethyl pyruvate may worsen survival in endotoxemia [26] depending on the dose and time of administration [27]. These results reveal the need to study the factors affecting the therapeutic potential and mechanism of action of ethyl pyruvate [27]. These studies are of particular interest since a phase II trial using ethyl pyruvate in cardiopulmonary bypass was recently terminated [17]. Unlike hemorrhage in critical care, classical experimental models of hemorrhage are characterized by the extensive use of analgesics and anesthetics that impinge directly upon the physiological responses. Thus, therapeutic strategies successful in anesthetized rats might not be beneficial in awake animals or in clinical settings. Phenobarbital increases extra-alveolar permeability and promotes neutrophil recruitment in the lung [28]. Morphine also induces immunosuppressive effects mediated by the induction of corticosterone [29]. These studies suggest that unlike classical experimental models of hemorrhage, awake hemorrhage can provide advantages for the study of anti-inflammatory strategies during hemorrhage and resuscitation. Here, we avoided the use of analgesics and anesthetics and also analyzed whether ethyl pyruvate could provide therapeutic benefits in experimental models of awake hemorrhage. Ethyl pyruvate was expected to provide an anti-inflammatory value to resuscitation fluids such as Hextend. If so, resuscitation with a small volume of Hextend supplemented with ethyl pyruvate could provide therapeutic benefits of significant value in clinical settings. In addition to survival, blood and organs were analyzed to determine a mechanism of action. Given the implications of TNF-α in hemorrhagic shock, we analyzed the potential of ethyl pyruvate to restrain TNF-α production during resuscitation.
Materials and methods
Animal experiments
Adult male Sprague–Dawley rats (Harlen Sprague–Dawley, Indianapolis, IN, USA) weighing 350–450 g were acclimated for 7 days at 25°C on a 12-h light/dark cycle. All animal experiments were performed in accordance with the National Institutes of Health Guidelines under protocols approved by the Institutional Animal Care and Use Committee of the North Shore University Hospital and the UMDNJ–New Jersey Medical School. Animals were randomly grouped and investigators were blinded to the experimental treatment.
Awake hemorrhage
Hemorrhagic shock in awake (unanesthetized) rats was performed similar to that described by Handrigan et al. [10] Briefly, animals underwent surgical catheter placement R-FAC (Braintree Scientific, Braintree, MA, USA) under sterile conditions in the femoral artery and vein under isoflurane anesthesia (5% induction, 2% maintenance; Minrad, Buffalo, NY, USA). The catheters were connected to the corresponding fluid reservoir and blood pressure monitor (BPA-400; Micro-Med, Louisville, KY, USA). This strategy allowed animal free movement after recovery from the surgical preparation and prevented the animal from biting or twisting the cannulae, thus enabling the experiment to be conducted in awake animals. No further anesthesia was administered during the experimental procedure in the anesthetized animals. Of note, the experiment in Fig. 3 compares awake to anesthetized animals. The anesthetized animals were kept anesthetized with isoflurane all the time until the end of the resuscitation treatment (Fig. 3). Anesthesia was stopped after catheter placement, and no further anesthesia was used on these animals. The animals were allowed to recover for approximately 1 h. At the time of the experiment, each enrolled animal was noted to be awake, alert, and without evidence of discomfort. The blood pressure and heart rate were monitored and recorded for 15 min as a physiological baseline prior to the hemorrhage procedure. Then, hemorrhage was initiated by bleeding the femoral artery for 15 min to reach a mean arterial blood pressure (MAP) of 35–40 mmHg and subsequent maintenance of this blood pressure by continuous blood withdrawal for another 15 min. After the shock phase, the animals to be resuscitated received specific resuscitation treatment over 40 min with a total volume of 15 mL/kg (equivalent to 1,000 mL of Hextend in a 70-kg man). Shed blood was considered lost and it was not reinfused. Heart rate, MAP, shed blood volume, and intravenous fluid volume were continuously monitored and recorded. After surgical procedure, the animals were housed individually in regular cages.
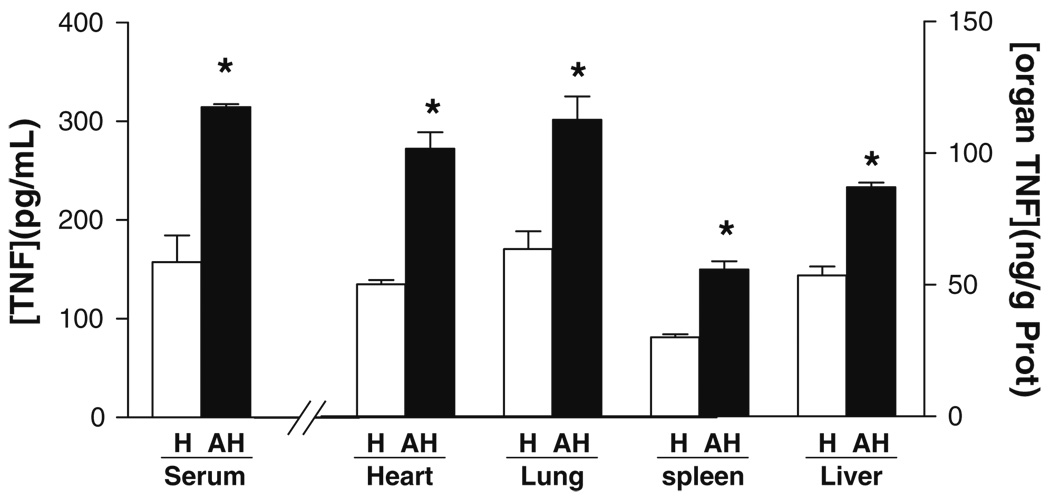
Fig. 3.
Systemic TNF-α responses in awake hemorrhage. Blood and organs from adult male Sprague–Dawley from anesthetized (H) or awake (AH) animals subjected to hemorrhage were collected at 2 h after the hemorrhagic load to analyze TNF-α protein concentration in the serum and specific organs. Asterisk represents p<0.05 vs. control (n=5/group; one-way ANOVA with Bonferroni’s corrections)
Blood chemistry and cytokine analyses
Blood was collected at 2 h after the hemorrhagic shock and analyzed using i-Stat blood analyzer (Abbot Laboratories, IL, USA). Metabolic changes in hemorrhagic shock were analyzed by the detection of electrolytes, pH, bicarbonate (HCO3), base excess of extracellular fluid (BEecf), anion gap (AnGap), glucose, hematocrit, and hemoglobin as well as total plasma protein. Lung and kidney functions were assessed by blood gases including total and partial carbon dioxide (TCO2, PCO2), and blood urea nitrogen. The systemic inflammatory status was assessed by TNF-α measured by enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).
NF-kB and PARP analyses
Specific p65NF-κB protein binding to DNA was analyzed by the TransAM DNA-binding ELISA assay (Active Motif; Cambridge, MA, USA) using specific p65NF-κB antibody. Poly(ADP-ribose) polymerase (PARP) activity in organ homogenates was analyzed using a commercially available kit (R&D Systems, Cat#4677-096K) following the manufacturer’s instructions. Protein levels were measured using the Bradford method (BIO-RAD) to normalize p65NF-κB and PARP activity to protein content.
Statistical analyses
Animals that died during the surgical preparation or before completion of the hemorrhage portion of the experiments were excluded from analyses. All data were expressed as mean±SD. Statistical analyses were performed using analysis of variance (ANOVA) with the Bonferroni’s correction. Analyses of normality and homogeneity of variance were performed to verify the assumptions of ANOVA. Data were log-transformed where applicable. ANOVA was used to compare all treatments and specific pairwise comparisons as stated in the experiments, particularly to compare control vs. Hextend, Hextend vs. HEP, and control vs. HEP. Student’s t test was used to compare mean values between the two experimental groups of Fig. 3. Statistical analyses of survival were determined using the log rank test. Kaplan–Meier product-limit estimates of the survival functions were plotted using Prism (version 5, San Diego, CA, USA). Tests resulting in p values of <0.05 were considered statistically significant.
Results
Ethyl pyruvate improved survival in experimental awake hemorrhage
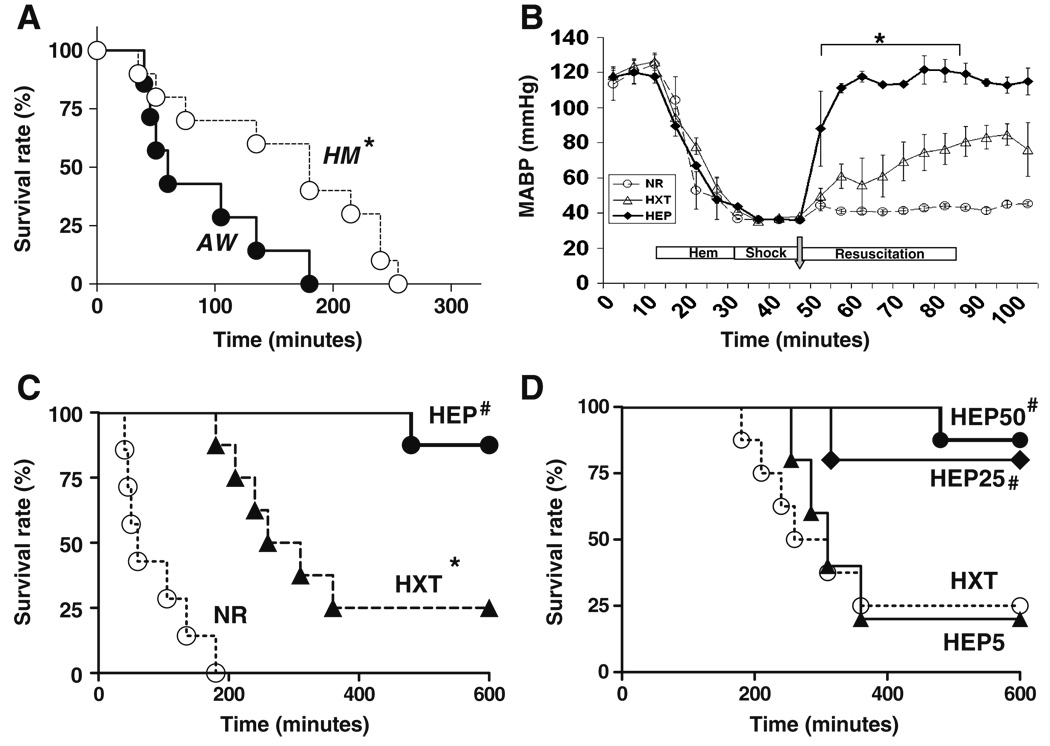
Awake (unanesthetized) rats were more resistant to hemorrhagic shock than anesthetized animals. Two groups of 25 adult male Sprague–Dawley rats of 420±30 g with no statistically significant differences in body weights or physiological blood pressures were used to compare the blood volume needed to induce the same hemorrhagic shock in awake or anesthetized animals. Hemorrhagic shock was induced by bleeding the femoral artery to obtain a mean arterial blood pressure (MABP) of 35–40 mmHg over 15 min and maintenance of this blood pressure for another 15 min. In anesthetized rats, this procedure required withdrawing ~21±4.3 mL blood/kg body weight, and the maintenance of that blood pressure for another 15 min required another 7±2.7 mL blood/kg body weight. The same hemorrhagic shock in awake rats required withdrawing approximately 31±5 mL blood/kg body weight, and the maintenance of that blood pressure required another 12±2.4 mL blood/kg body weight. In total, our experimental conditions required withdrawing 28±5 and 44±6 mL blood/kg body weight in anesthetized and awake rats, respectively (n=25, p<0.01). These data represent approximately 45% and 70% of the estimated blood volume, assuming normal blood volume of 60 mL/kg of body weight [30, 31]. This greater blood volume removed was associated with a faster mortality rate in the unanesthetized animals (Fig. 1a). All animals without resuscitation (NR), including both awake (n=8) and anesthetized (n=8) animals, did not reestablish normal blood pressure (Fig. 1b) and died within the first 5 h after hemorrhage (Fig. 1b). The average survival time after the hemorrhagic shock for awake and anesthetized rats was 60±26 and 135±85 min, respectively (n=8/group, p<0.01). Resuscitation with Hextend slowly reestablished mean arterial blood pressure and protected 25% of the animals (n=8/group, p<0.01 Hextend vs. NR, log rank survival test). The other 75% of the rats treated with Hextend died within 253±56 min after the hemorrhagic shock (Fig. 1c). The addition of ethyl pyruvate to the Hextend solution provided a statistically significant survival benefit during resuscitation. Resuscitation with ethyl pyruvate reestablished mean arterial blood pressure significantly earlier than resuscitation with Hextend (Fig. 1b) and protected 87% of the animals. Only one animal treated with ethyl pyruvate died (n=8/group, p<0.01 HEP50 vs. Hextend, log rank survival test). Ethyl pyruvate improved survival in hemorrhagic shock in a concentration-dependent manner (Fig. 1d). Addition of 25 mM ethyl pyruvate to Hextend significantly protected 80% of the hemorrhagic animals [p<0.01 HEP25 mM (n=5) vs. Hextend (n=8), log rank test], whereas 5 mM ethyl pyruvate failed to induce a statistically significant protection [p>0.5 HEP5 mM (n=5) vs. Hextend (n=8), log rank test]. All non-survival animals died within the first 8 h after hemorrhagic shock. All the animals that passed this critical period survived the hemorrhagic shock, and no late deaths were found even when the animals were followed for up to 5 days. These results suggest that ethyl pyruvate can induce a lasting protection and did not merely delay the pathologic onset.
Fig. 1.
Resuscitation with Hextend supplemented with ethyl pyruvate improved survival in awake hemorrhage. Adult male Sprague–Dawley hemorrhagic rats received no resuscitation treatment (NR) or were resuscitated with 15 mL/kg (i.v.) Hextend (HXT) or Hextend supplemented with 50 mM ethyl pyruvate (HEP). a Survival in experimental hemorrhage induced in awake, conscious (AW) or anesthetized (HM) animals. P<0.05 survival log rank test AH vs. HM (asterisk). b Arterial blood pressure was recorded during the hemorrhage and the resuscitation treatment. c Resuscitation with Hextend supplemented with 50 mM ethyl pyruvate (HEP50) statistically protected the animals from experimental hemorrhage in awake animals (n=7/group). d Ethyl pyruvate improved survival in hemorrhage in a concentration-dependent manner in the animals treated with 50 mM (HEP50), 25 mM (HEP25), or 5 mM (HEP5) ethyl pyruvate. P<0.05 survival log rank test vs. NR (asterisk) or HXT (number symbol)
Blood chemistry analyses during resuscitation
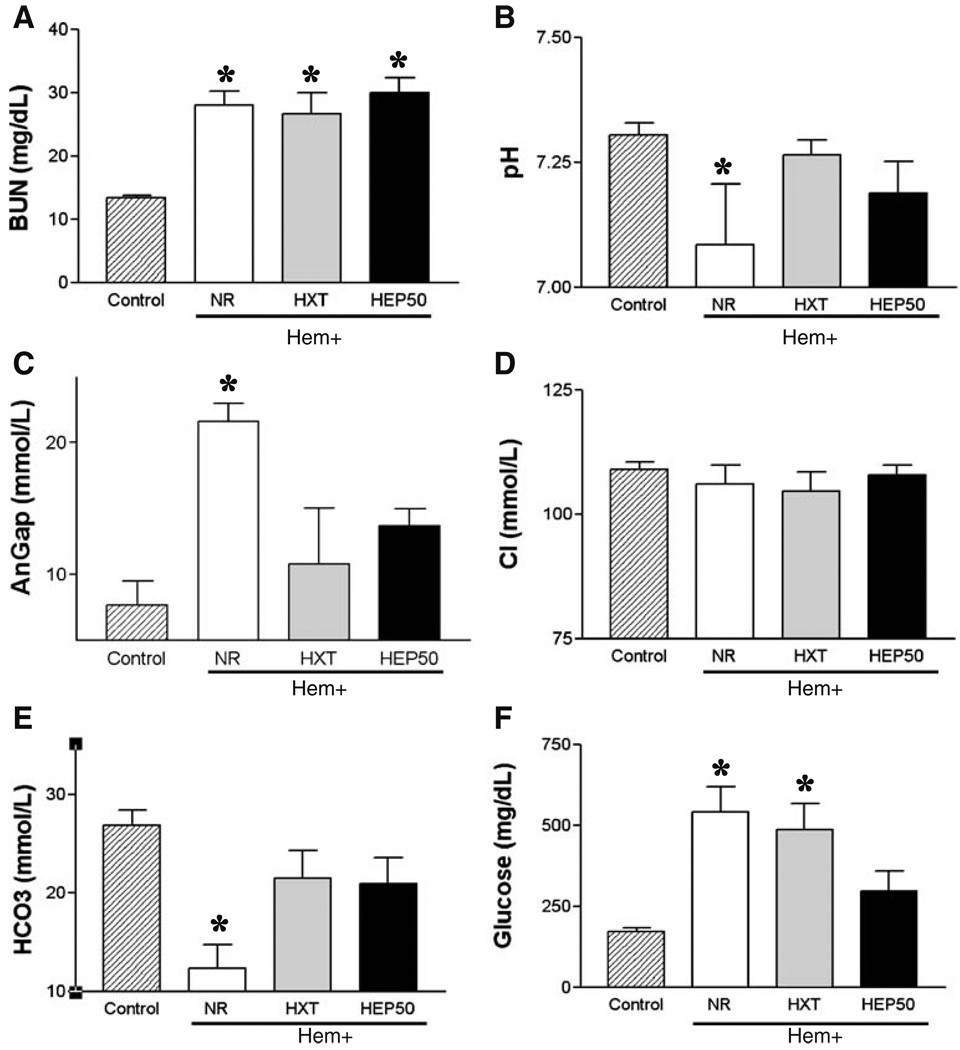
Characteristic pathological markers of hemorrhage were assessed by blood chemistry analyses. Blood was collected at 2 h after the hemorrhagic insult, which represents the average time of death for those animals without resuscitation treatment. Animals without resuscitation were characterized by uremia, metabolic acidosis, and hyperglycemia. Animals without resuscitation showed characteristic statistically significant high levels of blood urea nitrogen. Neither resuscitation with Hextend nor Hextend supplemented with 50 mM ethyl pyruvate prevented uremia, yet improved survival (Fig. 2a). Hemorrhagic animals without resuscitation showed metabolic acidosis (Fig. 2b) associated with elevated anion gap (AnGap; Fig. 2c) and low levels of bicarbonate (Fig. 2e). Both resuscitation with Hextend and Hextend supplemented with 50 mM ethyl pyruvate prevented metabolic acidosis and elevated anion gap to a similar extent. The animals resuscitated with Hextend or Hextend supplemented with 50 mM ethyl pyruvate showed statistically similar serum pH, anion gap, and bicarbonate levels. Hemorrhage also caused hyperglycemia (control=172±24 mg/dL vs. NR=541±78 mg/dL; n=5, p<0.005), which was not statistically inhibited by resuscitation with Hextend [NR=541±78 mg/dL (n=5) vs. Hextend=505±66 mg/dL (n=6), p>0.005; Fig. 2f]. Resuscitation with Hextend supplemented with 50 mM ethyl pyruvate tended to attenuate this response, but the effect was not statistically significant either using ANOVA (Newman–Keuls or Bonferroni’s correction) or Student’s t test analyses [NR=541±78 mg/dL (n=5) vs. HEP50=296±61 mg/dL (n=6), p>0.005]. Of note is that the glucose levels in those animals resuscitated with ethyl pyruvate were statistically similar to those in control naïve animals [control=172±24 mg/dL (n=5) vs. HEP50=296±61 mg/dL (n=6), p>0.05].
Fig. 2.
Blood chemistry analyses in awake hemorrhage. Blood from control adult male Sprague–Dawley rats or hemorrhagic awake animals without resuscitation treatment (NR) or resuscitated with 15 mL/kg (i.v.) Hextend (HXT) or Hextend supplemented with 50 mM ethyl pyruvate (HEP) was collected at 2 h after the hemorrhagic load to analyze a blood urea nitrogen (BUN), b pH, c the anion gap (AnGap), d chloride (Cl), e bicarbonate (HCO3), and f glucose. Number symbol represents p<0.05 for HEP vs. HXT. Asterisk represents p<0.05 vs. control (n=5/group; one-way ANOVA with Bonferroni’s corrections)
Inflammatory responses in awake hemorrhage
Tumor necrosis factor (TNF-α), a characteristic inflammatory and cardio-depressant factor contributing to cardiovascular shock, was analyzed in both anesthetized (isoflurane) and unanesthetized animals. TNF-α levels were analyzed at 2 h after the hemorrhagic insult, which represents the average time of death for those animals without resuscitation treatment. Unanesthetized animals had almost double serum TNF-α levels than anesthetized animals subjected to the same hemorrhagic procedure [anesthesized (H)=157±27 pg/mL vs. awake hemorrhage=314±29 pg/mL] (Fig. 3). TNF-α was also analyzed in the major organs, and TNF-α concentration was normalized according to total protein concentration. TNF-α concentrations were significantly higher in the lung, heart, and liver than in the spleen of anesthetized animals, similar as previously described [32]. Likewise, awake hemorrhagic animals exhibited a similar pattern and TNF-α concentrations that were particularly higher in the lung, heart, and liver than in the spleen. The higher serum TNF-α levels found in awake hemorrhagic animals were associated with statistically significant higher TNF-α levels in all the organs, namely lung, heart, liver, and spleen. All the organs had a statistically similar increase of TNF-α levels (~1.8- to 2.1-fold). Although this range of increase appears similar to that described in the serum, TNF-α concentrations in the organs are higher, as they are expressed in nanograms per gram as compared to the picograms per milliliter of serum. These results suggest that systemic TNF-α levels may not reflect the levels of that cytokine in major organs because of the TNF-α production by circulating immune cells.
Ethyl pyruvate attenuated systemic inflammation in awake hemorrhage
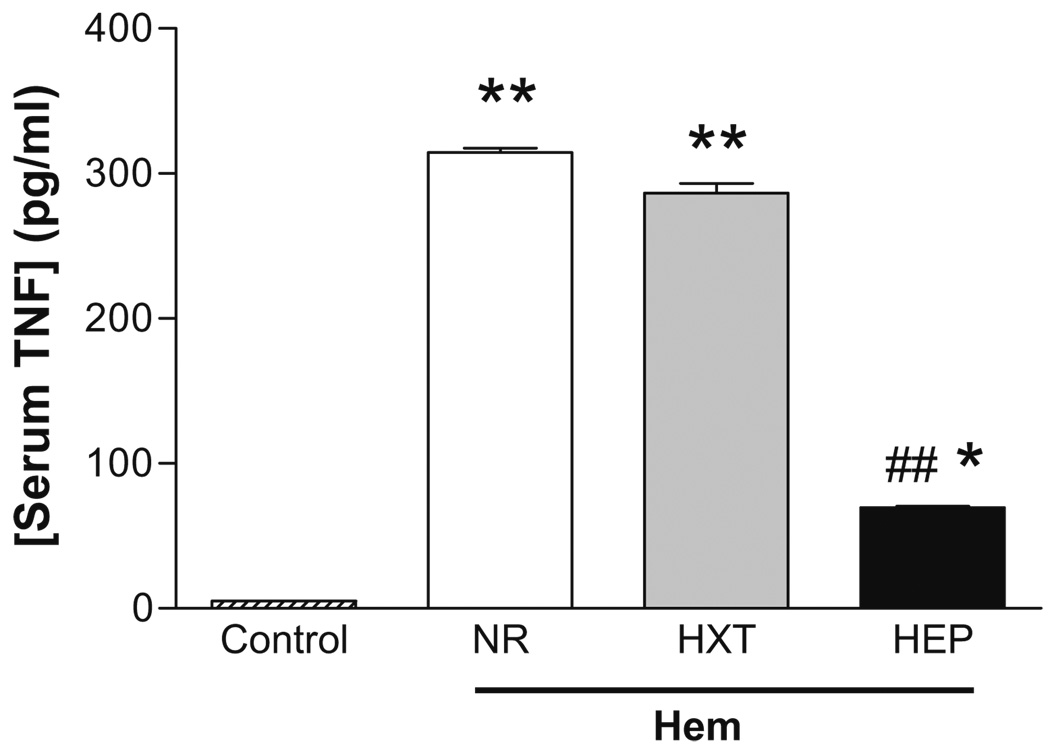
The most significant effects correlating with the therapeutic potential of ethyl pyruvate was its anti-inflammatory potential to restrain serum TNF-α levels. Hemorrhage induced characteristic lethal serum TNF-α responses that were not prevented by resuscitation with Hextend. Resuscitation with Hextend failed to attenuate serum TNF-α levels in a statistically significant manner (Fig. 4). However, resuscitation with Hextend-containing ethyl pyruvate statistically attenuated serum TNF-α levels by approximately 80%. Yet, TNF-α levels in the awake animals resuscitated with ethyl pyruvate remained significantly higher than those in normal animals (n=4, p<0.01). In order to characterize the anti-inflammatory mechanism of ethyl pyruvate, we analyzed its effects in the major organs (Fig. 5). Though Hextend did not inhibit serum TNF-α levels, resuscitation itself attenuated TNF-α levels in many organs including the lung, heart, and liver. This effect was particularly significant in the liver where resuscitation with Hextend attenuated serum TNF-α levels by over 85%. Resuscitation with Hextend also attenuated TNF-α levels by approximately 60% in both the lung and the heart. However, resuscitation with Hextend increased TNF-α levels in the spleen by almost twofold. In agreement with its effects on serum TNF-α, resuscitation with ethyl pyruvate blunted TNF-α in all the organs; this effect was statistically significant as compared to Hextend. The TNF-α levels in the organs of the animals resuscitated with ethyl pyruvate were statistically similar to those levels in normal (without hemorrhage) animals (p>0.01, n=4). The anti-inflammatory potential of ethyl pyruvate was particularly efficient in the spleen, where ethyl pyruvate completely blunted TNF-α as compared to Hextend. Thus, unlike Hextend, resuscitation with ethyl pyruvate prevented increases in serum TNF-α levels and blunted TNF-α responses in all the organs including the spleen.
Fig. 4.
Resuscitation with ethyl pyruvate attenuated serum TNF-α levels in awake hemorrhage. Blood from control adult male Sprague–Dawley rats or hemorrhagic animals without resuscitation treatment (NR) or resuscitated with 15 mL/kg (i.v.) Hextend (HXT) or Hextend supplemented with 50 mM ethyl pyruvate (HEP) was collected at 2 h after the hemorrhagic load to analyze serum TNF-α levels. Number symbols represent p<0.01 for HEP vs. HXT. Asterisk represents p<0.05 HEP vs. control. Two asterisks represent p<0.01 HEP vs. control (n=5/group; one-way ANOVA with Bonferroni’s corrections)
Fig. 5.
Resuscitation with ethyl pyruvate attenuated TNF-α levels in major organs in awake hemorrhage. Organs from control or animals subjected to hemorrhage were analyzed at 2 h to determine TNF-α concentration in the lung (a), heart (b), spleen (c), and liver (d). Number symbol represents p<0.05 for HEP vs. HXT. Asterisk represents p<0.05 as compared to control (n=5/group; one-way ANOVA with Bonferroni’s corrections)
Ethyl pyruvate inhibited NF-κB and PARP during resuscitation
P65NF-κB’s binding activity to DNA was analyzed in the spleen and liver at 2 h after hemorrhage. Anti-p65NF-κB antibody was used to analyze this particular NF-κB protein in the TransAM assay (Fig. 6a, b). Hemorrhage induced p65NF-κB binding to DNA in both the spleen and the liver by a similar magnitude of approximately twofold. Resuscitation with Hextend failed to inhibit p65NF-κB in either the spleen or liver. In contrast, resuscitation with Hextend-containing ethyl pyruvate significantly inhibited p65NF-κB in the liver, but not in the spleen. This inhibition in the liver was statistically significant when compared to the effect of Hextend alone (p<0.05). We also analyzed the effects of ethyl pyruvate on PARP, a characteristic regulator of the NF-κB pathway[33–36]. Hemorrhage induced a massive activation of the PARP in both the spleen (sixfold) and the liver (fourfold). Resuscitation with Hextend alone attenuated PARP in the liver, but not in the spleen (Fig. 6c, d). In contrast, ethyl pyruvate inhibited PARP in a statistically significant manner in both the spleen and liver.
Fig. 6.
Ethyl pyruvate inhibited in vivo NF-κB and PARP activation during resuscitation. Organs from control or hemorrhagic animals without resuscitation treatment (NR) or resuscitated with 15 mL/kg (i.v.) Hextend (HXT) or Hextend supplemented with 50 mM ethyl pyruvate (HEP) were analyzed at 2 h to analyze the activation of the p65NF-κB and PARP. Number symbol represents p<0.05 for HEP vs. HXT. Asterisk represents p<0.05 as compared to control (n=5/group; one-way ANOVA with Bonferroni’s corrections)
Discussion
Resuscitation with Hextend reestablishes the circulatory volume and improves survival in severe hemorrhage of unanesthetized rodents. Yet, over 75% of the animals resuscitated with Hextend died within the first few hours after hemorrhage. Ethyl pyruvate provided additional therapeutic potential, protecting over 87% of the unanesthetized hemorrhagic animals. There are three major considerations that may define the clinical implications of these studies as compared with previous studies [21]. First, our experimental models included about 70% of total blood volume loss in rats. Shed blood was considered lost and it was not reinfused. Resuscitation included a small volume of 15 mL/kg (equivalent to 1,000 mL of Hextend in a 70-kg patient) that represents approximately 33% of the shed blood volume. These considerations can have significant implications because recent studies indicate that high-volume, normotensive resuscitation may lead to increased hemorrhage volume and markedly higher mortality in shock associated with vascular injury [37]. These studies have particular implications in clinical settings of rural areas, military operations, or mass casualties characterized by limited supplies of resuscitation fluid, untimely evacuation to a medical facility, and hemorrhage associated with severe trauma [11, 38]. Second, our studies include awake hemorrhage with fixed hypotension. This is a significant difference as compared to the previous studies indicating that awake animals have better survival in hemorrhage with fixed blood volume. It has been suggested that this effect is, at least in part, due to the better ability of the awake animals to vasoconstrict and compensate blood loss. This effect should be neutralized with experimental models of hemorrhage with fixed hypotension. Third, similar to clinical standards where the patients die quickly or survive long term, all the control animals either died quickly within the first 8 h or survived over several days. The animals were followed for up to 5 days and no late deaths were observed. These observations imply that ethyl pyruvate can prevent early pathological responses contributing to cardiovascular collapse.
Ethyl pyruvate rendered survival benefits that did not correlate with significant changes in the metabolic markers but did correlate with an anti-inflammatory potential during resuscitation. Resuscitation with Hextend with or without ethyl pyruvate induced a similar effect in most of the standard metabolic markers representing a moderate organ dysfunction because ethyl pyruvate induced a lasting survival, although it did not affect these markers. Ethyl pyruvate failed to prevent hyperglycemia in a statistically significant manner. These results concur with previous studies indicating that ethyl pyruvate does not prevent hyperglycemia in a statistically significant manner in classical models of hemorrhage in rodents [32]. Similar studies also reported that ethyl pyruvate did not affect characteristic pathological markers or tissue energetics in anesthetized hemorrhagic swine [22]. One of the most significant effects of ethyl pyruvate was its potential to reestablish mean arterial blood pressure significantly earlier than resuscitation with Hextend alone. These results contrast with previous studies indicating that ethyl pyruvate did not improve early hemodynamics in anesthetized rodents [32] or swine [22]. Since TNF-α is a characteristic cardiodepressant factor contributing to cardiovascular shock [5, 6], this effect could be explained, at least in part, by lower serum TNF-α levels. Indeed, unanesthetized animals showed higher serum TNF-α levels during hemorrhage as compared to similar hemorrhagic shock in anesthetized animals. There are two major factors that may account for this effect. First, hemorrhage in unanesthetized animals required 25% higher blood withdrawal to achieve the same targeted mean arterial blood pressure. Second, anesthetics prevent characteristic inflammatory responses and interfere with the etiology of hemorrhage. For instance, phenobarbital increases extra-alveolar permeability and promotes neutrophil recruitment in the lung [28]. Morphine potentiates inflammatory responses by disrupting interleukin-1 modulation of the hypothalamic–pituitary–adrenal axis [39]. But depending on the experimental model, morphine can also induce immunosuppressive effects through the induction of corticosterone [29] and can promote macrophage apoptosis via p38 MAPK and Fas–Fas ligand interaction [40, 41]. These studies suggest that unlike classical experimental models of hemorrhage, awake hemorrhage can provide significant advantages for the study of anti-inflammatory strategies during hemorrhage and resuscitation. Thus, therapeutic strategies successful in anesthetized rats might not be beneficial in unanesthetized animals or clinical trials. These results may have additional implications, as a phase II trial using ethyl pyruvate in cardiopulmonary bypass was recently terminated [17]. According to our current results in unanesthetized animals, it may be possible to consider that different anesthetics used during cardiopulmonary bypass may affect the therapeutic potential of ethyl pyruvate.
Hemorrhage increased the serum TNF-α levels in unanesthetized animals by approximately twofold as compared to anesthetized animals. This response correlated with higher TNF-α levels in all the analyzed organs, namely heart, lung, spleen, and liver. All these organs increased TNF-α levels in a statistically similar pattern and magnitude of approximately twofold, very similar to that found in the serum. These results suggest that the increased inflammatory responses observed in unanesthetized animals are not due to the response of a single organ but produced by a general cytokine response in all of the organs. This notion contrasts with previous studies indicating that systemic TNF-α responses can be orchestrated by the spleen. Increased TNF-α produced by trauma associated with hemorrhage correlated with a particular TNF-α overproduction in the spleen. These results may suggest that trauma and anesthetics may impinge on the inflammatory responses through a different mechanism. The more significant results were that ethyl pyruvate improved survival, tissue perfusion (MABP), and prevented systemic TNF-α levels. Although resuscitation with Hextend alone failed to attenuate systemic TNF-α levels, it certainly inhibited TNF-α levels in the lung, heart, and liver, but not in the spleen. Unlike Hextend, ethyl pyruvate blunted TNF-α responses in all the organs including the spleen. These results could suggest that ethyl pyruvate may control systemic TNF-α levels during resuscitation in unanesthetized animlas by inhibiting TNF-α production in the spleen. Similar studies indicated that the spleen can be a major source of systemic TNF-α, and inhibition of TNF-α production in the spleen can prevent cardiovascular shock [42]. Moreover, the anti-inflammatory potential of ethyl pyruvate during resuscitation of anesthetized animals correlated with the inhibition of TNF-α production in the spleen [32]. The spleen also plays a critical role in controlling systemic inflammation in experimental models of sepsis. Splenectomy attenuated systemic inflammatory responses to bacterial endotoxemia and prevented the anti-inflammatory potential of general nicotinic agonists [42]. The vagus nerve and nicotine attenuate systemic inflammation in control but not in splenectomized endotoxemic rodents [42]. Nevertheless, our previous studies already indicated that ethyl pyruvate also attenuated systemic inflammation in splenectomized animals [32], suggesting that it can trigger a different anti-inflammatory mechanism independent of the spleen. In agreement with this hypothesis, our current studies indicate that ethyl pyruvate attenuates TNF-α levels in all the major organs.
Previous studies indicate that ethyl pyruvate prevents endotoxin-induced NF-κB activation in RAW264.7 macrophage cells [18, 19]. Similar studies in kidney 293 cells indicated that 40 mM of ethyl pyruvate inhibited p65RelA DNA binding [43]. In a cell-free system, ethyl pyruvate failed to inhibit the DNA-binding activity of both p50 homodimers and the mutant form of p65RelA with substitution of serine for cysteine 38 [43]. In this paper, we report that ethyl pyruvate modulated NF-κB and the PARP activity during resuscitation. One significant observation is that NF-κB did not mirror the pattern of TNF expression in these organs. The massive TNF production in the spleen and the liver did not correlate with a massive activation of NF-κB. Resuscitation with Hextend alone significantly attenuated hepatic TNF levels, but not hepatic NF-κB activity. Again, resuscitation with Hextend alone increased splenic TNF levels that did not correlate with increased splenic PARP activity. These potential discrepancies can be due, at least in part, to the implications of other factors required for TNF transcription. Indeed, recently published studies suggest that ethyl pyruvate can modulate diverse pathways. Ethyl pyruvate can modulate glyoxalase, which is important for detoxifying a reactive byproduct of intermediary metabolism, methylglyoxal [17]. Other studies suggest that the anti-inflammatory effects of ethyl pyruvate can be mediated by its antioxidant potential to scavenge hydrogen peroxide. Unlike p65NF-κB, PARP activity in the liver exhibited a pattern similar to TNF expression. These results could have significant implications, as PARP inhibition prevents organ injury and improves survival in hemorrhagic shock [34–36, 44]. Since PARP regulates NF-κB and PARP inhibitors suppress TNF-α production both in vitro and in vivo [36], it is possible that the therapeutic potential of ethyl pyruvate is, at least in part, mediated by modulating PARP and NF-κB. However, it is unknown how ethyl pyruvate can modulate these pathways. One hypothesis is that PARP can be modulated by the antioxidant potential of ethyl pyruvate [45]. The broad-spectrum antioxidant potential of ethyl pyruvate can reduce oxidative stress, and less DNA breakage can prevent PARP and NF-κB activation [36]. Similar studies indicated that ethyl pyruvate decreased NF-κB but worsened survival in lipopolysaccharide (LPS)-challenged mice [26]. Mice were treated with a single dose of ethyl pyruvate (0.01–100 mg/kg, n=204) at the same time point as LPS. The individual experiments did not provide a statistically significant effect, but it was statistically significant if all the experiments were combined together [26]. One potential explanation for this effect is that ethyl pyruvate was given in a single dose at the same time as LPS. Other studies reported therapeutic benefits with repeated administration of ethyl pyruvate started at different time points even before the endotoxic challenge [18, 27]. Another significant consideration is that NF-κB modulates cytokine production and also protects parenchyma cells from cytotoxicity and cell death [46–48]. The most characteristic example is that p65RelA [49–51] and IKKβ [52, 53] knockout mice exhibit embryonic death resulting from extensive TNF-α-mediated fetal hepatocyte apoptosis. Consistent with this hypothesis, TNF-α inhibition, either by removing TNF-α [54] or TNF-α-R1 [53], prevents this hepatocyte apoptosis in rela−/− mice, allowing embryonic development to birth. These studies suggest that ubiquitous NF-κB inhibition may not generate an overall beneficial effect especially in the liver, unless the therapy targets specific organs, immune cells, or NF-κB isoforms[55]. Together, these results warrant caution and reveal the need to study the factors affecting the mechanism and therapeutic potential of ethyl pyruvate in different experimental models before its clinical application [26, 27].
Acknowledgments
LU is supported by the faculty program of the Department of Surgery of the New Jersey Medical School, and grants from the US Army Medical Research Command (USAMRMC#05308004), the American Heart Association (AHA06352230N), and the NIH (RO1-GM084125).
Contributor Information
Bolin Cai, Laboratory of Anti-inflammatory Signaling and Surgical Immunology, Center of immunity and Infection, UMDNJ–New Jersey Medical School, Medical Science Building F-673, 185 South Orange Avenue, PO Box 1709, Newark, NJ 07103, USA.
Michael Brunner, Laboratory of Anti-inflammatory Signaling and Surgical Immunology, Center of immunity and Infection, UMDNJ–New Jersey Medical School, Medical Science Building F-673, 185 South Orange Avenue, PO Box 1709, Newark, NJ 07103, USA.
Haichao Wang, Department of Emergency Medicine, North Shore University Hospital, Manhasset, NY 11030, USA.
Ping Wang, Department of Surgery, North Shore University Hospital, Manhasset, NY 11030, USA.
Edwin A. Deitch, Department of Surgery, UMDNJ–New Jersey Medical School, Medical Science Building F-673, 185 South Orange Avenue, PO Box 1709, Newark, NJ 07103, USA
Luis Ulloa, Email: Mail@LuisUlloa.com, Laboratory of Anti-inflammatory Signaling and Surgical Immunology, Center of immunity and Infection, UMDNJ–New Jersey Medical School, Medical Science Building F-673, 185 South Orange Avenue, PO Box 1709, Newark, NJ 07103, USA; Department of Surgery, UMDNJ–New Jersey Medical School, Medical Science Building F-673, 185 South Orange Avenue, PO Box 1709, Newark, NJ 07103, USA.
References
- 1.Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med. 2007;356:2301–2311. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 2.Rushing GD, Britt LD. Reperfusion injury after hemorrhage: a collective review. Ann Surg. 2008;247:929–937. doi: 10.1097/SLA.0b013e31816757f7. [DOI] [PubMed] [Google Scholar]
- 3.Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 5.Tracey KJ, Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993;9:317–343. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- 6.Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 7.Gan TJ, Bennett-Guerrero E, Phillips-Bute B, Wakeling H, Moskowitz DM, Olufolabi Y, Konstadt SN, Bradford C, Glass PS, Machin SJ, Mythen MG. Hextend, a physiologically balanced plasma expander for large volume use in major surgery: a randomized phase III clinical trial. Hextend Study Group. Anesth Analg. 1999;88:992–998. doi: 10.1097/00000539-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen VG, Tan S, Brix AE, Baird MS, Parks DA. Hextend (hetastarch solution) decreases multiple organ injury and xanthine oxidase release after hepatoenteric ischemia–reperfusion in rabbits. Crit Care Med. 1997;25:1565–1574. doi: 10.1097/00003246-199709000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Kellum JA. Fluid resuscitation and hyperchloremic acidosis in experimental sepsis: improved short-term survival and acid–base balance with Hextend compared with saline. Crit Care Med. 2002;30:300–305. doi: 10.1097/00003246-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Handrigan MT, Bentley TB, Oliver JD, Tabaku LS, Burge JR, Atkins JL. Choice of fluid influences outcome in prolonged hypotensive resuscitation after hemorrhage in awake rats. Shock. 2005;23:337–343. doi: 10.1097/01.shk.0000156667.04628.1f. [DOI] [PubMed] [Google Scholar]
- 11.Mapstone J, Roberts I, Evans P. Fluid resuscitation strategies: a systematic review of animal trials. J Trauma. 2003;55:571–589. doi: 10.1097/01.TA.0000062968.69867.6F. [DOI] [PubMed] [Google Scholar]
- 12.Cordell AR. Milestones in the development of cardioplegia. Ann Thorac Surg. 1995;60:793–796. doi: 10.1016/0003-4975(95)00570-B. [DOI] [PubMed] [Google Scholar]
- 13.Baskett TF. The resuscitation greats: Sydney Ringer and lactated Ringer’s solution. Resuscitation. 2003;58:5–7. doi: 10.1016/s0300-9572(03)00209-0. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery CM, Fairhurst AS, Webb JL. Metabolic studies on heart mitochondria. III. The action of parapyruvate on –α-ketoglutaric oxidase. J Biol Chem. 1956;221:369–376. [PubMed] [Google Scholar]
- 15.Slovin PN, Huang CJ, Cade JR, Wood CE, Nasiroglu O, Privette M, Orbach P, Skimming JW. Sodium pyruvate is better than sodium chloride as a resuscitation solution in a rodent model of profound hemorrhagic shock. Resuscitation. 2001;50:109–115. doi: 10.1016/s0300-9572(01)00325-2. [DOI] [PubMed] [Google Scholar]
- 16.Vonkorff RW. Pyruvate-C14, purity and stability. Anal Biochem. 1964;8:171–178. doi: 10.1016/0003-2697(64)90043-0. [DOI] [PubMed] [Google Scholar]
- 17.Fink MP. Ethyl pyruvate. Curr Opin Anaesthesiol. 2008;21:160–167. doi: 10.1097/ACO.0b013e3282f63c2e. [DOI] [PubMed] [Google Scholar]
- 18.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002;99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulloa L, Fink MP, Tracey KJ. Ethyl pyruvate protects against lethal systemic inflammation by preventing HMGB1 release. Ann NY Acad Sci. 2003;987:319–321. [Google Scholar]
- 20.Tawadrous ZS, Delude RL, Fink MP. Resuscitation from hemorrhagic shock with Ringer’s ethyl pyruvate solution improves survival and ameliorates intestinal mucosal hyperpermeability in rats. Shock. 2002;17:473–477. doi: 10.1097/00024382-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Yang R, Gallo DJ, Baust JJ, Uchiyama T, Watkins SK, Delude RL, Fink MP. Ethyl pyruvate modulates inflammatory gene expression in mice subjected to hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2002;283:G212–G221. doi: 10.1152/ajpgi.00022.2002. [DOI] [PubMed] [Google Scholar]
- 22.Mulier KE, Beilman GJ, Conroy MJ, Taylor JH, Skarda DE, Hammer BE. Ringer’s ethyl pyruvate in hemorrhagic shock and resuscitation does not improve early hemodynamics or tissue energetics. Shock. 2005;23:248–252. [PubMed] [Google Scholar]
- 23.Sappington PL, Cruz RJ, Jr, Harada T, Yang R, Han Y, Englert JA, Ajami AA, Killeen ME, Delude RL, Fink MP. The ethyl pyruvate analogues, diethyl oxaloproprionate, 2-acetamidoacrylate, and methyl-2-acetamidoacrylate, exhibit anti-inflammatory properties in vivo and/or in vitro. Biochem Pharmacol. 2005;70:1579–1592. doi: 10.1016/j.bcp.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Su F, Wang Z, Cai Y, Remmelink M, Vincent JL. Beneficial effects of ethyl pyruvate in septic shock from peritonitis. Arch Surg. 2007;142:166–171. doi: 10.1001/archsurg.142.2.166. [DOI] [PubMed] [Google Scholar]
- 25.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 26.Su J, Li X, Cui X, Li Y, Fitz Y, Hsu L, Mani H, Quezado M, Eichacker PQ. Ethyl pyruvate decreased early nuclear factor-kappaB levels but worsened survival in lipopolysaccharide-challenged mice. Crit Care Med. 2008;36:1059–1067. doi: 10.1097/CCM.0B013E318164403B. [DOI] [PubMed] [Google Scholar]
- 27.Tenhunen JJ. Bull’s eye missed by the magic bullet: preclinical investigations, publication bias, and promising new interventions. Crit Care Med. 2008;36:1361–1363. doi: 10.1097/CCM.0b013e31816a1414. [DOI] [PubMed] [Google Scholar]
- 28.Peterson BT, Miller EJ, Griffith DE, Rowjee R, McWaters P. Modulation by pentobarbital of neutrophil responses to inhaled E. coli endotoxin in sheep: role of lung epithelium. Eur Respir J. 2000;16:697–703. doi: 10.1034/j.1399-3003.2000.16d22.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Charboneau R, Balasubramanian S, Barke RA, Loh HH, Roy S. The immunosuppressive effects of chronic morphine treatment are partially dependent on corticosterone and mediated by the mu-opioid receptor. J Leukoc Biol. 2002;71:782–790. [PubMed] [Google Scholar]
- 30.Giassi LJ, Poynter AK, Gainer JL. Trans sodium crocetinate for hemorrhagic shock: effect of time delay in initiating therapy. Shock. 2002;18:585–588. doi: 10.1097/00024382-200212000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Wu CH, Bogusky RT, Holcroft JW, Kramer GC. NMR monitoring of phosphate metabolism of rat skeletal muscle during hemorrhage and resuscitation. J Trauma. 1988;28:757–764. doi: 10.1097/00005373-198806000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Cai B, Chen F, Lin X, Levente K, Miller EJ, Szabo C, Deitch E, Ulloa L. Anti-inflammatory adjuvant in resuscitation fluids improves survival in hemorrhage. Crit Care Med. 2009 doi: 10.1097/CCM.0b013e31819b8237. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ditsworth D, Zong WX, Thompson CB. Activation of poly (ADP)-ribose polymerase (PARP-1) induces release of the pro-inflammatory mediator HMGB1 from the nucleus. J Biol Chem. 2007;282:17845–17854. doi: 10.1074/jbc.M701465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabo C. Potential role of the peroxynitrate-poly(ADP-ribose) synthetase pathway in a rat model of severe hemorrhagic shock. Shock. 1998;9:341–344. doi: 10.1097/00024382-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 36.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 37.Stern SA, Dronen SC, Birrer P, Wang X. Effect of blood pressure on hemorrhage volume and survival in a near-fatal hemorrhage model incorporating a vascular injury. Ann Emerg Med. 1993;22:155–163. doi: 10.1016/s0196-0644(05)80195-7. [DOI] [PubMed] [Google Scholar]
- 38.Institute of Medicine Committee on Fluid Resuscitation for Combat Casualties. Protocols of care at the cite of injury. Washington: D. N. A. P.; 1999. pp. 97–108. [Google Scholar]
- 39.House SD, Mao X, Wu G, Espinelli D, Li WX, Chang SL. Chronic morphine potentiates the inflammatory response by disrupting interleukin-1beta modulation of the hypothalamic–pituitary–adrenal axis. J Neuroimmunol. 2001;118:277–285. doi: 10.1016/s0165-5728(01)00337-x. [DOI] [PubMed] [Google Scholar]
- 40.Singhal PC, Bhaskaran M, Patel J, Patel K, Kasinath BS, Duraisamy S, Franki N, Reddy K, Kapasi AA. Role of p38 mitogen-activated protein kinase phosphorylation and Fas–Fas ligand interaction in morphine-induced macrophage apoptosis. J Immunol. 2002;168:4025–4033. doi: 10.4049/jimmunol.168.8.4025. [DOI] [PubMed] [Google Scholar]
- 41.Malik AA, Radhakrishnan N, Reddy K, Smith AD, Singhal PC. Morphine-induced macrophage apoptosis modulates migration of macrophages: use of in vitro model of urinary tract infection. J Endourol. 2002;16:605–610. doi: 10.1089/089277902320913314. [DOI] [PubMed] [Google Scholar]
- 42.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han Y, Englert JA, Yang R, Delude RL, Fink MP. Ethyl pyruvate inhibits nuclear factor-kappaB-dependent signaling by directly targeting p65. J Pharmacol Exp Ther. 2005;312:1097–1105. doi: 10.1124/jpet.104.079707. [DOI] [PubMed] [Google Scholar]
- 44.Liaudet L, Soriano FG, Szabo E, Virag L, Mabley JG, Salzman AL, Szabo C. Protection against hemorrhagic shock in mice genetically deficient in poly(ADP-ribose)polymerase. Proc Natl Acad Sci U S A. 2000;97:10203–10208. doi: 10.1073/pnas.170226797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woo YJ, Taylor MD, Cohen JE, Jayasankar V, Bish LT, Burdick J, Pirolli TJ, Berry MF, Hsu V, Grand T. Ethyl pyruvate preserves cardiac function and attenuates oxidative injury after prolonged myocardial ischemia. J Thorac Cardiovasc Surg. 2004;127:1262–1269. doi: 10.1016/j.jtcvs.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 46.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 48.Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 49.Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka M, Fuentes ME, Yamaguchi K, Durnin MH, Dalrymple SA, Hardy KL, Goeddel DV. Embryonic lethality, liver degeneration, and impaired NF-kappa B activation in IKK-beta-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- 52.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 53.Alcamo E, Hacohen N, Schulte LC, Rennert PD, Hynes RO, Baltimore D. Requirement for the NF-kappaB family member RelA in the development of secondary lymphoid organs. J Exp Med. 2002;195:233–244. doi: 10.1084/jem.20011885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doi TS, Marino MW, Takahashi T, Yoshida T, Sakakura T, Old LJ, Obata Y. Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proc Natl Acad Sci U S A. 1999;96:2994–2999. doi: 10.1073/pnas.96.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mantell LL, Parrish WR, Ulloa L. HMGB1 as a therapeutic target for infectious and inflammatory disorders. Shock. 2006;25:4–11. doi: 10.1097/01.shk.0000188710.04777.9e. [DOI] [PubMed] [Google Scholar]