Abstract
We previously observed marked downregulation of the mRNA for angiogenin, a potent inducer of neovascularization, in a mouse model of Parkinson’s disease (PD) based on overexpression of alpha-synuclein. Angiogenin has also been recently implicated in the pathogenesis of amyotrophic lateral sclerosis. In this study, we confirmed that mouse angiogenin-1 protein is dramatically reduced in this transgenic alpha-synuclein mouse model of PD, and examined the effect of angiogenin in cellular models of PD. We found that endogenous angiogenin is present in two dopamine-producing neuroblastoma cell lines, SH-SY5Y and M17, and that exogenous angiogenin is taken up by these cells and leads to phosphorylation of Akt. Applied angiogenin protects against the cell death induced by the neurotoxins MPP+ and rotenone and reduces the activation of caspase-3. Together our data supports the importance of angiogenin in protecting against dopaminergic neuronal cell death and suggests its potential as a therapy for PD.
Keywords: angiogenin, neuroprotection, MPP+, Parkinson’s Disease, alpha-synuclein, Akt
Introduction
Parkinson’s Disease (PD) is the most common neurodegenerative movement disorder, with approximately 1–2% of the population over 65 years of age affected (Eriksen et al. 2003). While the causes of PD are not well understood, recent studies have implicated the protein alpha-synuclein (α-syn): point mutations or gene multiplication of α-syn cause inherited forms of PD (Singleton et al. 2003, Polymeropoulos et al. 1997, Kruger et al. 1998, Athanassiadou et al. 1999), while in sporadic cases α-syn is found aggregated in the substantia nigra and other brain regions (Spillantini et al. 1997).
We have previously investigated how α-syn may cause toxicity through gene microarray analysis of transgenic mice overexpressing human α-syn under the PDGFβ promoter (Yacoubian et al. 2008). We found over 200 genes with alterations in gene expression at three months, but most of the changes were modest. However, one gene, mouse angiogenin1 (mAng1), was dramatically reduced by 7.5-fold compared to wildtype. In mice six isotypes of angiogenin exist, with mAng1 being the predominantly expressed form and a homolog to the only angiogenin gene in humans (Ibaragi et al. 2009). Angiogenin’s biological roles include cell migration, invasion, proliferation, angiogenesis, and neuroprotection (Kieran et al. 2008, Hu 1998, Hu et al. 1994, Gao & Xu 2008).
Angiogenin has been recently linked to the neurodegenerative disorder, amyotrophic lateral sclerosis (ALS). Several point mutations in angiogenin have been discovered in both sporadic and familial ALS (Greenway et al. 2004, Greenway et al. 2006, Wu et al. 2007). Wildtype angiogenin has been shown to reduce neuronal death in in vitro models of ALS, and knockdown of angiogenin expression by siRNA promotes cell death (Kieran et al. 2008). In contrast, mutant angiogenin inhibits neurite extension and promotes hypoxia-induced cell death in motoneurons (Subramanian & Feng 2007, Subramanian et al. 2008, Sebastia et al. 2009). In vivo, systemic treatment with wildtype angiogenin increases motoneuron survival, delays motor dysfunction, and increases lifespan of mutant superoxide dismutase 1 mice, a model for ALS (Kieran et al. 2008). This neuroprotective effect appears to be mediated by activation of the PI3K/Akt pathway, as inhibition of this pathway disrupts angiogenin’s neuroprotection in vitro (Kieran et al. 2008).
Because angiogenin levels are reduced in a transgenic α-syn mouse of PD and angiogenin is protective in models for ALS, we hypothesized that increasing angiogenin may also provide protection in PD. Here we examine whether exogenous angiogenin protects against cell death in cellular models for PD. We found that angiogenin reduces cell death in response to both rotenone and 1-methyl-4-phenylpyridinium (MPP+) in dopaminergic cell lines, and this protection appears to be mediated through inhibition of apoptosis via the PI3K-Akt signaling pathway.
Methods
Cell Culture
SH-SY5Y cells were a gift from J. Zhang (Birmingham, AL) and were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). SK-N-BE(2)-M17(M17) cells (ATCC, Manassas, VA) were cultured in a 1:1 mix of Eagle’s minimum essential media and F12K media supplemented with 10% FBS. Both cell lines were cultured at 37C with 5% CO2 and 95% air atmosphere in a humidified incubator.
Animals
α-Syn transgenic animals originally created by Masliah et al. (2000) were bred at Charles River Laboratories (Wilmington, MA). Three-month-old wildtype and transgenic mice were sacrificed by CO2 inhalation. The use of animals was supervised by the Massachusetts General Hospital and University of Alabama at Birmingham Animal Resources Programs in accordance with the PHS policy on the Humane Care and Use of Laboratory Animals. Cortical tissue from three-month-old α-syn knockout mice and wildtype littermates were a gift from Robert Nussbaum (Cabin et al. 2002).
Western Blot Analysis
SH-SY5Y cells were sonicated in lysis buffer (150nM NaCl, 10mm Tris-HCl (pH 7.4), 1mM EGTA, 1mM EDTA, 0.5%NP-40, protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN)) and centrifuged at 16000g for 10 min at 4C. Protein concentrations of supernatants were determined using the bicinchoninic assay (Pierce, Rockford, IL). Each sample was boiled for 5 min in 4 × DTT sample loading buffer (8% SDS, 0.25M Tris-HCl, 200mM DTT, 30% glycerol, and Bromophenol Blue), resolved on 12% SDS-polyacryladmide gels, and transferred electrophoretically to 0.45-μm nitrocellulose membranes at 100V for 1 hour. Following transfer, membranes were incubated for 30 min in 5% non-fat dry milk in TBST (25mM Tris-HCl pH 7.6, 137mM NaCl, 0.1% Tween 20) and then incubated overnight at 4C in primary goat polyclonal antibody against angiogenin (1:500; R&D Systems, Minneapolis, MN), rabbit polyclonal antibody against Akt phosphorylated at serine 473 (1:1500 Cell Signaling, Danvers, MA), rabbit polyclonal antibody against total Akt (1:1500; Cell Signaling, Danvers, MA), or mouse monoclonal antibody against α-tubulin (1:10000; Sigma, St. Louis, MO). After three washes in TBST, blots were incubated for two hours with HRP-conjugated goat anti-rabbit or anti-mouse secondary antibodies (1:2000; Jackson ImmunoResearch, West Grove, PA) and then washed in TBST six times for 10 minutes each. Super signal chemiluminescence (Pierce, Rockford, IL) was used to detect protein bands. Quantification of bands was performed using densitometry, and each band was normalized to the average of all bands on each blot.
For mouse brain samples, cortex was homogenized in TEVP buffer (10mM Tris-HCl, pH 7.4, 5mM sodium fluoride, 1mM sodium orthovanadate, 1mM EDTA, 1mM EGTA) with 320mM sucrose and centrifuged at 15000g for 16 minutes. Pellets were resuspended and sonicated in TEVP buffer supplemented with protease inhibitors (Roche). Immunoblotting against angiogenin was performed as described above, using a goat polyclonal antibody against angiogenin.
Immunocytochemistry
SH-SY5Y cells were washed and fixed in 4% paraformaldehyde for 30 min. After permeabilization in 0.1% Triton X-100 and blocking with 1% normal donkey serum, cells were incubated with goat polyclonal antibody against angiogenin overnight at 4C. After three washes in TBS, cells were incubated in cy3-conjugated donkey anti-goat secondary antibody (Jackson ImmunoResearch) for two hours at room temperature. Cells were rinsed with TBS for two times and once with distilled water prior to cover slipping. Internalization of angiogenin was visualized using a confocal scanning microscope (Leica Microsystems, Exton, PA).
LDH Assay
Cells were plated in 24-well plates for 8 hours, and then pretreated with angiogenin (100nM) for 12 hours. Rotenone (0.2μM) or MPP+ (1mM) was then added with angiogenin into serum-free media for another 24 hours. Cell death was measured by lactate dehydrogenase (LDH) release into media using a LDH assay kit (Roche). LDH release was normalized to maximal LDH release for each well.
Statistical Analysis
All analysis of experiments were performed with GraphPad Prism5 (GraphPad Inc., LaJolla, CA). LDH assay experiments were analyzed with one-way ANOVA followed by post-hoc Bonferonni’s test. Protein blot analysis was performed using either unpaired t-test, one group t-test with Bonferroni correction, or one-way ANOVA with a post-hoc Tukey’s test.
Results
Angiogenin expression is reduced in α-syn transgenic mice
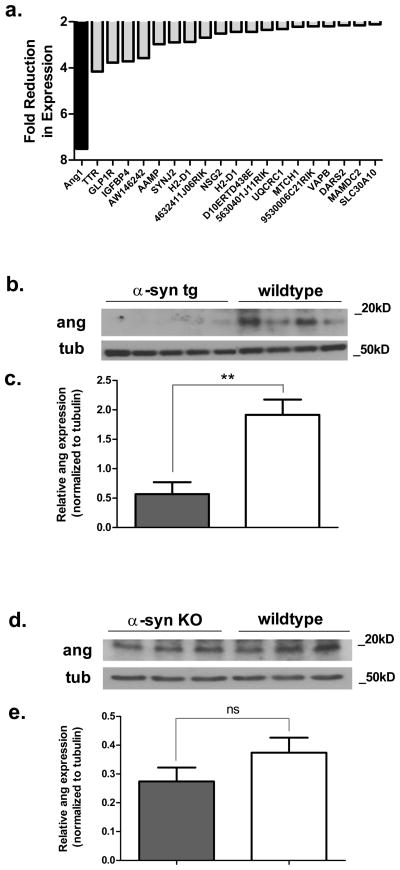
Our previous microarray analysis of α-syn transgenic mice revealed a substantial reduction in the mRNA for mAng1, the mouse homolog for human angiogenin, at three months (Supplemental Table 1 in Yacoubian et al. 2008). Among all genes evaluated in this study, mAng1 was the most altered and was reduced by 7.5-fold (Fig. 1a). We extended this analysis by studying the expression of angiogenin protein in the same mouse model. Cell lysates from the cortex of transgenic and wildtype littermates were run on a SDS-polyacrylamide gel and immunoblotted with an antibody against angiogenin (Fig. 1b). We found that protein levels of angiogenin were reduced by 70% in transgenic mice at three months by Western blotting (Fig. 1c). To further examine a relationship between α-syn and angiogenin, we analyzed angiogenin expression in the cortices of α-syn knockout mice. We found no significant differences in angiogenin expression between α-syn knockout mice and wildtype littermates (Fig. 1d, e).
Figure 1. Mouse angiogenin 1 levels are reduced in transgenic mice overexpressing α-syn.
a. mAng1 is the most downregulated gene in a gene microarray study evaluating gene expression in the substantia nigra of transgenic α-syn mice compared to wildtype littermates. The top 20 most downregulated genes are shown from three-month-old mice. This data was extracted from the supplemental data of our previous publication (Yacoubian et al. 2008).
b. mAng1 protein levels are also reduced in the cortex of three-month-old transgenic mice. Representative Western blot from lysates from transgenic and wildtype mouse cortices.
c. Quantification of Western blotting against angiogenin by densitometry. Tubulin was used as a loading control, and angiogenin levels were normalized to tubulin. **p<0.01 (unpaired t-test). Error bars reflect SEM.
d. Angiogenin expression is not altered in the cortex of three-month-old α-syn knockout mice. Representative Western blot from lysates of knockout and wildtype mouse cortex homogenate.
e. Quantification of Western blotting against angiogenin by densitometry. Tubulin was used as a loading control, and angiogenin levels were normalized to tubulin. ns = no significant difference (unpaired t-test). Error bars reflect SEM.
SH-SY5Y and M17 cells express and respond to angiogenin
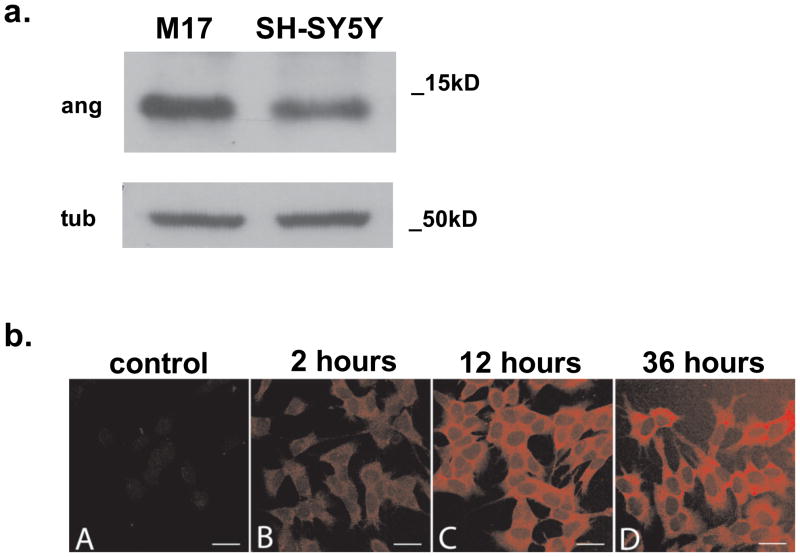
To evaluate the potential neuroprotective role of angiogenin in cell culture models of PD, we first examined whether commonly used dopaminergic (DA) cell lines can synthesize and respond to angiogenin. Using both quantitative PCR and Western blotting, we found that both the mRNA and the protein for angiogenin were detectable in SH-SY5Y and SK-N-BE(2)-M17 (M17) dopamine-producing neuroblastoma cell lines (Fig. 2a). Immunostaining of native SH-SY5Y cells revealed a low level of intracellular angiogenin protein with a punctate distribution (Fig. 2b).
Figure 2. Dopaminergic cell lines express and take up angiogenin.
a. Western blotting reveals endogenous levels of angiogenin produced by both M17 and SH-SY5Y dopaminergic cell lines. Tubulin was used as loading control.
b. A robust increase in intracellular angiogenin staining is observed after treatment with exogenous angiogenin. SH-SY5Y cells were immunostained using a goat polyclonal antibody against angiogenin and a cy3-conjugated donkey anti-goat antibody at A) control, B) 2 hours, C) 12 hours, and D) 36 hours after treatment with 100nM recombinant angiogenin. Scale bar represents 25μm.
We next examined if the SH-SY5Y dopaminergic cell line could take up exogenous angiogenin. Recombinant angiogenin was applied at 100nM to SH-SY5Y cells in culture, and the cells were then fixed and immunostained for angiogenin at varying time points. We detected a clear increase in angiogenin staining at two hours in these cells, and the intensity of the intracellular angiogenin staining continued to increase for up to 36 hours (Fig. 2b). Mild increase in angiogenin staining was detected as early as 15 minutes.
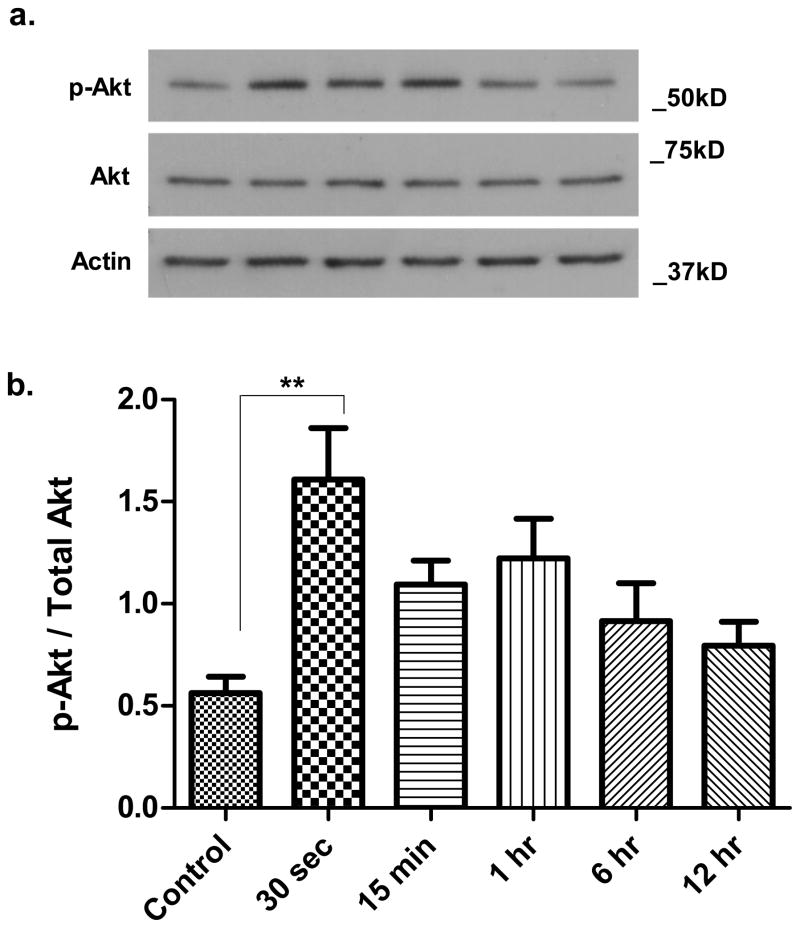
Published studies have demonstrated that angiogenin can increase phosphorylation of Akt, a downstream target of PI3K (Kieran et al. 2008, Kim et al. 2007, Trouillon et al 2010.). We examined the effect of exogenous angiogenin on Akt phosphorylation in SH-SY5Y cells. Cells were treated with 100nM angiogenin and cell lysates were collected at 30 seconds, 1 minute, 15 minutes, 6 hours, and 12 hours. Levels of phosphorylated (Ser473) Akt were determined by Western blotting (Fig. 3a). As early as 30 seconds after application, angiogenin increased phospho-Akt significantly and the level of phospho-Akt remained elevated for several hours (Fig. 3b). The increase in phospho-Akt was not secondary to changes in total Akt levels, as levels of total Akt remained stable at all time points evaluated.
Figure 3. Angiogenin induces Akt phosphorylation at serine 473.
a. Representative Western blot demonstrates increased Akt phosphorylation at serine 473 within 30 seconds following treatment of SH-SY5Y cells with recombinant angiogenin (100nM). This Akt phosphorylation is maintained for several hours. Total Akt levels are unchanged with angiogenin treatment. Actin is used as a loading control.
b. Densitometric quantification of Western blots against phosphorylated Akt. Results reflect three independent experiments. **p<0.01 (One-way ANOVA with Tukey’s post-hoc test). Error bars reflect SEM.
Angiogenin is protective against the neurotoxin MPP+
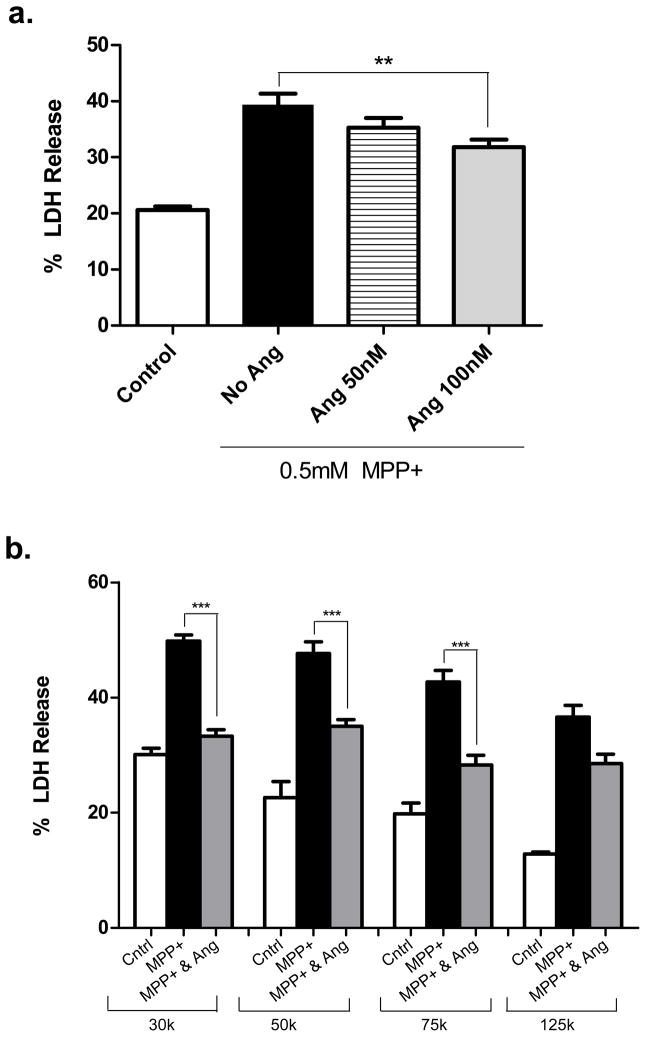
Having shown that SH-SY5Y cells can respond to angiogenin, we next investigated whether angiogenin can reduce toxicity in response to the neurotoxin MPP+, the active metabolite of MPTP which induces a Parkinsonian-like syndrome in animals and humans (Przedborski et al. 2001). SH-SY5Y cells were first pretreated with angiogenin for 12 hours and then co-treated with MPP+ and angiogenin for another 24 hours. Cell death was determined by LDH release into the media. Angiogenin reduced cell death in response to MPP+ in a dose-dependent manner (Fig. 4a).
Figure 4. Exogenous angiogenin reduces MPP+ toxicity in SH-SY5Y cells.
a. Angiogenin demonstrates a concentration-dependent protective response to MPP+ induced cell death. Cells were plated 150,000 cells/well and pretreated with varying concentrations of angiogenin for 12 hours prior to treatment with 0.5mM MPP+. Cell death was assayed by LDH release into media. n=6. ***p<0.001 (one-way ANOVA followed by post-hoc Bonferroni’s test). Error bars reflect SEM.
b. Angiogenin’s neuroprotective effect against MPP+ varies with cell density. SH-SY5Y cells were plated at cell densities ranging from 30,000 cells/well up to 125,000 cells/well and pretreated with 100nM angiogenin for 12 hours prior to treatment with 1 mM MPP+. Cell death was assayed by LDH release. n=4. ***p<0.001 (one-way ANOVA followed by post-hoc Bonferroni’s test). Error bars reflect SEM.
Prior research has demonstrated an inverse relationship between angiogenin’s biological activity and cell density. Biological activity is reduced with increasing cell density, an effect which may be related to alterations in angiogenin receptor levels (Moroianu & Riordan 1994, Hatzi et al. 2000). Here we tested the relationship of cell density to angiogenin’s neuroprotective effect in SH-SY5Y cells. Angiogenin (100nM) reduced cell death induced by MPP+ at all densities tested; however, the effect was significantly greater at lower cell densities. At the lowest density tested (30,000 cells/well), cell death induced by MPP+ was blocked almost entirely by the addition of angiogenin (Fig. 4b)
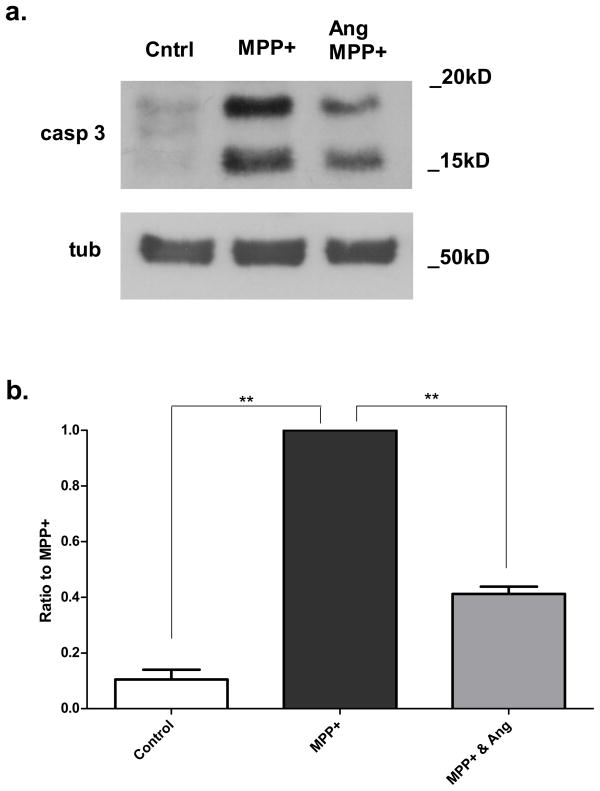
Since MPP+ induces apoptosis in SH-SY5Y cells (Gomez et al. 2001), we next tested whether angiogenin alters the apoptotic cascade by evaluating caspase-3 activation. SH-SY5Y cells were pretreated with angiogenin for 12 hours followed by treatment with 1mM MPP+ with or without angiogenin for 24 hours, and cleaved caspase-3 levels were determined by Western blotting of cell lysates (Fig. 5a). Cells pretreated with angiogenin showed a significant reduction in the induction of cleaved caspase-3 by MPP+ (Fig. 5b).
Figure 5. Exogenous angiogenin reduces caspase-3 cleavage in response to MPP+ treatment.
a. Representative Western blot against cleaved caspase-3 of SH-SY5Y cells treated with 1mM MPP+ with or without 100nM angiogenin. Tubulin was used as a loading control.
b. Densitometric quantification of Western blots against cleaved caspase-3. Results reflect three independent experiments. Each condition was normalized to MPP+-treated condition for the given blot. **p<0.01 (one group t-test with Bonferroni correction). Error bars reflect SEM.
Angiogenin is also protective against rotenone
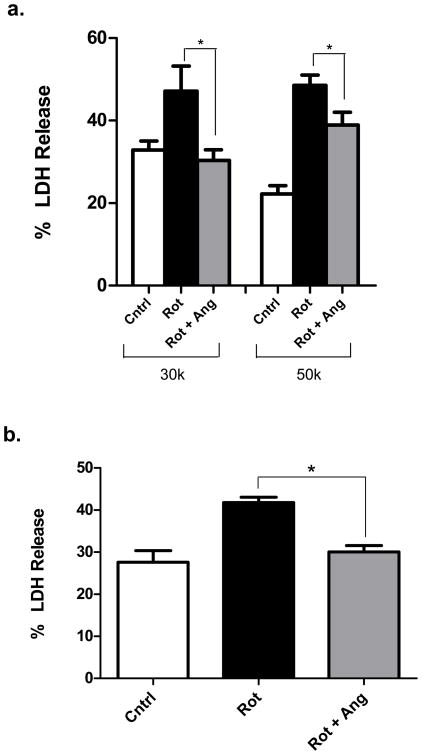
We next asked whether angiogenin’s neuroprotective effect in SH-SY5Y cells was limited to MPP+ or whether it could protect against other neurotoxins. We turned to the pesticide rotenone, which has been shown to induce a Parkinsonian syndrome in rodents (Betarbet et al. 2000). In SH-SY5Y cells, we found that pretreatment with 100nM angiogenin markedly reduced cell death in response to rotenone (0.2μM), as determined by LDH release. Protection was seen at both cell densities tested, with more complete protection at the lower cell density (Fig. 6a). A similar protective effect of angiogenin against rotenone toxicity was found in M17 cells (Fig. 6b).
Figure 6. Angiogenin reduces cell death induced by rotenone.
a. Exogenous angiogenin reduces rotenone-induced toxicity in SH-SY5Y cells in a cell-density-dependent manner. Cells were plated at either 30,000 or 50,000 cells/well and pretreated with angiogenin (100nM) prior to treatment with 0.2μM rotenone. n=4.
b. Angiogenin reduces rotenone toxicity in a different dopaminergic cell line, M17. n=4. *p<0.05 (one-way ANOVA followed by post-hoc Tukey’s test). Error bars reflect SEM
DISCUSSION
In this study, we provide evidence that angiogenin plays a protective role in PD models. We demonstrate that mouse angiogenin levels are reduced in a transgenic mouse model in which α-syn is overexpressed. Dopaminergic cell lines can take up exogenous angiogenin in vitro, and application of angiogenin to these cell lines reduces rotenone and MPP+ toxicity in a dose and cell density-dependent manner. This reduction in toxicity is associated with a reduction in apoptosis, as determined by caspase-3 cleavage. Treatment of cells with angiogenin is also associated with Akt phosphorylation, suggesting that angiogenin may reduce apoptotic signaling through activation of this survival pathway.
We first discovered a potential link of angiogenin to PD through our gene microarray study which investigated alterations in gene expression in a transgenic α-syn mouse. As determined by in situ hybridization, mAng1 is normally expressed at highest levels in the granule cell layer of the cerebellum, the dentate granule cells and CA1 and CA3 pyramidal neurons of the hippocampus, and the main olfactory bulb in mouse brain, while lower levels are detected ubiquitously in the brain, including the substantia nigra and cortex (Lein et al. 2007). In the rat brain, angiogenin protein has been detected in the cortex and subcortical regions (Huang et al. 2009). In the microarray study, we found that mAng1 was the gene most reduced in expression in the substantia nigra of three-month-old transgenic mice (Yacoubian et al. 2008). Here we confirm that angiogenin protein expression is also reduced in the cortex of α-syn transgenic mice. At three months of age these transgenic mice show minimal pathological or behavioral changes but with age develop α-syn inclusions, reduction in striatal dopamine levels, and motor deficits (Masliah et al. 2000). Since the loss of angiogenin expression is an early feature of this transgenic model, it is possible that the reduction in this protein contributes to the subsequent degenerative changes. This view, while speculative, is consistent with our finding that exogenous angiogenin promotes cell survival in the face of PD toxins such as MPP+ and suggests that endogenous angiogenin may play a role in dopaminergic cell function and survival in vivo.
It is unclear how overexpression of α-syn causes decreased angiogenin expression in this mouse model. One possibility is that α-syn normally negatively regulates angiogenin expression in the brain. Indeed, α-syn has been shown to affect transcription via inhibition of histone acetylation (Kontopoulos et al. 2006). Therefore,α-syn overexpression could cause a reduction in angiogenin levels via this mechanism. We did evaluate whether a reciprocal increase in angiogenin levels is seen in an α-syn knockout mouse, but we did not detect any differences between wildtype and knockout mice. This finding suggests that α-syn does not regulate angiogenin levels under normal circumstances. Instead, an alteration in angiogenin expression is likely a pathological consequence of α-syn overexpression. Consistent with this, functional genomic analysis of our microarray study has shown that the main consequence of α-syn overexpression in the α-syn mouse is alteration of genes involved in transcriptional processes (Yacoubian et al. 2008).
Regardless whether or not reduced angiogenin levels play a role in the pathogenesis of PD pathology, the ability of exogenous angiogenin to reduce cell death in PD models suggests that increasing angiogenin levels could serve as a means to slow degeneration in PD. The mechanism for angiogenin’s neuroprotective effects in this and other model systems is unclear, but the PI3K/Akt pathway may play an important role. In ALS models blockade of the PI3K/Akt pathway prevents the neuroprotective effects of angiogenin (Kieran et al. 2008). Activation of this pathway has been previously shown to be protective in the 6-OHDA mouse model (Ries et al. 2006), and interestingly postmortem tissue from PD patients demonstrates depletion of phosphorylated Akt (Malagelada et al. 2008). Consistent with these observations, we find that angiogenin at neuroprotective concentrations induces phosphorylation of Akt at serine 473 in SH-SY5Y cells. There may also be additional downstream pathways activated by angiogenin in our model, such as ERK1/2 (Cho et al. 2010, Kieran et al. 2008)
Angiogenin could activate Akt phosphorylation by binding an extracellular receptor, or, alternatively, it first becomes endocytosed and then activates an intracellular signaling cascade. We see Akt phosphorylation within seconds as measured by Western blot, whereas we observe angiogenin uptake after minutes by immunocytochemistry. This rapid activation of Akt may indicate a potential extracellular receptor-mediated signaling response, rather than an induction of Akt phosphorylation after endocytotic uptake of angiogenin. An unknown 170 kDa receptor that binds angiogenin has been proposed to be capable of activating PI3K/Akt signaling in endothelial cells and to respond in a cell density dependent manner similar to our findings (Hu et al. 1997, Kim et al. 2007, Trouillon et al. 2010). However, it is also possible that a low level of angiogenin uptake occurs faster than we could observe by immunocytochemistry such that this uptake is sufficient to induce Akt phosphorylation within seconds.
Akt signaling can result in downstream inhibition of apoptosis through phosphorylation of its substrates, such as Bad, caspase-9, and FOXO (Burke 2007, Nakaso et al. 2008, Woodgett 2005, Zhou et al. 2000). We demonstrate that caspase-3 cleavage, a marker of apoptosis, was increased in SH-SY5Y cells following MPP+ treatment and that angiogenin inhibited this effect. Caspase-3 activation has been linked to PD. Adult mice show increased caspase-3 cleavage in the substantia nigra following MPTP administration (Turmel et al. 2001, Yu et al. 2010). Similarly, postmortem PD brains demonstrate apoptotic cell death with increased caspase-3 cleavage in nigral neurons (Hartmann et al. 2001). Our results indicate the protective effect of angiogenin may be mediated through inhibition of apoptosis.
In summary our findings demonstrate a neuroprotective role of angiogenin in dopaminergic cell lines, similar to findings in motoneurons. It is possible that angiogenin may act as a general pro-survival factor, yet our findings of the decrease in angiogenin levels in a mouse model of PD suggest that alterations in angiogenin may play a specific role in disease pathophysiology. The neuroprotective mechanism includes inhibition of the apoptotic cascade and likely involves induction of PI3K/Akt signaling. In clinical trials of ALS, angiogenin has been delivered by gene therapy approaches (Zavalishin et al. 2008). Systemic delivery may also be feasible: in vivo studies show that angiogenin administered by intraperitoneal injections can cross the blood-brain barrier and have therapeutic effects (Kieran et al. 2008). Either of these approaches might be used to deliver angiogenin to PD patients, making it a potentially promising approach as a new therapy to slow neurodegeneration.
Acknowledgments
We thank Mary Ballestas at the UAB Neuroscience Core (P30 NS47466) and Michelle Gray for advice and discussion. We thank Robert Nussbaum for providing mouse α-syn knockout and wildtype cortices. This research was supported by The Parkinson Association of Alabama and the American Parkinson Disease Association, and by a NIH-NINDS K08 award (NS060948).
Abbreviations
- 6-OHDA
6-hydroxydopamine
- α-syn
alpha-synuclein
- ALS
Amyotrophic Lateral Sclerosis
- DA
dopamine
- ERK
extracellular-signal-regulated kinases
- LDH
lactate dehydrogenase
- mAng1
mouse angiogenin-1
- MPP+
1-Methyl-4-phenylpyridinium
- PD
Parkinson’s disease
- PDGFβ
platelet-derived growth factor beta
- PI3K
Phosphatidylinositol 3-phosphate
Footnotes
Laboratory of Origin: Talene Yacoubian, M.D., Ph.D.
Conflicts of Interest:
Trent Steidinger, Talene Yacoubian, and David Standaert declare no potential conflict of interest.
References
- Athanassiadou A, Voutsinas G, Psiouri L, Leroy E, Polymeropoulos MH, Ilias A, Maniatis GM, Papapetropoulos T. Genetic analysis of families with Parkinson disease that carry the Ala53Thr mutation in the gene encoding alpha-synuclein. Am J Hum Genet. 1999;65:555–558. doi: 10.1086/302486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Burke RE. Inhibition of mitogen-activated protein kinase and stimulation of Akt kinase signaling pathways: Two approaches with therapeutic potential in the treatment of neurodegenerative disease. Pharmacol Ther. 2007;114:261–277. doi: 10.1016/j.pharmthera.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho GW, Kang BY, Kim SH. Human angiogenin presents neuroprotective and migration effects in neuroblastoma cells. Mol Cell Biochem. 2010;340:133–41. doi: 10.1007/s11010-010-0410-0. [DOI] [PubMed] [Google Scholar]
- Eriksen JL, Dawson TM, Dickson DW, Petrucelli L. Caught in the act: alpha–synuclein is the culprit in Parkinson's disease. Neuron. 2003;40:453–456. doi: 10.1016/s0896-6273(03)00684-6. [DOI] [PubMed] [Google Scholar]
- Gao X, Xu Z. Mechanisms of action of angiogenin. Acta Biochim Biophys Sin (Shanghai) 2008;40:619–624. doi: 10.1111/j.1745-7270.2008.00442.x. [DOI] [PubMed] [Google Scholar]
- Gomez C, Reiriz J, Pique M, Gil J, Ferrer I, Ambrosio S. Low concentrations of 1-methyl-4-phenylpyridinium ion induce caspase-mediated apoptosis in human SH–SY5Y neuroblastoma cells. J Neurosci Res. 2001;63:421–428. doi: 10.1002/1097-4547(20010301)63:5<421::AID-JNR1037>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Greenway MJ, Alexander MD, Ennis S, Traynor BJ, Corr B, Frost E, Green A, Hardiman O. A novel candidate region for ALS on chromosome 14q11.2. Neurology. 2004;63:1936–1938. doi: 10.1212/01.wnl.0000144344.39103.f6. [DOI] [PubMed] [Google Scholar]
- Greenway MJ, Andersen PM, Russ C, et al. ANG mutations segregate with familial and 'sporadic' amyotrophic lateral sclerosis. Nat Genet. 2006;38:411–413. doi: 10.1038/ng1742. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Michel PP, Troadec JD, Mouatt-Prigent A, Faucheux BA, Ruberg M, Agid Y, Hirsch EC. Is Bax a mitochondrial mediator in apoptotic death of dopaminergic neurons in Parkinson's disease? J Neurochem. 2001;76:1785–1793. doi: 10.1046/j.1471-4159.2001.00160.x. [DOI] [PubMed] [Google Scholar]
- Hatzi E, Bassaglia Y, Badet J. Internalization and processing of human angiogenin by cultured aortic smooth muscle cells. Biochem Biophys Res Commun. 2000;267:719–725. doi: 10.1006/bbrc.1999.2015. [DOI] [PubMed] [Google Scholar]
- Hu G, Riordan JF, Vallee BL. Angiogenin promotes invasiveness of cultured endothelial cells by stimulation of cell-associated proteolytic activities. Proc Natl Acad Sci U S A. 1994;91:12096–12100. doi: 10.1073/pnas.91.25.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu GF. Neomycin inhibits angiogenin-induced angiogenesis. Proc Natl Acad Sci U S A. 1998;95:9791–9795. doi: 10.1073/pnas.95.17.9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu GF, Riordan JF, Vallee BL. A putative angiogenin receptor in angiogenin-responsive human endothelial cells. Proc Natl Acad Sci U S A. 1997;94:2204–2209. doi: 10.1073/pnas.94.6.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Huang Y, Guo H. Dominant expression of angiogenin in NeuN positive cells in the focal ischemic rat brain. J Neurol Sci. 2009;285:220–223. doi: 10.1016/j.jns.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Ibaragi S, Yoshioka N, Kishikawa H, Hu JK, Sadow PM, Li M, Hu GF. Angiogenin-stimulated rRNA transcription is essential for initiation and survival of AKT-induced prostate intraepithelial neoplasia. Mol Cancer Res. 2009;7:415–424. doi: 10.1158/1541-7786.MCR-08-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieran D, Sebastia J, Greenway MJ, King MA, Connaughton D, Concannon CG, Fenner B, Hardiman O, Prehn JH. Control of motoneuron survival by angiogenin. J Neurosci. 2008;28:14056–14061. doi: 10.1523/JNEUROSCI.3399-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Kang DK, Kim HY, Kang SS, Chang SI. Angiogenin-induced protein kinase B/Akt activation is necessary for angiogenesis but is independent of nuclear translocation of angiogenin in HUVE cells. Biochem Biophys Res Commun. 2007;352:509–513. doi: 10.1016/j.bbrc.2006.11.047. [DOI] [PubMed] [Google Scholar]
- Kontopoulos E, Parvin JD, Feany MB. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum Mol Genet. 2006;15:3012–3023. doi: 10.1093/hmg/ddl243. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Malagelada C, Jin ZH, Greene LA. RTP801 is induced in Parkinson's disease and mediates neuron death by inhibiting Akt phosphorylation/activation. J Neurosci. 2008;28:14363–14371. doi: 10.1523/JNEUROSCI.3928-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Moroianu J, Riordan JF. Nuclear translocation of angiogenin in proliferating endothelial cells is essential to its angiogenic activity. Proc Natl Acad Sci U S A. 1994;91:1677–1681. doi: 10.1073/pnas.91.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaso K, Ito S, Nakashima K. Caffeine activates the PI3K/Akt pathway and prevents apoptotic cell death in a Parkinson's disease model of SH-SY5Y cells. Neurosci Lett. 2008;432:146–150. doi: 10.1016/j.neulet.2007.12.034. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem. 2001;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- Ries V, Henchcliffe C, Kareva T, Rzhetskaya M, Bland R, During MJ, Kholodilov N, Burke RE. Oncoprotein Akt/PKB induces trophic effects in murine models of Parkinson's disease. Proc Natl Acad Sci U S A. 2006;103:18757–18762. doi: 10.1073/pnas.0606401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastia J, Kieran D, Breen B, King MA, Netteland DF, Joyce D, Fitzpatrick SF, Taylor CT, Prehn JH. Angiogenin protects motoneurons against hypoxic injury. Cell Death Differ. 2009;16:1238–47. doi: 10.1038/cdd.2009.52. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Crabtree B, Acharya KR. Human angiogenin is a neuroprotective factor and amyotrophic lateral sclerosis associated angiogenin variants affect neurite extension/pathfinding and survival of motor neurons. Hum Mol Genet. 2008;17:130–149. doi: 10.1093/hmg/ddm290. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Feng Y. A new role for angiogenin in neurite growth and pathfinding: implications for amyotrophic lateral sclerosis. Hum Mol Genet. 2007;16:1445–1453. doi: 10.1093/hmg/ddm095. [DOI] [PubMed] [Google Scholar]
- Trouillon R, Kang DK, Park H, Chang SI, O'Hare D. Angiogenin induces nitric oxide synthesis in endothelial cells through PI-3 and Akt kinases. Biochemistry. 2010;49:3282–3288. doi: 10.1021/bi902122w. [DOI] [PubMed] [Google Scholar]
- Turmel H, Hartmann A, Parain K, Douhou A, Srinivasan A, Agid Y, Hirsch EC. Caspase-3 activation in 1–methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. Mov Disord. 2001;16:185–189. doi: 10.1002/mds.1037. [DOI] [PubMed] [Google Scholar]
- Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Wu D, Yu W, Kishikawa H, Folkerth RD, Iafrate AJ, Shen Y, Xin W, Sims K, Hu GF. Angiogenin loss–of-function mutations in amyotrophic lateral sclerosis. Ann Neurol. 2007;62:609–617. doi: 10.1002/ana.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoubian TA, Cantuti-Castelvetri I, Bouzou B, Asteris G, McLean PJ, Hyman BT, Standaert DG. Transcriptional dysregulation in a transgenic model of Parkinson disease. Neurobiol Dis. 2008;29:515–528. doi: 10.1016/j.nbd.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Zheng W, Xin N, Chi ZH, Wang NQ, Nie YX, Feng WY, Wang ZY. Curcumin prevents dopaminergic neuronal death through inhibition of the c-Jun N-terminal kinase pathway. Rejuvenation Res. 2010;13:55–64. doi: 10.1089/rej.2009.0908. [DOI] [PubMed] [Google Scholar]
- Zavalishin IA, Bochkov NP, Suslina ZA, et al. Gene therapy of amyotrophic lateral sclerosis. Bull Exp Biol Med. 2008;145:483–486. doi: 10.1007/s10517-008-0124-4. [DOI] [PubMed] [Google Scholar]
- Zhou H, Li XM, Meinkoth J, Pittman RN. Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol. 2000;151:483–494. doi: 10.1083/jcb.151.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]