Abstract
Background
A novel method to quantify dyssynchrony has been developed using phase analysis of gated single-photon emission computed tomography perfusion imaging. We report on the effect of variability in image reconstruction on the phase analysis results (repeatability) and on the interobserver and intraobserver reproducibility of the technique.
Methods
Phase standard deviation (SD) and bandwidth are phase indices that quantify dyssynchrony. To evaluate repeatability, raw data sets were processed twice in 50 patients with left ventricular dysfunction and 50 normal controls. To determine the optimal processing method, two replicated phase analysis results were obtained using automated and manual base parameter placement. Reproducibility of the phase analysis was determined using the data from 20 patients.
Results
In normal controls, manual base parameter placement improves repeatability of the phase analysis as measured by the mean absolute difference between two reads for phase SD (12.0° vs. 1.2°, P< 0.0001) and bandwidth (33.7° vs. 3.6°, P< 0.0001). Repeatability is better for normal controls than for patients with left ventricular dysfunction for phase SD (1.2° vs. 6.0°, P < 0.0001) and bandwidth (3.6° vs. 26.5°, P < 0.0001). Reproducibility of the phase analysis is high as measured by the intraclass correlation coefficients for phase SD and bandwidth of 0.99 and 0.99 for the interobserver comparisons and 1.00 and 1.00 for the intraobserver comparisons.
Conclusion
A novel method to quantify dyssynchrony has been developed using gated single-photon emission computed tomography perfusion imaging. Manual base parameter placement reduces the effect that variability in image reconstruction has on phase analysis. A high degree of reproducibility of phase analysis is observed.
Keywords: dyssynchrony, heart failure, single-photon emission computed tomography
Introduction
Congestive heart failure affects more than five million people in the United States [1–3]. Cardiac resynchronization therapy (CRT) with the use of a biventricular pacemaker is approved for the treatment of patients with advanced New York Heart Association class III–IV symptoms, with ejection fractions ≤ 35%, and with QRS durations ≥ 120 ms on surface electrocardiograms. Improvements in quality of life, functional class, exercise capacity, and ejection fraction have been reported with CRT [4–7]. Two recent studies have gone beyond showing symptomatic benefit to show an additional mortality benefit for patients undergoing CRT [8,9].
Approximately 20–30% of patients fail to benefit from CRT when the QRS duration is used to determine dyssynchrony. Electrical dyssynchrony may not adequately describe the degree of mechanical dyssynchrony present; and therefore, the QRS duration may not be the best predictor of patient response to CRT [10–12]. Efforts have been made to improve the selection of patients who might benefit from CRT by using imaging modalities to define cardiac dyssynchrony more precisely. These efforts have primarily focused on echocardiographic techniques to determine the degree of left ventricular mechanical dyssynchrony [13].
A novel method for describing left ventricular mechanical dyssynchrony has been developed, which uses phase analysis of ECG-gated single-photon emission computed tomography (SPECT) myocardial perfusion imaging to describe the timing of the regional left ventricular onset of mechanical contraction (OMC) [14–16]. The repeatability and reproducibility of this technique are not known. Our study evaluates the effect of variability associated with image processing and reconstruction on the phase analysis (repeatability) and reports the intraobserver and interobserver reproducibility of the phase analysis technique.
Methods
Patients selection
Our study retrospectively examined cohorts of 50 consecutive patients with left ventricular dysfunction with an ejection fraction ≤ 35% and 50 normal controls. Both cohorts of patients had undergone routine gated SPECT perfusion imaging for clinical indications at Duke University Medical Center. Normal controls were defined as patients with ejection fractions ≥ 50% on gated SPECT myocardial perfusion imaging, without evidence of perfusion defects, without clinical history of coronary artery disease, with QRS durations ≤ 120 ms, and who were in normal sinus rhythm. Baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics
| Normal controls (n = 50) | LV dysfunction (n = 50) | P value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 62 | 62 | 0.77 |
| Race (White, Black, others) (%) | 64.0, 36.0, 0.0 | 46.0, 52.0, 2.0 | 0.14 |
| Sex (male) (%) | 40.0 | 78.0 | 0.0001 |
| History of coronary artery disease (%) | 0.0 | 72.0 | < 0.0001 |
| History of CABG (%) | 0.0 | 36.0 | < 0.0001 |
| History of diabetes mellitus (%) | 30.0 | 52.0 | 0.03 |
| History of hypertension (%) | 64.0 | 86.0 | 0.02 |
| History of renal insufficiency (%) | 18.0 | 44.0 | 0.01 |
| History of atrial fibrillation (%) | 8.0 | 30.0 | 0.01 |
| History of cardiomyopathy (NICM, ICM) (%) | 0.0, 0.0 | 26.0, 74.0 | < 0.0001 |
| ECG data | |||
| QRS (ms) | 84.5 | 114.5 | < 0.0001 |
| Sinus rhythm (%) | 100.0 | 72.0 | 0.001 |
| SPECT data | |||
| Ejection fraction (%) | 71.3 | 34.1 | < 0.0001 |
| End diastolic volume (ml) | 88.7 | 217.1 | < 0.0001 |
| End systolic volume (ml) | 26.5 | 144.7 | < 0.0001 |
| Mass (g) | 115.2 | 199.3 | < 0.0001 |
CABG, coronary artery bypass graft; ICM, ischemic cardiomyopathy; LV, left ventricular; NICM, nonischemic cardiomyopathy; SPECT, single-photon emission computed tomography.
Onset of mechanical contraction determination and phase analysis
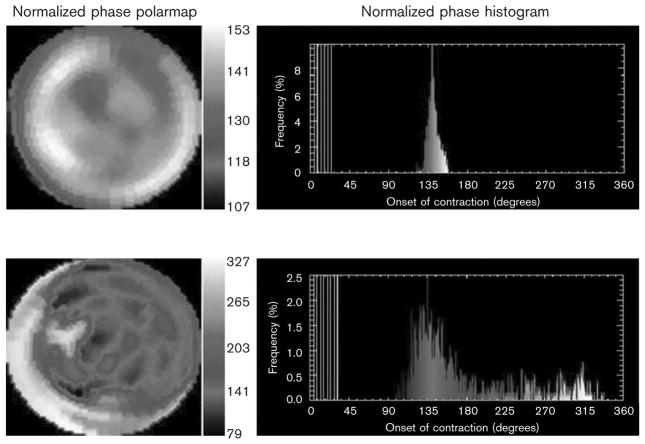
Each patient underwent a standard ECG-gated SPECT myocardial perfusion scan for clinical indications at Duke University Medical Center. Data were acquired at eight frames per cardiac cycle. The short-axis data sets were generated by Butterworth filtering followed by filtered back projection reconstruction and oblique reorientation. Three-dimensional count distributions were then extracted from each of the eight left ventricular short-axis data sets and submitted to Fourier phase analysis. The analysis applied one-dimensional fast Fourier transform to the count variation over time of each voxel to calculate the phase of the first Fourier harmonics. Then, the analysis generated a three-dimensional phase distribution that described the timing of the left ventricular regional OMC as a function of degrees, with the 360° range representing the entire length of the R–R interval. Once the phase distribution was generated, it was displayed on the polar map as well as in histogram format. Examples of a phase histogram from a patient with and without mechanical dyssynchrony are shown in Fig. 1. The x-axis represents the timing of one cardiac cycle (R–R interval) in degrees. The y-axis represents the percentage of myocardium, which demonstrated the OMC during any particular time of the cardiac cycle.
Fig. 1.
Representative phase analysis histogram from a patient with (bottom) and without (top) dyssynchrony is shown. The x-axis represents the duration of one cardiac cycle (R–R interval). The y-axis represents the percentage of myocardium demonstrating the onset of mechanical contraction for each phase of the cardiac cycle.
The method calculates quantitative indices used to describe the phase dispersion of the left ventricular regional OMC. Phase SD is the standard deviation of phase distribution. Phase histogram bandwidth represents the range of degrees during which 95% of the myocardium is initiating contraction. Therefore, higher degrees of phase SD and histogram bandwidth indicate higher degrees of mechanical dyssynchrony. A phase SD of 43° and a bandwidth of 135° have been shown to be predictive of patient response to CRT [17]. A comprehensive description of the method has recently been published, and the software has been implemented in the Emory Cardiac Toolbox (Emory University/Syntermed, Atlanta, Georgia, USA) for analysis of gated SPECT myocardial perfusion imaging [14].
Comparison of the effect of variability in image reconstruction on the phase analysis (repeatability) using automated and manual base parameter placement in normal controls
The raw image data set acquired from each gated SPECT myocardial perfusion study was processed twice by the same individual to create two gated SPECT data sets for each of the 50 normal controls. The raw image data processing consisted of optimization of the region of interest to exclude extracardiac counts as permissible without reducing cardiac counts and alignment of the horizontal and vertical axes of the images.
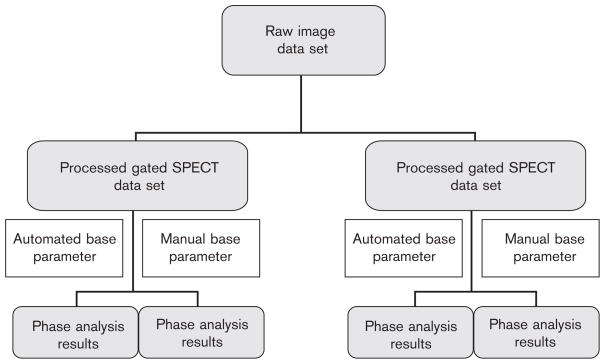
Next, two different methods for the positioning of the base, apex, radius, and center parameters before the phase analysis algorithm were used to create replicated phase analysis results for each of the two processed gated SPECT data sets. The first technique was the automated base parameter technique. The base parameter was determined by the software and no changes were made manually. Manual corrections to the center, radius, and apex parameters were made only if there was gross visual misalignment of these parameters. The second technique used was manual base parameter placement. This was done by placing the base parameter for each of the eight gated images at the slice, two slices toward the apex from the basal most slice with perfusion counts seen (within the membranous interventricular septum). Manual corrections to the center, radius, and apex parameters were made only if there was gross visual misalignment of these parameters. The phase analysis algorithm was then applied to each of these processed gated SPECT data sets. The end result of the image data processing was the creation of four sets of phase analysis results from each patient. Two of these phase analysis results were the result of automated base parameter placement processing and two were the result of manual base parameter placement processing (Fig. 2). These analyses enable the assessment of repeatability for each of the automated and manual base parameter placement methods.
Fig. 2.
Processing methods which are used to create replicated phase analysis results using both automated and manual base parameter placement processing methods used to compare the repeatability of the phase analysis.
Comparison of the effect of variability in image reconstruction on the phase analysis (repeatability) in patients with left ventricular dysfunction and normal controls using manual base parameter placement
The raw image data set acquired from each gated SPECT myocardial perfusion study was processed twice by the same individual using the same methods described for the normal controls. The phase analysis algorithm was then applied to each of the two replicated gated SPECT data sets using the manual base parameter placement processing method described above for each patient. These analyses enable the assessment of repeatability using the manual base parameter placement method in the cohort of patients with left ventricular dysfunction and for comparison with repeatability in normal controls.
Determination of intraobserver and interobserver reproducibility of the phase analysis
Ten consecutive patients with left ventricular dysfunction and 10 consecutive normal controls were used for the determination of intraobserver and interobserver reproducibility. One gated SPECT data set for each patient was used in the comparisons. To determine intraobserver reproducibility of phase analysis, one reader performed phase analysis after manual placement of the base parameter on two different occasions. To determine interobserver reproducibility, a second reader performed phase analysis after manual placement of the base parameter and this result was compared with the first reading from the first reader. The second reader was blinded to the results of phase analysis performed by the first reader.
Statistical methods
Baseline characteristics were compared between the 50 normal controls and 50 patients with left ventricular dysfunction using χ2 test for categorical variables and Wilcoxon two-sample tests for continuous variables. Fisher’s exact test was used for categorical variables if the frequency in one of the categories was less than 10. Results of the SPECT imaging phase indices were presented as mean ± SD and Wilcoxon signed-rank test for paired data was used to test the difference in means between the two groups. Repeatability is expressed either by the mean absolute difference between two replicated readings or by intraclass correlation coefficients. Wilcoxon signed-rank test for paired data and Wilcoxon two-sample test, were used to test the difference in the mean absolute difference between the two processing methods (automated vs. manual methods) and between two cohorts of patients (normal vs. left ventricular dysfunction), respectively. Intraclass correlation coefficients and mean absolute differences between two readings were used to assess the intraobserver and interobserver reproducibility of phase analysis.
This study was approved by the Duke University Institutional Review Board.
Results
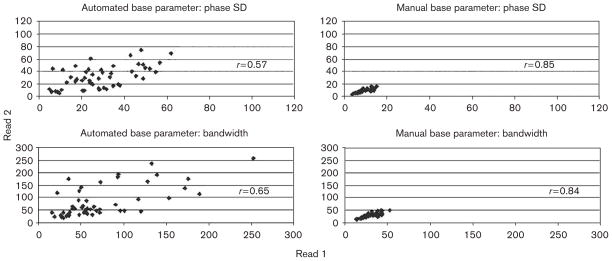
In normal controls, the phase indices of phase SD (30.0°±14.5° vs. 8.6°±2.9°, P<0.0001) and bandwidth (78.0°±50.8° vs. 27.9°±8.9°, P<0.0001) of the first reading were significantly reduced and demonstrated a narrower SD of values when manual base parameter placement was used as compared with automated base parameter placement. As demonstrated in Fig. 3, manual base parameter placement improves the repeatability of phase analysis in normal controls as measured by the intraclass correlation coefficient for phase SD (r=0.57 vs. r=0.85) and for bandwidth (r=0.65 vs. r=0.84). Improvement of the repeatability using manual base parameter placement is further demonstrated by an improvement in the mean absolute difference between the two reads for phase SD (12.0°±9.6° vs. 1.2°±1.2°, P<0.0001) and for bandwidth (33.7°±34.5° vs. 3.6°±3.7°, P<0.0001).
Fig. 3.
Improvement in repeatability of the phase analysis as measured by intraclass correlation coefficients using manual base parameter placement in 50 normal controls.
Table 2 demonstrates the phase analysis indices in patients with left ventricular dysfunction as compared with normal controls when the manual base parameter processing method is used. Phase SD (41.6°±24.2° vs. 8.6°±2.9°, P<0.0001) and histogram bandwidth (115.4°±60.5° vs. 27.9°±8.9°, P<0.0001) were significantly different between patients with left ventricular dysfunction and normal controls when the first reading was analyzed. With the manual base parameter placement, the degree of repeatability of the phase analysis indices in patients with left ventricular dysfunction as compared with normal controls is also demonstrated in Table 2. The mean absolute difference between the two reads for phase SD (6.0°±7.3° vs. 1.2°±1.2°, P<0.0001) and bandwidth (26.5°±40.2° vs. 3.6°±3.7°, P<0.0001) was higher in patients with left ventricular dysfunction compared with normal controls. The correlation coefficients of the two reads in patients with left ventricular dysfunction were 0.93 for phase SD and 0.73 for bandwidth, which are similar to the correlation coefficients of the two reads in normal controls (r=0.85 for phase SD and r=0.84 for bandwidth).
Table 2.
Comparison of the repeatability of the phase analysis indices in patients with left ventricular dysfunction and normal controls
| Normal controls | LV dysfunction | P value | |
|---|---|---|---|
| Manual base | Manual base | ||
| N = 50 | N = 50 | ||
| Phase SD (°) | |||
| Mean ± SD of the first reading | 8.6 ± 2.9 | 41.6 ± 24.2 | < 0.0001 |
| Absolute difference (mean ± SD) of the two readings | 1.2 ± 1.2 | 6.0 ± 7.3 | < 0.0001 |
| Intraclass correlation coefficient of the two readings | 0.85 | 0.93 | |
| Bandwidth (°) | |||
| Mean ± SD of the first reading | 27.9 ± 8.9 | 115.4 ± 60.5 | < 0.0001 |
| Absolute difference (mean ± SD) of the two readings | 3.6 ± 3.7 | 26.5 ± 40.2 | < 0.0001 |
| Intraclass correlation coefficient of the two readings | 0.84 | 0.73 | |
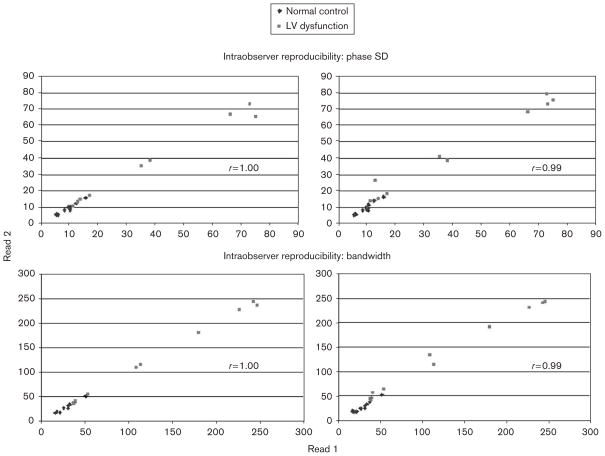
Intraobserver and interobserver reproducibility of phase analysis is shown in Fig. 4. For intraobserver reproducibility, the intraclass correlation coefficient for phase SD and bandwidth were 1.00 and 1.00, respectively, and the mean absolute difference between the two reads was 0.8° and 1.4°, respectively. For interobserver reproducibility, the intraclass correlation coefficient for phase SD and bandwidth were 0.99 and 0.99, respectively, and the mean absolute difference between the two reads was 2.0° and 5.4°, respectively.
Fig. 4.
High degree of intraobserver and interobserver reproducibility as measured by intraclass correlation coefficients of the phase analysis in 20 patients (LV-dysfunction, n=10; normal controls, n=10). LV, left ventricular.
Discussion
CRT with the use of biventricular pacing is approved for the treatment of patients with New York Heart Association class III–IV heart failure symptoms who have ejection fractions ≤ 35% and a QRS duration ≥ 120 ms. Several studies have shown clinical and mortality benefits from CRT when added to optimal medical therapy for groups of patients who meet the above selection criteria [4–9].
It is difficult to accurately select individual patients who would benefit from CRT. Data from randomized trials evaluating CRT demonstrate that a significant percentage of patients (20–30%) does not respond to CRT. Efforts have been made to utilize imaging techniques to more precisely define cardiac dyssynchrony in the hope of more accurately predicting which patients would benefit from CRT. These efforts have primarily focused on utilizing advanced echocardiographic techniques to determine left ventricular mechanical dyssynchrony [18–39].
A novel nuclear method for the evaluation of left ventricular mechanical dyssynchrony has recently been developed and normal databases have been reported using phase analysis of ECG-gated SPECT myocardial perfusion imaging [14]. Clinical validation of the technique began by demonstrating that differences in the phase analysis indices exist between patients with left ventricular dysfunction or conduction disturbances and normal controls [15]. Additionally, phase indices were shown to correlate with dyssynchrony as determined by tissue Doppler echocardiography [16]. Our study sought to further the validation of this technique by describing the effect of variability in image processing and reconstruction on phase analysis (repeatability), by describing the optimal image processing method to improve repeatability, and by describing intraobserver and interobserver reproducibility of phase analysis.
Our study first determined the effect that variability in processing and reconstruction of the SPECT myocardial perfusion data, has on the repeatability of phase analysis results in a cohort of normal controls. Several steps are involved in the processing of the raw data to arrive at phase analysis results. These include determining the region of interest and limit boundaries to isolate cardiac counts from noncardiac counts, reorienting and aligning the horizontal and vertical axes, processing of data through the gated SPECT algorithm, determining the placement of the center, radius, base, and apex parameters, and processing of gated SPECT data through the phase analysis algorithm. Each of these steps can introduce variation in the final phase analysis results.
We next sought to refine the processing methods used to improve the degree of repeatability of phase analysis. We observed that a significant amount of variability came from differences in the amounts of low-frequency ‘noise’ located at the base of the ventricle. By adjusting the processing protocol to include placing the base parameter at the slice, two slices toward the apex from the basal most slice with perfusion counts in each of the eight gated image data sets (a level usually within the membranous ventricular septum), all ‘noise’ was eliminated from the phase histogram. The elimination of low-frequency noise resulted in lower values of phase analysis indices and in significant improvement in the repeatability of phase analysis indices. Our results indicate that manual base parameter placement should be used in future investigations.
Furthermore, our study compared the repeatability of phase analysis using the manual base parameter placement in patients with left ventricular dysfunction and normal controls. Higher amounts of variability were observed in the processing of patients with left ventricular dysfunction as compared with normal controls as measured by the mean absolute difference between the two reads. This was expected. It is more difficult to determine the limits of the region of interest, to align and reorient the horizontal and vertical axes, and to determine the appropriate placement of the center, radius, apex, and base parameters in these patients. This cohort includes patients with dense perfusion defects that can increase processing variability. Our data are consistent with data previously reported demonstrating that patients with perfusion defects have a greater degree of processing variability, specifically with regard to placement of the apex parameter and in the alignment of the horizontal and vertical axes [40].
Our study demonstrated a high degree of intraobserver and interobserver reproducibility when using manual base parameter placement. The mean absolute differences observed for the intraobserver and interobserver reproducibility comparisons were much smaller than those observed for the repeatability comparisons. Furthermore, the degree of variability we demonstrated is small in comparison with the large differences between normal controls and patients with dyssynchrony, and therefore, should not adversely influence interpretation of the phase analysis results.
The use of gated SPECT myocardial perfusion imaging has several potential advantages in the evaluation of dyssynchrony in patients with left ventricular dysfunction. The technique is automated and takes less than 1 min to perform. This is in contrast with currently used echocardiographic techniques, which require significant offline data processing and analysis. Second, additional myocardial perfusion information could be helpful in the prediction of patient response to CRT. The presence, location, and severity of myocardial scar have been shown to impact patient response to CRT in a retrospective study using cardiac magnetic resonance imaging [41]. Recently, the extent of myocardial variability as assessed by F-FDG SPECT has also been shown to predict response to CRT [42]. Finally, the addition of dyssynchrony evaluation in patients having gated SPECT perfusion studies performed for other indications would be very cost effective and could potentially obviate the need for additional diagnostic testing.
Limitations do exist to our study. Although a thorough assessment of the effect of variability associated with image processing and reconstruction on phase analysis is described, it is not known to what degree each of the steps contributes to the variability seen. The only additional processing steps involved in phase analysis as compared with routine-gated SPECT image processing are the placement of the base parameter and the actual phase analysis algorithm. Through our determination of the very high intraobserver and interobserver reproducibility, we have demonstrated that the additional variability involved in these steps is minimal and should not have an impact on the usefulness of phase analysis data.
Acknowledgments
This study was funded by research grants from the Medtronic-Duke Strategic Alliance of which Salvador Borges-Neto, MD and Mark A. Trimble, MD are primary investigators.
Footnotes
Dr Garcia reports an ownership interest in Syntermed Inc., the remaining authors report no conflicts of interest.
References
- 1.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–e82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, et al. Heart disease and stroke statistics–2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 3.O’Connell JB, Bristow MR. Economic impact of heart failure in the United States: time for a different approach. J Heart Lung Transplant. 1994;13:S107–S112. [PubMed] [Google Scholar]
- 4.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 5.Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42:1454–1459. doi: 10.1016/s0735-1097(03)01042-8. [DOI] [PubMed] [Google Scholar]
- 6.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 7.McAlister F, Ezekowitz J, Wiebe N, Rowe B, Spooner C, Crumley E, et al. Cardiac resynchronization therapy for congestive heart failure. Evid Rep Technol Assess (Summ) 2004;106:1–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 9.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 10.Leclercq C, Faris O, Tunin R, Johnson J, Kato R, Evans F, et al. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation. 2002;106:1760–1763. doi: 10.1161/01.cir.0000035037.11968.5c. [DOI] [PubMed] [Google Scholar]
- 11.Achilli A, Sassara M, Ficili S, Pontillo D, Achilli P, Alessi C, et al. Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and ‘narrow’ QRS. J Am Coll Cardiol. 2003;42:2117–2124. doi: 10.1016/j.jacc.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Brecker SJ, Xiao HB, Sparrow J, Gibson DG. Effects of dual-chamber pacing with short atrioventricular delay in dilated cardiomyopathy. Lancet. 1992;340:1308–1312. doi: 10.1016/0140-6736(92)92492-x. [DOI] [PubMed] [Google Scholar]
- 13.Bax JJ, Abraham T, Barold SS, Breithardt OA, Fung JW, Garrigue S, et al. Cardiac resynchronization therapy: Part 1 – issues before device implantation. J Am Coll Cardiol. 2005;46:2153–2167. doi: 10.1016/j.jacc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol. 2005;12:687–695. doi: 10.1016/j.nuclcard.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 15.Trimble M, Borges-Neto S, Smallheiser S, Chen J, Honeycutt EF, Shaw LK, et al. Evaluation of left ventricular mechanical dyssynchrony as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging in patients with left ventricular dysfunction and conduction disturbances. J Nuc Cardiol. 2007;14:298–307. doi: 10.1016/j.nuclcard.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 16.Henneman MM, Chen J, Ypenburg C, Dibbets P, Bleeker GB, Boersma E, et al. Phase analysis of gated myocardial perfusion single-photon emission computed tomography compared with tissue Doppler imaging for the assessment of left ventricular dyssynchrony. J Am Coll Cardiol. 2007;49:1708–1714. doi: 10.1016/j.jacc.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 17.Henneman M, Chen J, Dibbets-Schneider P, Stokkel M, Bleeker G, Ypenburg C, et al. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J Nucl Med. 2007;48:1104–1111. doi: 10.2967/jnumed.107.039925. [DOI] [PubMed] [Google Scholar]
- 18.Pitzalis MV, Iacoviello M, Romito R, Massari F, Rizzon B, Luzzi G, et al. Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. J Am Coll Cardiol. 2002;40:1615–1622. doi: 10.1016/s0735-1097(02)02337-9. [DOI] [PubMed] [Google Scholar]
- 19.Pitzalis MV, Iacoviello M, Romito R, Guida P, De Tommasi E, Luzzi G, et al. Ventricular asynchrony predicts a better outcome in patients with chronic heart failure receiving cardiac resynchronization therapy. J Am Coll Cardiol. 2005;45:65–69. doi: 10.1016/j.jacc.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 20.Marcus GM, Rose E, Viloria EM, Schafer J, De Marco T, Saxon LA, et al. Septal to posterior wall motion delay fails to predict reverse remodeling or clinical improvement in patients undergoing cardiac resynchronization therapy. J Am Coll Cardiol. 2005;46:2208–2214. doi: 10.1016/j.jacc.2005.05.095. [DOI] [PubMed] [Google Scholar]
- 21.Penicka M, Bartunek J, De Bruyne B, Vanderheyden M, Goethals M, De Zutter M, et al. Improvement of left ventricular function after cardiac resynchronization therapy is predicted by tissue Doppler imaging echocardiography. Circulation. 2004;109:978–983. doi: 10.1161/01.CIR.0000116765.43251.D7. [DOI] [PubMed] [Google Scholar]
- 22.Ansalone G, Giannantoni P, Ricci R, Trambaiolo P, Laurenti A, Fedele F, et al. Doppler myocardial imaging in patients with heart failure receiving biventricular pacing treatment. Am Heart J. 2001;142:881–896. doi: 10.1067/mhj.2001.117324. [DOI] [PubMed] [Google Scholar]
- 23.Garrigue S, Reuter S, Labeque JN, Jais P, Hocini M, Shah DC, et al. Usefulness of biventricular pacing in patients with congestive heart failure and right bundle branch block. Am J Cardiol. 2001;88:1436–1441. A8. doi: 10.1016/s0002-9149(01)02131-2. [DOI] [PubMed] [Google Scholar]
- 24.Bordachar P, Lafitte S, Reuter S, Sanders P, Jais P, Haissaguerre M, et al. Echocardiographic parameters of ventricular dyssynchrony validation in patients with heart failure using sequential biventricular pacing. J Am Coll Cardiol. 2004;44:2157–2165. doi: 10.1016/j.jacc.2004.08.065. [DOI] [PubMed] [Google Scholar]
- 25.Yu CM, Chau E, Sanderson JE, Fan K, Tang MO, Fung WH, et al. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation. 2002;105:438–445. doi: 10.1161/hc0402.102623. [DOI] [PubMed] [Google Scholar]
- 26.Bax JJ, Marwick TH, Molhoek SG, Bleeker GB, van Erven L, Boersma E, et al. Left ventricular dyssynchrony predicts benefit of cardiac resynchronization therapy in patients with end-stage heart failure before pacemaker implantation. Am J Cardiol. 2003;92:1238–1240. doi: 10.1016/j.amjcard.2003.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Yu CM, Fung WH, Lin H, Zhang Q, Sanderson JE, Lau CP. Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol. 2003;91:684–688. doi: 10.1016/s0002-9149(02)03404-5. [DOI] [PubMed] [Google Scholar]
- 28.Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834–1840. doi: 10.1016/j.jacc.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Notabartolo D, Merlino JD, Smith AL, DeLurgio DB, Vera FV, Easley KA, et al. Usefulness of the peak velocity difference by tissue Doppler imaging technique as an effective predictor of response to cardiac resynchronization therapy. Am J Cardiol. 2004;94:817–820. doi: 10.1016/j.amjcard.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 30.Yu CM, Fung JW, Zhang Q, Chan CK, Chan YS, Lin H, et al. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation. 2004;110:66–73. doi: 10.1161/01.CIR.0000133276.45198.A5. [DOI] [PubMed] [Google Scholar]
- 31.Sogaard P, Egeblad H, Kim WY, Jensen HK, Pedersen AK, Kristensen BO, et al. Tissue Doppler imaging predicts improved systolic performance and reversed left ventricular remodeling during long-term cardiac resynchronization therapy. J Am Coll Cardiol. 2002;40:723–730. doi: 10.1016/s0735-1097(02)02010-7. [DOI] [PubMed] [Google Scholar]
- 32.Sogaard P, Egeblad H, Pedersen AK, Kim WY, Kristensen BO, Hansen PS, et al. Sequential versus simultaneous biventricular resynchronization for severe heart failure: evaluation by tissue Doppler imaging. Circulation. 2002;106:2078–2084. doi: 10.1161/01.cir.0000034512.90874.8e. [DOI] [PubMed] [Google Scholar]
- 33.Breithardt OA, Stellbrink C, Herbots L, Claus P, Sinha AM, Bijnens B, et al. Cardiac resynchronization therapy can reverse abnormal myocardial strain distribution in patients with heart failure and left bundle branch block. J Am Coll Cardiol. 2003;42:486–494. doi: 10.1016/s0735-1097(03)00709-5. [DOI] [PubMed] [Google Scholar]
- 34.Sun JP, Chinchoy E, Donal E, Popovic ZB, Perlic G, Asher CR, et al. Evaluation of ventricular synchrony using novel Doppler echocardiographic indices in patients with heart failure receiving cardiac resynchronization therapy. J Am Soc Echocardiogr. 2004;17:845–850. doi: 10.1016/j.echo.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Popovic ZB, Grimm RA, Perlic G, Chinchoy E, Geraci M, Sun JP, et al. Noninvasive assessment of cardiac resynchronization therapy for congestive heart failure using myocardial strain and left ventricular peak power as parameters of myocardial synchrony and function. J Cardiovasc Electrophysiol. 2002;13:1203–1208. doi: 10.1046/j.1540-8167.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- 36.Dohi K, Suffoletto MS, Schwartzman D, Ganz L, Pinsky MR, Gorcsan J., III Utility of echocardiographic radial strain imaging to quantify left ventricular dyssynchrony and predict acute response to cardiac resynchronization therapy. Am J Cardiol. 2005;96:112–116. doi: 10.1016/j.amjcard.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Gorcsan J, III, Kanzaki H, Bazaz R, Dohi K, Schwartzman D. Usefulness of echocardiographic tissue synchronization imaging to predict acute response to cardiac resynchronization therapy. Am J Cardiol. 2004;93:1178–1181. doi: 10.1016/j.amjcard.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 38.Yu CM, Zhang Q, Fung JW, Chan HC, Chan YS, Yip GW, et al. A novel tool to assess systolic asynchrony and identify responders of cardiac resynchronization therapy by tissue synchronization imaging. J Am Coll Cardiol. 2005;45:677–684. doi: 10.1016/j.jacc.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Kapetanakis S, Kearney MT, Siva A, Gall N, Cooklin M, Monaghan MJ. Real-time three-dimensional echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation. 2005;112:992–1000. doi: 10.1161/CIRCULATIONAHA.104.474445. [DOI] [PubMed] [Google Scholar]
- 40.Akesson L, Svensson A, Edenbrandt L. Operator dependent variability in quantitative analysis of myocardial perfusion images. Clin Physiol Funct Imaging. 2004;24:374–379. doi: 10.1111/j.1475-097X.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- 41.Bleeker GB, Kaandorp TA, Lamb HJ, Boersma E, Steendijk P, de Roos A, et al. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113:969–976. doi: 10.1161/CIRCULATIONAHA.105.543678. [DOI] [PubMed] [Google Scholar]
- 42.Ypenburg C, Schalij MJ, Bleeker GB, Steendijk P, Boersma E, Dibbets-Schneider P, et al. Extent of viability to predict response to cardiac resynchronization therapy in ischemic heart failure patients. J Nucl Med. 2006;47:1565–1570. [PubMed] [Google Scholar]