Abstract
The mechanism by which the FERM domain protein Merlin, encoded by the tumor suppressor NF2, restrains cell proliferation is poorly understood. Prior studies have suggested that Merlin exerts its antimitogenic effect by interacting with multiple signaling proteins located at or near the plasma membrane. We have recently observed that Merlin translocates into the nucleus and binds to and inhibits the E3 ubiquitin ligase CRL4DCAF1. Genetic evidence indicates that inactivation of Merlin induces oncogenic gene expression, hyperproliferation, and tumorigenicity by unleashing the activity of CRL4DCAF1. In addition to providing a potential explanation for the diverse effects that loss of Merlin exerts in multiple cell types, these findings suggest that compounds inhibiting CRL4DCAF1 may display therapeutic efficacy in Neurofibromatosis type 2 and other cancers driven by Merlin inactivation.
Key words: Merlin, NF2, E3 ubiquitin ligase, CRL4, DCAF1, FERM domain protein
Introduction
Increasing evidence indicates that the NF2 gene, originally identified because of its inactivation in the familial cancer predisposition syndrome Neurofibromatosis type 2, has a broad tumor-suppressor function.1,2 NF2 encodes Merlin, a member of the Ezrin/Radixin/Moesin (ERM) family of proteins, which mediate linkage of cell adhesion receptors, such as CD44 and ICAM, to cortical actin. Because of sequence homology to ERM proteins and apparently prevalent localization to the cortical cytoskeleton, it has been suggested that functions at or near the plasma membrane to inhibit the transmission of promitogenic signals. In apparent agreement with this general model, it has been reported that Merlin interacts with multiple signaling proteins located at these cellular compartments and opposes activation of several pro-mitogenic signaling pathways.1 Some reported interactions appear to be of low affinity, are not supported by convincing mutational analysis, and some of the effects observed are cell-type specific and perhaps irrelevant to the tumor suppressor function of Merlin. However, it seems clear that exogenous Merlin inhibits multiple signaling pathways in Schwannoma and meningioma cells carrying loss-of-function mutations at the NF2 locus. The major pathways that appear to be modulated include membrane recruitment and activation of Rac and thereby PAK,3–5 activation of mTORC1 independently of Akt,6,7 the EGFR-Ras-ERK pathway, the PI3K-Akt pathway, and focal adhesion kinase (FAK)-Src signaling.8–14 In addition, Merlin cooperates with Expanded and Kibra to activate the Hippo tumor-suppressor pathway in Drosophila15–19 and a recent study has implicated YAP, the oncoprotein that lies downstream of the core Hippo pathway in mammalian cells, as a mediator of Merlin-dependent tumorigenesis in a mouse model of liver tumorigenesis.20 In spite of these significant advances, the biochemical function of Merlin and hence the mechanism through which it suppresses tumorigenesis have remained, until recently, elusive.
Merlin Suppresses Tumorigenesis by Inhibiting CRL4DCAF1 in the Nucleus
We have used tandem affinity purification followed by mass spectrometry to identify proteins interacting with wild type but not a tumor-derived mutant form of Merlin. Our findings revealed that wild type but not mutant Merlin binds with high affinity to the E3 ubiquitin ligase CRL4DCAF1.21 Biochemical analyses indicated that Merlin binds directly to DCAF1, the substrate receptor subunit of CRL4DCAF1, and inhibits CRL4DCAF1-mediated ubiquitylation of target proteins. Further studies provided evidence that the unphoshorylated form of Merlin, which is presumably stabilized in the closed conformation and able to mediate growth inhibition, translocates into the nucleus and binds to CRL4DCAF1, whereas the inactive phosphorylated form remains in the cytoplasm. To examine if Merlin mediates growth inhibition and suppresses tumorigenesis by inhibiting CRL4DCAF1, we conducted genetic epistasis experiments in mammalian cells. Depletion of DCAF1 blocked the hyperproliferation caused by inactivation of Merlin in Schwann cells and mesothelial cells. Conversely, enforced expression of a Merlin-insensitive mutant of DCAF1 counteracted the antimitogenic effect of Merlin in mesothelioma cells. In addition, re-expression of Merlin and silencing of DCAF1 induced an overlapping tumor suppressive program of gene expression in Merlin-deficient Schwannoma cells, suggesting that inactivation of Merlin induces an oncogenic program of gene expression by deregulating CRL4DCAF1 activity. To further test if Merlin suppresses tumorigenesis through inhibition of CRL4DCAF1, we conducted a detailed biochemical and functional analysis of several tumor-derived mutants of Merlin. We found that the pathogenic mutants under scrutiny fall into three major classes: some of the missense mutants mapping to the FERM domain exhibited defective translocation into the nucleus, others failed to bind to DCAF1, while the C-terminal truncation mutants accumulated in the nucleus and bound to DCAF1 but failed to suppress E3 ligase activity. Collectively, this study provided evidence that Merlin needs to enter the nucleus, bind to DCAF1 and suppress CRL4DCAF1 in order to suppress tumorigenesis. Finally, we examined if CRL4DCAF1 mediates the oncogenicity of Merlin-deficient cells. Depletion of DCAF1 suppressed the ability of Merlin-deficient Schwannoma cells to hyperproliferate in vitro, to grow in soft agar, and to form tumors upon subcutaneous injection in nude mice. Together, these results provided strong evidence that Merlin suppresses tumorigenesis by inhibiting CRL4DCAF1 in the nucleus.
Potential Connections to Other Signaling Pathways
CRL4DCAF1 belongs to a large subfamily of cullin-ring E3 ligases that consist of Roc1/Rbx1 (catalytic subunit), Cullin 4 (scaffold), DDB1 (adaptor) and one of multiple WD40 domain-containing substrate receptors.22,23 The substrate receptor of CRL4DCAF1 is DCAF1 (see Fig. 1). CRL4 ligases have been implicated in regulating chromatin remodeling, DNA replication and the DNA damage response. Although the physiological substrates of CRL4DCAF1 have not yet been identified, our gene expression analysis suggests that CRL4DCAF1 regulates a broad program of gene expression, consisting of more than 1,000 genes.21 Therefore, it is conceivable that CRL4DCAF1 exerts this effect by promoting the poly- or mono-ubiquitylation of histones, chromatin-remodeling factors or transcription factors, as it has been established for other members of the CRL4 subfamily.23–25 Irrespective of the specific mechanism by which CRL4DCAF1 regulates gene expression, the breadth of the oncogenic gene expression program it induces and the identity of some of the genes regulated suggest that Merlin may suppress multiple mitogenic signaling pathways by inhibiting CRL4DCAF1.
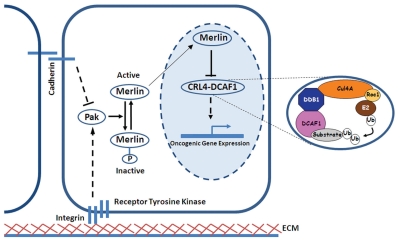
Figure 1.
Merlin suppresses tumorigenesis by translocating into the nucleus and inhibiting the E3 ligase CRL4DCAF1. Signals regulating cell growth from neighboring cells or extracellular matrix (ECM) regulate the phosphorylation status of Merlin through Pak. The active form of Merlin enters the nucleus and binds to the E3 ubiquitin ligase CRL4DCAF1, thereby inhibiting its activity. Inset shows a model of the molecular architecture of the ligase. We posit that deregulated CRL4DCAF1 drives the oncogenicity of Merlin-deficient cells by upregulating the expression of multiple oncogenic genes.
Receptor tyrosine kinase signaling.
Multiple mechanisms have been invoked to explain the inhibitory effect of Merlin on the EGFR-Ras-ERK signaling pathway. It has been proposed that Merlin suppresses EGFR signaling by binding to the receptor and sequestering it in an inactive conformation at cell-to-cell junctions.13,26 Furthermore, whereas studies in the fly have indicated that Merlin accelerates the endocytosis of the EGFR and other signaling receptors,27 studies in Schwannoma cells suggest that Merlin inhibits the export of the EGFR and other receptor tyrosine kinases to the plasma membrane.28 Finally, it has been proposed that Merlin interferes with Ras activation downstream of receptor tyrosine kinases.14 Our gene expression analysis has revealed that Merlin can regulate the transcription of several genes encoding components or regulators of signaling pathways jointly regulated by receptor tyrosine kinases and integrins.21 Interestingly, expression of Merlin and silencing of DCAF1 cause a concordant up or downregulation of these genes, suggesting that Merlin regulates their expression by inhibiting CRL4DCAF1. This observation provides a potential unitary explanation to the multiple effects that Merlin appears to exert on the signaling pathways activated by receptor tyrosine kinases and integrins.
The hippo pathway.
The Hippo pathway is an evolutionarily conserved signaling cascade which controls organ size and suppresses tumorigenesis by inhibiting cell proliferation and promoting apoptosis.29–31 The core pathway consists of two serine/threonine kinases and their adaptors—Hippo(MST1/2)-Salvador(WW45) and Warts(Lats1/2)-Mats(MOBKL1A/B)—and of a transcriptional co-activator Yorkie (YAPTAZ). Hippo phosphorylates and thereby activates Warts, which in turn phosphorylates Yorkie, promoting its exclusion from the nucleus. Yorkie promotes cell proliferation and survival by inducing the expression of various genes, including Cyclin E and DIAP. Although the function of the core Hippo pathway has been elucidated, the mechanism by which it is activated is not as well understood. In Drosophila, genetic analysis suggests that Merlin and another FERM domain protein Expanded function upstream of the Hippo kinase cascade to transduce extracellular signals.16 Interestingly, Expanded can regulate the Hippo pathway by directly interacting with Yorkie, the downstream target of the Hippo kinase cascade.32 How Merlin activates the Hippo pathway is not known. Recently, another component of the Hippo pathway, Kibra, was identified.17–19 Genetic analysis suggests that Kibra functions upstream of the Hippo kinase cascade and is partially redundant with Expanded and Merlin. Furthermore, the Merlin-Expanded-Kibra complex interacts directly with the Hippo-Salvador complex in transfected cells, suggesting that Merlin-Expanded-Kibra might impinge directly on Hippo. In mammals, overexpression of Merlin activates the Hippo pathway and inactivates Yap, a homolog of Yorkie.33 However, it remains to be determined whether Merlin suppresses cell proliferation and tumorigenesis, at least in part, through activation of the Hippo pathway and whether Merlin activates the pathway at or near the plasma membrane, as it appears to do in Drosophila. We found that re-expression of Merlin or inactivation of CRL4DCAF1 in mouse Schwannoma cells induces the expression of a set of genes which are known to be regulated by Yap.21,33 This finding supports the hypothesis that Yap is an effector of Merlin and that CRL4DCAF1 is involved in its regulation. However, since our results suggest that Merlin needs to enter the nucleus and inhibit CRL4DCAF1 to suppress cell proliferation and tumorigenesis, it will be interesting to investigate whether CRL4DCAF1 regulates Hippo signaling in mammalian cells, the mechanism involved, and the nuclear function of Merlin is conserved in Drosophila.
Potential Drug Targets for NF2-Related Tumors
The NF2 gene is inactivated in familial as well as in sporadic Schwannomas, meningiomas and ependymomas and in a large fraction of malignant mesotheliomas. Although Schwannomas, meningiomas, and ependymomas are slow-growing tumors and do not invade adjacent tissue, they arise in the brain and spinal cord and therefore can cause significant morbidity. In contrast, malignant mesotheliomas are aggressive tumors and do not respond well to classical chemotherapy.34 Development of effective targeted therapies for these diseases requires a complete definition of the biochemical function of Merlin and the identification of the major signaling pathways that drive the expansion of Merlin-deficient tumors. We have found that inactivation of CRL4DCAF1 through silencing of DCAF1 inhibits the proliferation of primary human Schwannoma cells but has no inhibitory effect on normal primary human Schwann cells.21 In addition, depletion of DCAF1 suppresses the tumorigenic properties of NF2-deficient tumor cells but not of cells carrying other oncogenic mutations. These observations indicate that Merlin-deficient cells are dependent upon the signaling output of CRL4DCAF1, suggesting that targeting this ligase or the signaling pathways activated by the ligase may constitute an effective therapeutic strategy. In addition, since depletion of CRL4DCAF1 does not appear to inhibit the proliferation of cells expressing wild-type Merlin to a large extent, this strategy is predicted to afford a large therapeutic window. Modification of the cullin protein by NEDD8 is required for the cullin-RING ubiquitin ligase activity. Therefore, interfering with the neddylation of the ubiquitin ligase will affect the ubiquitinylation-dependent cellular processes, such as cell cycle and cell survival. Notably, a potent and selective inhibitor of the NEDD8-activating enzyme, MLN4924, has recently been developed and shown to inhibit human colon tumor and lung tumor growth in mouse xenograft models.35 It would be interesting to test whether this neddylation inhibitor could also be applied to inhibit the growth of schwannomas or other cancers caused by loss of Merlin function.
Perspective
Since the NF2 gene was identified as a tumor suppressor inactivated in Neurofibromatosis type 2 and several sporadic cancers, intensive research has been carried out to elucidate the functions of its protein product, Merlin, and to understand how inactivation of this protein leads to tumorigenesis. Most anti-mitogenic functions of Merlin, especially related to contact inhibition of cell growth, were attributed to its roles in cortical cytoskeleton organization and signaling regulation. Whether these roles are critical in tumor suppression is unclear. We found that the closed/active form of Merlin translocates to the nucleus and binds to CRL4DCAF1, thereby inhibiting its E3 ligase activity. This unexpected function is essential for tumor suppression by Merlin and might be responsible for regulating multiple mitogenic signaling pathways, some of which may also be affected by Merlin's functions at the cortical cytoskeleton. Identification of the substrates of this E3 ligase should help in understanding the relationship between Merlin's inhibitory effect on CRL4DCAF1 and its role in regulating multiple mitogenic signaling pathways and ultimately may lead to generation of targeted therapies for tumors driven by loss of Merlin.
Acknowledgements
We thank members of our laboratory for discussions. This work was supported by National Institutes of Health (NIH) Grant R01 CA152975 (to F.G.G.) and Cancer Center Support Grant P30 CA08748. W.L. is a recipient of the Young Investigator Award from the Children's Tumor Foundation.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/13838
References
- 1.Okada T, You L, Giancotti FG. Shedding light on Merlin's wizardry. Trends Cell Biol. 2007;17:222–229. doi: 10.1016/j.tcb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 2.McClatchey AI, Giovannini M. Membrane organization and tumorigenesis—the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19:2265–2277. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- 3.Kaempchen K, Mielke K, Utermark T, Langmesser S, Hanemann CO. Upregulation of the Rac1/JNK signaling pathway in primary human schwannoma cells. Hum Mol Genet. 2003;12:1211–1221. doi: 10.1093/hmg/ddg146. [DOI] [PubMed] [Google Scholar]
- 4.Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin. The product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell. 2003;12:841–849. doi: 10.1016/s1097-2765(03)00382-4. [DOI] [PubMed] [Google Scholar]
- 5.Okada T, Lopez-Lago M, Giancotti FG. Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J Cell Biol. 2005;171:361–371. doi: 10.1083/jcb.200503165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James MF, Han S, Polizzano C, Plotkin SR, Manning BD, Stemmer-Rachamimov AO, et al. NF2/Merlin Is a Novel Negative Regulator of mTOR Complex 1 and Activation of mTORC1 Is Associated with Meningioma and Schwannoma Growth. Mol Cell Biol. 2009;29:4250–4261. doi: 10.1128/MCB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Lago MA, Okada T, Murillo MM, Socci N, Giancotti FG. Loss of the Tumor Suppressor Gene NF2, Encoding Merlin, Constitutively Activates Integrin-Dependent mTORC1 Signaling. Mol Cell Biol. 2009;29:4235–4249. doi: 10.1128/MCB.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ammoun S, Flaiz C, Ristic N, Schuldt J, Hanemann CO. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 2008;68:5236–5245. doi: 10.1158/0008-5472.CAN-07-5849. [DOI] [PubMed] [Google Scholar]
- 9.Jin H, Sperka T, Herrlich P, Morrison H. Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature. 2006;442:576–579. doi: 10.1038/nature04856. [DOI] [PubMed] [Google Scholar]
- 10.Poulikakos PI, Xiao GH, Gallagher R, Jablonski S, Jhanwar SC, Testa JR. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006;25:5960–5968. doi: 10.1038/sj.onc.1209587. [DOI] [PubMed] [Google Scholar]
- 11.Rong R, Tang X, Gutmann DH, Ye K. Neurofibromatosis 2 (NF2) tumor suppressor merlin inhibits phosphatidylinositol 3-kinase through binding to PIKE-L. Proc Natl Acad Sci USA. 2004;101:18200–18205. doi: 10.1073/pnas.0405971102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akhmametyeva EM, Mihaylova MM, Luo H, Kharzai S, Welling DB, Chang LS. Regulation of the neurofibromatosis 2 gene promoter expression during embryonic development. Dev Dyn. 2006;235:2771–2785. doi: 10.1002/dvdy.20883. [DOI] [PubMed] [Google Scholar]
- 13.Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison H, Sperka T, Manent J, Giovannini M, Ponta H, Herrlich P. Merlin/neurofibromatosis type 2 suppresses growth by inhibiting the activation of Ras and Rac. Cancer Res. 2007;67:520–527. doi: 10.1158/0008-5472.CAN-06-1608. [DOI] [PubMed] [Google Scholar]
- 15.Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, Irvine KD. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 16.Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 17.Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D. Kibra functions as a tumor suppressor protein that regulates hippo signaling in conjunction with Merlin and expanded. Dev Cell. 2010;18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the salvador/warts/hippo signaling network. Dev Cell. 2010;18:300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H. The WW domain protein Kibra acts upstream of hippo in Drosophila. Dev Cell. 2010;18:309–316. doi: 10.1016/j.devcel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, et al. The Merlin/NF2 Tumor Suppressor Functions through the YAP Oncoprotein to Regulate Tissue. Homeostasis in Mammals. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140:477–490. doi: 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Zhou P. DCAFs, the Missing Link of the CUL4-DDB1. Ubiquitin Ligase. 2007;26:775–780. doi: 10.1016/j.molcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 23.O'Connell BC, Harper JW. Ubiquitin proteasome system (UPS): what can chromatin do for you? Current Opinion in Cell Biology. 2007;19:206–214. doi: 10.1016/j.ceb.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Lee S, Zhang J, Peters SB, Hannah J, Zhang Y, et al. CUL4A abrogation augments DNA damage response and protection against skin carcinogenesis. Mol Cell. 2009;34:451–460. doi: 10.1016/j.molcel.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, Zhou P. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol Cell. 2007;26:775–780. doi: 10.1016/j.molcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Cole BK, Curto M, Chan AW, McClatchey AI. Localization to the cortical cytoskeleton is necessary for Nf2/merlin-dependent epidermal growth factor receptor silencing. Mol Cell Biol. 2008;28:1274–1284. doi: 10.1128/MCB.01139-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maitra S, Kulikauskas RM, Gavilan H, Fehon RG. The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol. 2006;16:702–709. doi: 10.1016/j.cub.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 28.Lallemand D, Manent J, Couvelard A, Watilliaux A, Siena M, Chareyre F, et al. Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene. 2009;28:854–865. doi: 10.1038/onc.2008.427. [DOI] [PubMed] [Google Scholar]
- 29.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 30.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway—an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- 31.Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes & Development. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badouel C, Gardano L, Amin N, Garg A, Rosenfeld R, Le Bihan T, et al. The FERM-domain protein Expanded regulates Hippo pathway activity via direct interactions with the transcriptional activator Yorkie. Dev Cell. 2009;16:411–420. doi: 10.1016/j.devcel.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stahel RA, Weder W. Improving the outcome in malignant pleural mesothelioma: nonaggressive or aggressive approach? Curr Opin Oncol. 2009;21:124–130. doi: 10.1097/CCO.0b013e328324bc30. [DOI] [PubMed] [Google Scholar]
- 35.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]