Abstract
The monocyte chemoattractant protein-1 (MCP-1)/CC-chemokine receptor 2 (CCR2) pathway plays a critical role in the development of antiglomerular basement membrane (anti-GBM) nephritis. We recently showed angiotensin II (Ang II) infusion in rats activated MCP-1 and transforming growth factor-β1 (TGF-β1), which in turn induced macrophage infiltration of renal tissues. This study was performed to demonstrate that combination therapy with a CCR2 antagonist (CA) and an Ang II type 1 receptor blocker (ARB) ameliorated renal injury in the anti-GBM nephritis model. An anti-GBM nephritis rat model developed progressive proteinuria and glomerular crescent formation, accompanied by increased macrophage infiltration and glomerular expression of MCP-1, angiotensinogen, Ang II, and TGF-β1. Treatment with CA alone or ARB alone moderately ameliorated kidney injury; however, the combination treatment with CA and ARB dramatically prevented proteinuria and markedly reduced glomerular crescent formation. The combination treatment also suppressed the induction of macrophage infiltration, MCP-1, angiotensinogen, Ang II, and TGF-β1 and reversed the fibrotic change in the glomeruli. Next, primary cultured glomerular mesangial cells (MCs) stimulated by Ang II showed significant increases in MCP-1 and TGF-β1 expression. Furthermore, cocultured model consisting of MCs, parietal epithelial cells, and macrophages showed an increase in Ang II-induced cell proliferation and collagen secretion. ARB treatment attenuated these augmentations. These data suggest that Ang II enhances glomerular crescent formation of anti-GBM nephritis. Moreover, our results demonstrate that inhibition of the MCP-1/CCR2 pathway with a combination of ARB effectively reduces renal injury in anti-GBM nephritis.
Keywords: renin-angiotensin system, crescentic glomerulonephritis, MCP-1, CCR2 antagonist, TGF-β1
Crescentic glomerulonephritis (GN), also known as antiglomerular basement membrane (anti-GBM) disease or Goodpasture’s syndrome, is characterized by the formation and deposition of antibodies on the basement membranes of glomeruli and alveoli.1 The disease progresses rapidly, and patients present with renal failure, dyspnea, hemoptysis, a sudden decrease in the hemoglobin level, pallor, and circulatory disturbances. Most patients with advanced disease do not respond to plasmapheresis or immunosuppression therapy.2 While kidney transplantation is an option, because of the risk of recurrence, a patient should wait for 6 months or after the disappearance of serum anti-GBM antibodies before undergoing kidney transplantation.1 Therefore, a novel therapeutic strategy is needed. Studies based on anti-GBM antibody have focused on elucidating the molecular and cellular mechanisms involved in the pathogenesis of this disease. Understanding the mechanisms of proinflammatory responses help facilitate the identification of therapeutic targets for arresting the progression of anti-GBM disease.
In Wistar-Kyoto (WKY) rats, the administration of a minute dose of anti-GBM antibodies induces severe proliferative and necrotizing GN with crescent formation.3 In rat models of anti-GBM disease, glomerular infiltration by T lymphocytes, monocytes/macrophages, and a few neutrophils is the earliest and the most prominent pathological change.3 Recent studies have revealed that monocyte chemoattractant protein-1 (MCP-1) is involved in the pathogenesis of crescentic GN.4 Various methods for blocking the actions of proinflammatory cytokines and chemokines have been evaluated in animal models3,5,6 However, the current knowledge regarding effective therapies for anti-GBM disease and the mechanism underlying its pathogenesis is still limited.
Recently, we showed that chronic angiotensin II (Ang II) infusion in rats activated MCP-1 and transforming growth factor-β1 (TGF-β1), which in turn induced macrophage infiltration in renal tissues.7 Furthermore, we reported that TGF-β1 is associated with crescent formation in GN.8 MCP-1 plays a pivotal role in crescentic GN. The release of MCP-1 and TGF-β1 is mediated by the renin-angiotensin system (RAS), and these molecules are considered to be key targets in the treatment of anti-GBM disease. In this study, we hypothesize that the therapeutic management of anti-GBM disease could focus on blocking the MCP-1/CC chemokine receptor 2 (CCR2) signaling pathway and RAS. Our central hypothesis is that the interaction between the MCP-1/CCR2 pathway and the RAS is important for the development of anti-GBM disease.
Methods
Animal Preparation (In Vivo Study)
The Institutional Animal Care and Use Committee of the Tulane University Health Sciences Center approved all procedures and protocols used in this study. To investigate whether the blockade of MCP-1 and RAS attenuates anti-GBM disease, we treated anti-GBM disease-affected rats with a CCR2 antagonist (CA) and an Ang II type 1 (AT1) receptor blocker (ARB). Progressive anti-GBM GN was induced in 7-week–old male WKY rats by a single intraperitoneal injection of 100 μg of rat monoclonal GBM antibody.9 RS102895 (CA, 10 mg/kg per day) and/or olmesartan (ARB, 10 mg/kg per day) were mandatory injected into their mouths from disease induction. All rats were killed under anesthesia (pentobarbital) at 2 weeks after the injection of anti-GBM antibodies. RS102895 (Tocris Bioscience) is a novel member of specific CA, and this compound has been shown to inhibit MCP-1/CCR2 signaling in vivo in rodents.10 The dose of olmesartan that was used in this experiment was high enough to inhibit Ang II receptor binding to kidney tissue.11 Control rats were nondiseased rats without anti-GBM antibodies or any drug treatment. The urine was obtained in a 24-hour collection using metabolic cages. The amount of protein excreted into the urine was measured by the pyrogallol red method. Blood samples were obtained from all rats at the time of sacrifice. Urinary concentrations of angiotensinogen (AGT) were measured with a commercially available ELISA kit (IBL).12 Serum creatinine levels were measured by quantitative colorimetric determination (BioAssay Systems). Systolic blood pressure was measured in conscious rats using tail-cuff plethysmography (Visitech) as described previously.7,12 All rats were euthanized at 2 weeks after the injection of anti-GBM antibodies, and kidneys were immediately harvested for protein or RNA extraction or for histological analysis.
Cell Preparation (In Vitro Study)
Rat cultured mesangial cells (MCs) were established from intact glomeruli as described previously.13 The normal alveolar macrophages NR8383 were purchased from American Type Culture Collection (ATCC). Parietal epithelial cells (PECs) and macrophages were placed into the lower compartment of Transwell cluster plates (Costa Corning) separated by the chamber with a 0.4-μm polyester membrane filter. MCs were added to the upper chamber and incubated at 37°C in a humidified 5% CO2 incubator. Supernatants were collected from MCs, and the MCP-1 or TGF-β1 concentration was measured with a commercially available ELISA kit. A 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (WST-1) assay was performed to determine the cell proliferation ability, and the Sircol collagen assay kit was used to measure the collagen concentration from cultured cells.
Kidney Histology and Immunohistochemistry, Quantitative Real-Time RT-PCR, Western Blot Analysis, Rat PEC Culture, Cytokine Determination by ELISA, and Cell Proliferation Assay and Collagen Measurement
See http://hyper.ahajournals.org for the detailed Methods.
Statistical Analysis
Statistical analysis was performed using a one-way factorial ANOVA with the post hoc Scheffe’s F test. All data are presented as means±SEM, and probability values <0.05 were considered significant.
Results
Effects of Treatment With the CA and/or the ARB on Systolic Blood Pressure, Urinary Protein Excretion, and Plasma Creatinine Levels
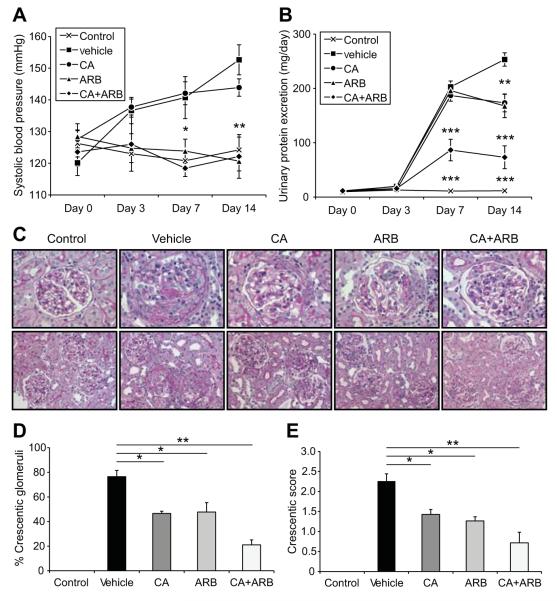
In contrast to the non-GN rats, systolic blood pressure of the vehicle-treated GN rats was elevated. Treatment with the CA alone showed slightly lower systolic blood pressure than the vehicle-treated GN rats. In comparison, the systolic blood pressure was significantly reduced in the groups treated with the ARB alone or with the combination of CA plus ARB (Figure 1A). As shown in Figure 1B, the vehicle-treated GN rats developed time-dependent, progressive proteinuria, with the urinary protein excretion level increased almost 20-fold at day 14. CA or ARB treatment moderately ameliorated the development of proteinuria. However, CA plus ARB treatment appeared to be attenuated better than other treatments. Consistently, plasma creatinine levels at day 14 were elevated in vehicle-treated rats, which were significantly reduced by CA plus ARB treatment (supplemental Figure S1A). Body weight, daily total water intake, and urine volume were comparable among all groups (supplemental Figure S1B, S1C, and S1D).
Figure 1.
Effects of CA, ARB, or combination therapy (CA+ARB) on systolic blood pressure, urinary protein excretion, and crescent formation in an anti-GBM disease rat model. A and B, Systolic blood pressure (A) and urinary protein excretion (B). Data are mean±SEM. *P<0.05 (vs vehicle); **P<0.01 (vs vehicle); ***P<0.001 (vs vehicle). C, Periodic acid-Schiff-stained sections. Original magnification ×400 (top) and ×200 (bottom). D, Percentage of glomeruli with crescentic formation. E, Quantitative analyses of the crescent score. Data are mean±SEM. *P<0.05 and **P<0.01 between groups as indicated.
Histological Study
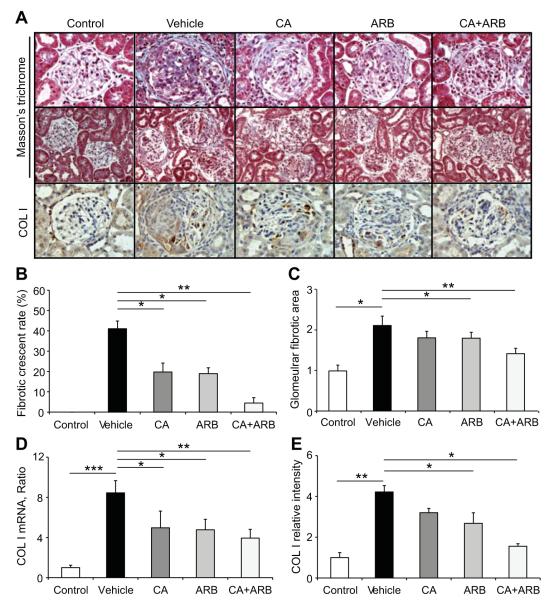
We examined the effects of treatment with the CA and/or the ARB on renal histology by using periodic acid-Schiff-stained sections (Figure 1C). The most prominent change in the vehicle-treated GN rats was severe glomerular crescent formation. The degree of crescent formation was lower in the CA- or the ARB-treated rats than in rats treated with the vehicle (Figure 1D). Moreover, the combination of the CA and the ARB significantly inhibit crescent formation and GN. The crescentic score was also highest in vehicle-treated GN rats, and the combination therapy with a CA and an ARB showed the significant reduction of the score (Figure 1E). Furthermore, we quantified the fibrotic changes in glomeruli (Figure 2). Compared with the non-GN rats, vehicle-treated GN rats showed elevation of the percentage of fibrocellular crescent in counted glomerular crescent (Figure 2B) and glomerular fibrotic area (Figure 2C) as well as collagen type I expression (Figure 2A, 2D, and 2E). The inductions of these factors were suppressed by CA and ARB treatment, consistent with the improved level of urinary protein in cotreated rats. Therefore, combination therapy with a CA and an ARB markedly prevents the development of glomerular fibrosis during the course of crescentic GN.
Figure 2.
Effects of the treatments on the development of glomerular fibrosis in an anti-GBM disease rat model. A, Masson’s trichrome-stained sections and collagen type I (COL I)-immunostained kidney sections. Original magnification ×400 (top, Masson’s trichrome; bottom, COL I); ×200 (middle, Masson’s trichrome). B, Percentage of fibro-cellular crescent glomeruli. C, Quantitative assessment of the glomerular fibrotic area. D, Quantitative real-time reverse transcription-PCR of COL I mRNA in the isolated glomeruli of all treatment groups. E, Densitometric analysis of COL I expression determined by immunostaining. Data are mean±SEM *P<0.05, **P<0.01, and ***P<0.001 between groups as indicated.
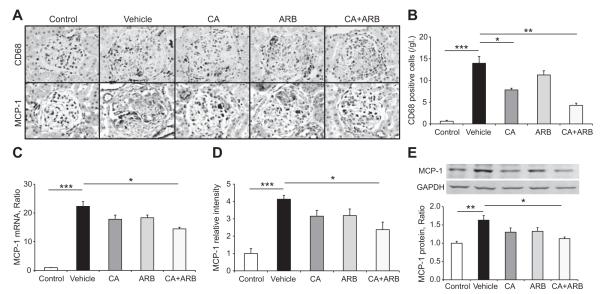
Glomerular Infiltration by Macrophage and Expression of MCP-1
The number of CD68-positive macrophages in nephritic glomeruli was markedly increased in vehicle-treated GN rats (Figure 3A and 3B) compared with the non-GN rats. Treatment with CA significantly inhibited the macrophage accumulation in glomeruli. Moreover, CA plus ARB treatment dramatically reduced the macrophage infiltration into glomeruli. Consistently, glomerular expression of MCP-1 was induced in vehicle-treated GN rats (Figure 3A, 3C, 3D, and 3E), as determined by quantitative polymerase chain reaction (PCR) (Figure 3C), immunostaining (Figure 3A and 3D), and Western blotting (Figure 3E). Treatment with the CA and ARB combination prevented the induction of MCP-1 at the both the mRNA and protein levels in the GN rats.
Figure 3.
Macrophage infiltrations and MCP-1 expression in crescentic glomeruli. A, CD68-positive macrophage infiltration (top) and MCP-1-immunostained (bottom) kidney sections. Original magnification ×400. B, Number of CD68-positive macrophages per glomerular cross-section. C, Quantitative real-time reverse transcription-PCR of MCP-1 mRNA in the isolated glomeruli of all treatment groups. D, MCP-1 expression determined by immunostaining. E, Western blot analyses of MCP-1 protein levels in isolated glomeruli of all treatment groups. Data are mean±SEM. *P<0.05, **P<0.01, and ***P<0.001 between groups as indicated.
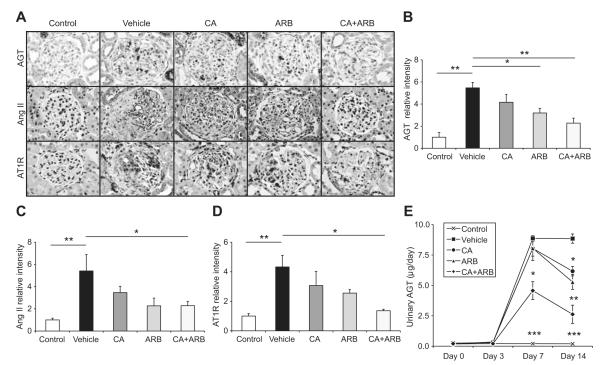
RAS Activation in Crescentic GN
To determine whether CA plus ARB treatment more effectively suppressed the activation of RAS, Ang II expression levels in glomeruli were examined. Immunostaining showed marked Ang II accumulation in nephritic glomeruli (Figure 4A and 4C). While CA or ARB treatment moderately reduced the increase of Ang II expression level in glomeruli, CA plus ARB treatment further prevented the Ang II accumulation. Consistently, CA plus ARB treatment drastically suppressed AGT expression in glomeruli, whereas CA or ARB treatment mildly attenuated the AGT increase in glomeruli (Figure 4A and 4B). These observations were confirmed by urinary AGT levels (Figure 4E). Furthermore, AT1 receptor expression levels were paralleled with Ang II and AGT expression levels (Figure 4A and 4D).
Figure 4.
Effects of the treatments on intrarenal AGT, Ang II, and AT1 receptor (AT1R) expressions and urinary AGT excretion in an anti-GBM disease rat model. A, AGT (top)-, Ang II (middle)-, and AT1R (bottom)-immunostained kidney sections. Original magnification ×400. B, Densitometric analyses of AGT expressions determined by immunostaining. C, Densitometric analyses of Ang II expressions determined by immunostaining. D, Densitometric analyses of AT1R expressions determined by immunostaining. Data are mean±SEM. *P<0.05 and **P<0.01 between groups as indicated. E, Urinary AGT excretion. Data are mean±SEM. *P<0.05 (vs vehicle); **P<0.01 (vs vehicle); ***P<0.001 (vs vehicle).
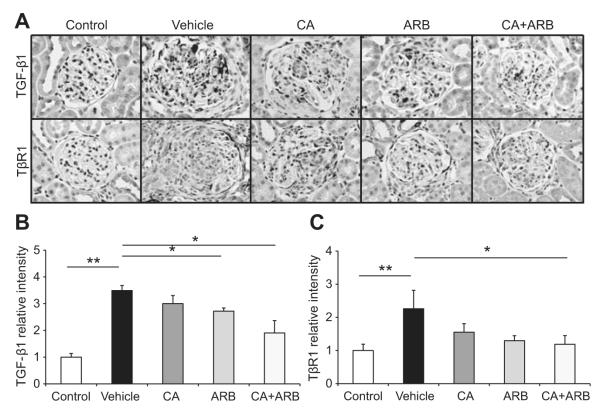
Expression of TGF-β1 in Crescentic GN
We quantified the expression of TGF-β1 by immunostaining (Figure 5A and B), quantitative PCR (supplemental Figure S2A), and Western blotting (supplemental Figure S2B). Compared with control rats, vehicle-treated GN rats showed elevation of TGF-β1 expression. The drug treatments, particularly CA plus ARB treatment, mostly attenuate these augmentations, consistent with the improved glomerular fibrotic levels and RAS activation. TGF-β1 type 1 receptor (TβR1) expression levels in glomeruli were closely paralleled with TGF-β1 expressions (Figure 5A and 5C).
Figure 5.
Effects of the treatments on TGF-β1 expression in an anti-GBM disease rat model. A, TGF-β1-immunostained (top) and TGF-β1 type 1 receptor (TβR1)-immunostained (bottom) kidney sections. Original magnification ×400. B, Densitometric analysis of TGF-β1 expression determined by immunostaining. C, Densitometric analysis TβR1 expression determined by immunostaining. Data are mean±SEM. *P<0.05 and **P<0.01 between groups as indicated.
Effects of Ang II on MCP-1 and TGF-β1 mRNA Levels in Cultured MCs
Following incubation of quiescent MCs with each concentration of Ang II, quantitative PCR revealed an increase in MCP-1 and TGF-β1 mRNA levels (supplemental Figure S3A and S3B). ARB treatment reduced the elevated expression levels of MCP-1 and TGF-β1 (supplemental Figure S3C and S3D). These observations were confirmed by ELISA (supplemental Figure S3E and S3F).
Effects of Ang II on Cell Proliferation and Collagen Secretion in Cocultured PECs and Macrophages With/Without MCs
We examined cell proliferation and collagen secretion in the cocultured PECs and macrophages with/without MCs stimulated with Ang II using the WST-1 assay or Sircol collagen assay, respectively. As a result, ARB treatment completely prevented an increase in cell proliferation or collagen secretion stimulated by Ang II, while CA or pan-specific neutralizing TGF-β antibody alone moderately reduced increases (supplemental Figure S4A and S4B). On the other hand, there were no significant differences between each group in cocultured PECs and macrophages without MCs (supplemental Figure S4C and S4D).
Discussion
To our knowledge, our results provided the first evidence that the combination therapy of CA and ARB markedly attenuates proteinuria and the progression of crescentic GN. MCP-1 is presumed to be a key molecule in chemotaxis and activation of macrophages.14 CCR2, a cognate receptor of MCP-1 expressed mainly on monocytes, has been reported to be involved in human crescentic GN.15 The strategy of blocking MCP-1/CCR2 interaction might be effective in preventing macrophage-induced tissue damage. Supporting this notion, neutralization of MCP-1 has been reported to reduce macrophage infiltration and progressive kidney damage.3,16,17 Newly developed antagonists against chemokine receptors are now available and have been used as therapeutic agents in kidney injury.18,19 In addition, RS102895 also has the capacity to inhibit MCP-1–induced chemotaxis and renal inflammation in the hypertensive rat model, where MCP-1 plays a role.10 However, few studies have provided direct evidence that the blockade of CCR2 might be effective for the treatment of crescentic GN.20
The RAS plays an important role in the development of hypertension, in fluid and electrolyte homeostasis, and in the progression of renal disease.21,22 Recently, the focus of interest on the RAS has shifted toward the role of the local/tissue RAS in specific tissues.23 The local RAS in the kidney has several pathophysiologic functions, for not only regulating blood pressure but also renal cell growth and production of glomerulosclerosis, which is included in the development of renal fibrosis.24,25 Indeed, previous studies have shown that RAS blockades have beneficial effects in rats and in humans with various renal diseases, and these effects are often considerably more significant than their suppressive effects on blood pressure.26,27 Based on these principles, here we demonstrated that combination administration of a CA and an ARB very effectively blocks the development of crescentic GN in the anti-GBM disease animal model.
Glomerular crescents are defined as the presence of ≥2 layers of cells in the Bowman space. Monocyte/macrophages and PECs are the principle mediators of crescent formation.3 The presence of crescents in glomeruli is a marker of severe injury.28 In the present study, we demonstrated that CA or ARB alone moderately normalized the crescent formation. The dose of CA or ARB was determined by previous reports10,11 and could be adequate to preclude the effects of the MCP-1/CCR2 signal pathway and RAS. Their combination significantly blocked the development of crescent formation, preventing the infiltration of macrophages. Consistently, the combination therapy markedly reduced proteinuria. Interestingly, the expression of MCP-1 was significantly reduced by the combination therapy. This reduction may be proportional to the decline in macrophage infiltration into the glomerular crescent. It was reported that a positive feedback loop between monocyte and MCP-1 expression depends on MCP-1 stimulation.29 Also, the interaction between macrophages and renal resident cells would be important. Activated macrophages by MCP-1/CCR2 signaling produce proinflammatory cytokines and chemokines including MCP-1, which in turn stimulate renal resident cells to produce cytokines and chemokines.19
It is well known that intrarenal RAS activation is a major mediator of progressive renal injury in GN.30-33 In this anti-GBM disease model, the glomerular expression levels of RAS components were increased compared to control rats. The disturbance in the expression of these components likely plays an important role in the pathogenesis of the crescentic formation in GN. Furthermore, it is reported that Ang II upregulated AGT and Ang II receptor expressions and ARB prevents the increase of AGT, suggesting positive Ang II feedback in kidney.34 Interestingly, ARB treatment prevented increases in kidney and renal interstitial fluid Ang II concentration in the Ang II-infused rat.35 Thus, in our study, combination therapy suppressed these expressions more effectively than CA or ARB alone, cutting intrarenal RAS activation. In previous studies, the RAS activation was shown to be involved in the formation of glomerular crescent.36,37 Together, these data clearly indicate that blocking the RAS is a key target in the treatment of anti-GBM disease. Our results cannot utterly exclude that the lowering blood pressure level by ARB treatment affects the present data. However, it has been shown that RAS blockade has the protective effect for renal injury independent of systemic blood pressure.24,26,27 Furthermore, we have clarified ARB suppressed fibrotic changes of glomerular crescent and the expression of collagen type 1 in the in vivo study. Also, ARB inhibited cell proliferation and collagen secretion for PECs in the in vitro study. From these findings, we believe that suppressing RAS activation is effective for the attenuation of the progression of crescentic GN.
The reversibility of crescents correlates with the relative predominance of cellular components.38 The progression or resolution of crescents may depend on the integrity of the Bowman capsule and the resulting cellular composition of the crescent. The progression of crescents to the fibrous stage is commonly observed in a ruptured capsule and also in the presence of dominant fibrous lesion and macrophages in the Bowman space. The presence of fibrous crescents usually correlates with irreversible glomerular sclerosis. The combination of CA and ARB also reduced fibrous changes in the glomerular crescent, which was accompanied by suppression of collagen type I induction at both mRNA and protein levels. TGF-β1 is a major profibrotic factor that plays a key role in glomerular sclerosis in GN.39 Moreover, it is reported that Ang II is the important inducer for TGF-β1.24 In the present study, ARB treatment reduced the elevated level of TGF-β1 in the crescentic glomeruli. Based on these findings, it suggests that RAS activation enhanced TGF-β1 expression in crescentic GN. Monocyte/macrophages infiltration into the kidney, stimulated by MCP-1 produced from kidney cells in GN, promotes kidney fibrosis and renal injury through secretion of inflammatory cytokines. Although the MCP-1 blockade alone can directly suppress the crescent formation,3,17 this study showed that cotreatment with ARB and CA achieved better inhibition for the progression of the disease. Because TGF-β1 and MCP-1 are upregulated by Ang II in cultured MCs, the suppression of these factors by the cotreatment is likely to arrest macrophage infiltrations and fibrous changes for glomerular crescent effectively. From these findings, combination therapy with ARB and CA may confer strong, synergistic effects on glomerular crescent formations by suppressing inflammatory process with macrophage infiltrations and by preventing fibrous changes associated with TGF-β1 overexpressions in crescentic GN.
The next study was performed to determine the molecular and cellular mechanisms underlying the involvement of MCP-1 and the RAS in anti-GBM disease. Glomerular MCs express the relevant receptors for immune complexes and inflammatory cytokines that are responsible for initiation and progression of crescentic GN.40 In addition, through the stimulation of these cell-surface receptors, MCs produce a range of chemokines and inflammatory mediators that are relevant to the pathogenesis of crescentic GN.40 The mechanisms from cultured MCs have been shown to be important in subsequent intervention studies in crescentic GN.3 Furthermore, it is well known that cellular crescents consist of PECs and macrophage.41-43 In our hypothesis, MCP-1 and TGF-β1 released from Ang II-stimulated MCs, induces PEC proliferation and fibrosis with macrophage in the course of crescentic GN. Therefore, this in vitro study was designed to elucidate the mechanism of crescent formation, MCP-1 and RAS signal mediators, via glomerular MCs, leading to PEC proliferation and fibrosis. Our in vitro data suggested that Ang II induces the expression of MCP-1 and TGF-β1 in MCs, MCP-1–stimulated monocytes induce PEC proliferation, and TGF-β1 induces fibrous change of PECs. Recent studies have reported that MCP-1, which is induced by interleukin-1β and TNF-α and is released by CD8-positive T cells, plays a pivotal role in crescentic GN3-6 In this study, we investigated the involvement of the Ang II-induced expression of MCP-1 and TGF-β1 in the pathogenesis of anti-GBM disease. By performing immunohistochemical analysis, it has been confirmed that Ang II, MCP-1, and TGF-β1 are expressed in crescentic glomeruli. The study is the first to demonstrate that MCP-1 and TGF-β1 expression in Ang II-stimulated MCs induces PEC proliferation.
Perspectives
The present study reveals that a combination therapy of RAS inhibition with MCP-1/CCR2 signal inhibition can be used to block the MCP-1 and TGF-β1 increases, and thus mitigate renal injury in crescentic GN. Additional studies will be needed to determine the relationship between RAS activation and MCP-1/CCR2 signal in crescentic GN and clarify the mechanism for Ang II-induced fibrotic change of glomeruli. Furthermore, it will be important to determine whether MCP-1/CCR2 signal affects MCP-1 itself, TGF-β1 expression, and which glomerular cell components, such as MCs, PECs, or macrophages, mainly proliferate by the stimulation of MCP-1 in the glomerular crescent. However, it is clear that the combination therapy prevents the infiltration of macrophage and crescent formation in this animal model. We propose that therapeutic effects of the combination could provide a novel pharmacological strategy of anti-GBM disease.
Supplementary Material
Supplemental Figure S1. The level of plasma creatinine, body weight, total water intake, and daily urine volume in anti-GBM disease rat model. (A) The level of plasma creatinine, (B) body weight, (C) total water intake, and (D) daily urinary volume. Data are mean +/− SEM. * P < 0.05, and ** P < 0.01 between groups as indicated.
Supplemental Figure S2. Effects of the treatment on TGF-β1 expression in anti-GBM disease rat model. (A) Quantitative real-time RT-PCR of TGF-β1 mRNA in the isolated glomerulo of all tratment groups. (B) Western blot analyses of TGF-β1 protein levels in isolated glomeruli of all treatment groups. Data are mean +/− SEM. * P < 0.05, and ** P < 0.01 between groups as indicated.
Supplemental Figure S3. Effects of angiotensin II (Ang II) on cultured mesangial cells (MCs). (A and B) mRNA levels of MCP-1 (A) and TGF-β1 (B) in cultured MCs. MCs were stimulated with Ang II for indicated concentrations and analyzed by quantitative real-time RT-PCR. (C and D) mRNA levels of MCP-1 (C) and TGF-β1 (D) in cultured MCs. MCs were pretreated with Ang II type 1 receptor blocker (ARB) and subsequently stimulated with 100 nM Ang II. Sandwich ELISA for secretion of MCP-1 (E) and TGF-β1 (F) in MCs. MCs were pretreated with ARB (100 nM) and subsequently stimulated with 100 nM Ang II. Data are mean +/− SEM. ** P < 0.01 between groups as indicated. N.S., not Significant.
Supplemental Figure S4. Effects of angiotensin II (Ang II) on cultured mesangial cells (MCs) and cultured parietal epithelial cells (PECs) co-cultured with macrophages with/without MCs. (A) Cell proliferation in PECs co-cultured with macriphages and MCs using WST-1 assay. (B) Collagen secretion in PECs co-cultured with macrophages and MCs using Sircol assay. (C) Cell proliferation in PECs co-cultured with macrophages without MCs using WST-1 assay. (D) Collagen secretion in PECs co-cultured with macrophages without MCs using Sircol assay. PECs co-cultured with macrophages were pretreated with ARB (100 nM), CCR2 antagonist (CA, 10 μM) or pan-specific TGF-β3 neutralizing antibody (TGFβ Ab, 10 μg/ml), and subsequently stimulated with 100 nM ang II. Data are mean +/− SEM. ** P <0.01 between groups as indicated. N.S., not significant.
Acknowledgments
We acknowledge critical discussion and/or excellent technical assistance from L. Gabriel Navar, Toshie Saito, Masumi Kamiyama, Akemi Katsurada, M. Patrick Sweeny, G. Michael Upchurch, Nina A. Perrault, Jessica L. Mucci, and Salem I. Elkhayat.
Sources of Funding This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK072408) and the National Center for Research Resources (P20RR017659).
Footnotes
Disclosures None.
References
- 1.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med. 2003;348:2543–2556. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- 2.Jindal KK. Management of idiopathic crescentic and diffuse proliferative glomerulonephritis: evidence-based recommendations. Kidney Int Suppl. 1999;70:S33–S40. doi: 10.1046/j.1523-1755.1999.07005.x. [DOI] [PubMed] [Google Scholar]
- 3.Wada T, Yokoyama H, Furuichi K, Kobayashi KI, Harada K, Naruto M, Su SB, Akiyama M, Mukaida N, Matsushima K. Intervention of crescentic glomerulonephritis by antibodies to monocyte chemotactic and activating factor (MCAF/MCP-1) FASEB J. 1996;10:1418–1425. [PubMed] [Google Scholar]
- 4.Wada T, Furuichi K, Sakai N, Iwata Y, Yoshimoto K, Shimizu M, Kobayashi K, Mukaida N, Matsushima K, Yokoyama H. A new anti-inflammatory compound, FR167653, ameliorates crescentic glomerulonephritis in Wistar-Kyoto rats. J Am Soc Nephrol. 2000;11:1534–1541. doi: 10.1681/ASN.V1181534. [DOI] [PubMed] [Google Scholar]
- 5.Lan HY, Nikolic-Paterson DJ, Zarama M, Vannice JL, Atkins RC. Suppression of experimental crescentic glomerulonephritis by the interleukin-1 receptor antagonist. Kidney Int. 1993;43:479–485. doi: 10.1038/ki.1993.70. [DOI] [PubMed] [Google Scholar]
- 6.Le Hir M, Haas C, Marino M, Ryffel B. Prevention of crescentic glomerulonephritis induced by anti-glomerular membrane antibody in tumor necrosis factor-deficient mice. Lab Invest. 1998;78:1625–1631. [PubMed] [Google Scholar]
- 7.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol. 2007;292:F330–F339. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu M, Kondo S, Urushihara M, Takamatsu M, Kanemoto K, Nagata M, Kagami S. Role of integrin-linked kinase in epithelial-mesenchymal transition in crescent formation of experimental glomerulonephritis. Nephrol Dial Transplant. 2006;21:2380–2390. doi: 10.1093/ndt/gfl243. [DOI] [PubMed] [Google Scholar]
- 9.Kohda T, Okada S, Hayashi A, Kanzaki S, Ninomiya Y, Taki M, Sado Y. High nephritogenicity of monoclonal antibodies belonging to IgG2a and IgG2b subclasses in rat anti-GBM nephritis. Kidney Int. 2004;66:177–186. doi: 10.1111/j.1523-1755.2004.00719.x. [DOI] [PubMed] [Google Scholar]
- 10.Elmarakby AA, Quigley JE, Olearczyk JJ, Sridhar A, Cook AK, Inscho EW, Pollock DM, Imig JD. Chemokine receptor 2b inhibition provides renal protection in angiotensin II - salt hypertension. Hypertension. 2007;50:1069–1076. doi: 10.1161/HYPERTENSIONAHA.107.098806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koike H, Sada T, Mizuno M. In vitro and in vivo pharmacology of olmesartan medoxomil, an angiotensin II type AT1 receptor antagonist. J Hypertens Suppl. 2001;19:S3–S14. doi: 10.1097/00004872-200106001-00002. [DOI] [PubMed] [Google Scholar]
- 12.Kobori H, Katsurada A, Miyata K, Ohashi N, Satou R, Saito T, Hagiwara Y, Miyashita K, Navar LG. Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am J Physiol Renal Physiol. 2008;294:F1257–F1263. doi: 10.1152/ajprenal.00588.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urushihara M, Takamatsu M, Shimizu M, Kondo S, Kinoshita Y, Suga K, Kitamura A, Matsuura S, Yoshizumi M, Tamaki T, Kawachi H, Kagami S. ERK5 activation enhances mesangial cell viability and collagen matrix accumulation in rat progressive glomerulonephritis. Am J Physiol Renal Physiol. 2010;298:F167–F176. doi: 10.1152/ajprenal.00124.2009. [DOI] [PubMed] [Google Scholar]
- 14.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segerer S, Cui Y, Hudkins KL, Goodpaster T, Eitner F, Mack M, Schlondorff D, Alpers CE. Expression of the chemokine monocyte chemoattractant protein-1 and its receptor chemokine receptor 2 in human crescentic glomerulonephritis. J Am Soc Nephrol. 2000;11:2231–2242. doi: 10.1681/ASN.V11122231. [DOI] [PubMed] [Google Scholar]
- 16.Wada T, Furuichi K, Sakai N, Iwata Y, Kitagawa K, Ishida Y, Kondo T, Hashimoto H, Ishiwata Y, Mukaida N, Tomosugi N, Matsushima K, Egashira K, Yokoyama H. Gene therapy via blockade of monocyte chemoattractant protein-1 for renal fibrosis. J Am Soc Nephrol. 2004;15:940–948. doi: 10.1097/01.asn.0000120371.09769.80. [DOI] [PubMed] [Google Scholar]
- 17.Fujinaka H, Yamamoto T, Takeya M, Feng L, Kawasaki K, Yaoita E, Kondo D, Wilson CB, Uchiyama M, Kihara I. Suppression of anti-glomerular basement membrane nephritis by administration of anti-monocyte chemoattractant protein-1 antibody in WKY rats. J Am Soc Nephrol. 1997;8:1174–1178. doi: 10.1681/ASN.V871174. [DOI] [PubMed] [Google Scholar]
- 18.Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, Ishiwata Y, Asano M, Wang H, Matsushima K, Takeya M, Kuziel WA, Mukaida N, Yokoyama H. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J Am Soc Nephrol. 2003;14:2503–2515. doi: 10.1097/01.asn.0000089563.63641.a8. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, Takeya M, Kuziel WA, Matsushima K, Mukaida N, Yokoyama H. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol. 2004;165:237–246. doi: 10.1016/S0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burt D, Salvidio G, Tarabra E, Barutta F, Pinach S, Dentelli P, Camussi G, Perin PC, Gruden G. The monocyte chemoattractant protein-1/cognate CC chemokine receptor 2 system affects cell motility in cultured human podocytes. Am J Pathol. 2007;171:1789–1799. doi: 10.2353/ajpath.2007.070398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest. 1986;77:1993–2000. doi: 10.1172/JCI112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navar LG, Harrison-Bernard LM, Imig JD, Wang CT, Cervenka L, Mitchell KD. Intrarenal angiotensin II generation and renal effects of AT1 receptor blockade. J Am Soc Nephrol. 1999;10(suppl 12):S266–S272. [PubMed] [Google Scholar]
- 23.Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circulation. 1994;89:493–498. doi: 10.1161/01.cir.89.1.493. [DOI] [PubMed] [Google Scholar]
- 24.Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz-Ortega M, Egido J. Angiotensin II modulates cell growth-related events and synthesis of matrix proteins in renal interstitial fibroblasts. Kidney Int. 1997;52:1497–1510. doi: 10.1038/ki.1997.480. [DOI] [PubMed] [Google Scholar]
- 26.Horita Y, Tadokoro M, Taura K, Suyama N, Taguchi T, Miyazaki M, Kohno S. Low-dose combination therapy with temocapril and losartan reduces proteinuria in normotensive patients with immunoglobulin a nephropathy. Hypertens Res. 2004;27:963–970. doi: 10.1291/hypres.27.963. [DOI] [PubMed] [Google Scholar]
- 27.Ravid M, Brosh D, Levi Z, Bar-Dayan Y, Ravid D, Rachmani R. Use of enalapril to attenuate decline in renal function in normotensive, normoal-buminuric patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1998;128:982–988. doi: 10.7326/0003-4819-128-12_part_1-199806150-00004. [DOI] [PubMed] [Google Scholar]
- 28.Hudson BG, Kalluri R, Gunwar S, Noelken ME, Mariyama M, Reeders ST. Molecular characteristics of the Goodpasture autoantigen. Kidney Int. 1993;43:135–139. doi: 10.1038/ki.1993.22. [DOI] [PubMed] [Google Scholar]
- 29.Sakai N, Wada T, Furuichi K, Shimizu K, Kokubo S, Hara A, Yamahana J, Okumura T, Matsushima K, Yokoyama H, Kaneko S. MCP-1/CCR2-dependent loop for fibrogenesis in human peripheral CD14-positive monocytes. J Leukoc Biol. 2006;79:555–563. doi: 10.1189/jlb.0305127. [DOI] [PubMed] [Google Scholar]
- 30.Brunner HR. ACE inhibitors in renal disease. Kidney Int. 1992;42:463–479. doi: 10.1038/ki.1992.311. [DOI] [PubMed] [Google Scholar]
- 31.Kohan DE. Angiotensin II and endothelin in chronic glomerulonephritis. Kidney Int. 1998;54:646–647. doi: 10.1046/j.1523-1755.1998.00038.x. [DOI] [PubMed] [Google Scholar]
- 32.Lafayette RA, Mayer G, Park SK, Meyer TW. Angiotensin II receptor blockade limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1992;90:766–771. doi: 10.1172/JCI115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giatras I, Lau J, Levey AS. Effect of angiotensin-converting enzyme inhibitors on the progression of nondiabetic renal disease: a meta-analysis of randomized trials. Angiotensin-Converting-Enzyme Inhibition and Progressive Renal Disease Study Group. Ann Intern Med. 1997;127:337–345. doi: 10.7326/0003-4819-127-5-199709010-00001. [DOI] [PubMed] [Google Scholar]
- 34.Ingelfinger JR, Jung F, Diamant D, Haveran L, Lee E, Brem A, Tang SS. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol. 1999;276:F218–F227. doi: 10.1152/ajprenal.1999.276.2.F218. [DOI] [PubMed] [Google Scholar]
- 35.Nishiyama A, Seth DM, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of renal interstitial fluid angiotensin II in angiotensin II-induced hypertension. J Hypertens. 2003;21:1897–1903. doi: 10.1097/00004872-200310000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Hisada Y, Sugaya T, Yamanouchi M, Uchida H, Fujimura H, Sakurai H, Fukamizu A, Murakami K. Angiotensin II plays a pathogenic role in immune-mediated renal injury in mice. J Clin Invest. 1999;103:627–635. doi: 10.1172/JCI2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki Y, Shirato I, Okumura K, Ravetch JV, Takai T, Tomino Y, Ra C. Distinct contribution of Fc receptors and angiotensin II-dependent pathways in anti-GBM glomerulonephritis. Kidney Int. 1998;54:1166–1174. doi: 10.1046/j.1523-1755.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- 38.Couser WG. Glomerulonephritis. Lancet. 1999;353:1509–1515. doi: 10.1016/S0140-6736(98)06195-9. [DOI] [PubMed] [Google Scholar]
- 39.Border WA, Noble NA. Transforming growth factor-beta in glomerular injury. Exp Nephrol. 1994;2:13–17. [PubMed] [Google Scholar]
- 40.Satriano JA, Hora K, Shan Z, Stanley ER, Mori T, Schlondorff D. Regulation of monocyte chemoattractant protein-1 and macrophage colony-stimulating factor-1 by IFN-gamma, tumor necrosis factor-alpha, IgG aggregates, and cAMP in mouse mesangial cells. J Immunol. 1993;150:1971–1978. [PubMed] [Google Scholar]
- 41.Magil AB. Histogenesis of glomerular crescents. Immunohistochemical demonstration of cytokeratin in crescent cells. Am J Pathol. 1985;120:222–229. [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshioka K, Takemura T, Akano N, Miyamoto H, Iseki T, Maki S. Cellular and non-cellular compositions of crescents in human glomerulonephritis. Kidney Int. 1987;32:284–291. doi: 10.1038/ki.1987.205. [DOI] [PubMed] [Google Scholar]
- 43.Liu ZH, Chen SF, Zhou H, Chen HP, Li LS. Glomerular expression of C-C chemokines in different types of human crescentic glomerulonephritis. Nephrol Dial Transplant. 2003;18:1526–1534. doi: 10.1093/ndt/gfg172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. The level of plasma creatinine, body weight, total water intake, and daily urine volume in anti-GBM disease rat model. (A) The level of plasma creatinine, (B) body weight, (C) total water intake, and (D) daily urinary volume. Data are mean +/− SEM. * P < 0.05, and ** P < 0.01 between groups as indicated.
Supplemental Figure S2. Effects of the treatment on TGF-β1 expression in anti-GBM disease rat model. (A) Quantitative real-time RT-PCR of TGF-β1 mRNA in the isolated glomerulo of all tratment groups. (B) Western blot analyses of TGF-β1 protein levels in isolated glomeruli of all treatment groups. Data are mean +/− SEM. * P < 0.05, and ** P < 0.01 between groups as indicated.
Supplemental Figure S3. Effects of angiotensin II (Ang II) on cultured mesangial cells (MCs). (A and B) mRNA levels of MCP-1 (A) and TGF-β1 (B) in cultured MCs. MCs were stimulated with Ang II for indicated concentrations and analyzed by quantitative real-time RT-PCR. (C and D) mRNA levels of MCP-1 (C) and TGF-β1 (D) in cultured MCs. MCs were pretreated with Ang II type 1 receptor blocker (ARB) and subsequently stimulated with 100 nM Ang II. Sandwich ELISA for secretion of MCP-1 (E) and TGF-β1 (F) in MCs. MCs were pretreated with ARB (100 nM) and subsequently stimulated with 100 nM Ang II. Data are mean +/− SEM. ** P < 0.01 between groups as indicated. N.S., not Significant.
Supplemental Figure S4. Effects of angiotensin II (Ang II) on cultured mesangial cells (MCs) and cultured parietal epithelial cells (PECs) co-cultured with macrophages with/without MCs. (A) Cell proliferation in PECs co-cultured with macriphages and MCs using WST-1 assay. (B) Collagen secretion in PECs co-cultured with macrophages and MCs using Sircol assay. (C) Cell proliferation in PECs co-cultured with macrophages without MCs using WST-1 assay. (D) Collagen secretion in PECs co-cultured with macrophages without MCs using Sircol assay. PECs co-cultured with macrophages were pretreated with ARB (100 nM), CCR2 antagonist (CA, 10 μM) or pan-specific TGF-β3 neutralizing antibody (TGFβ Ab, 10 μg/ml), and subsequently stimulated with 100 nM ang II. Data are mean +/− SEM. ** P <0.01 between groups as indicated. N.S., not significant.