Abstract
Background
Using phase analysis of gated single photon emission computed tomography (SPECT) imaging, we examined the relation between myocardial perfusion, degree of electrical dyssynchrony, and degree of SPECT-derived mechanical dyssynchrony in patients with left ventricular (LV) dysfunction.
Methods and Results
We retrospectively examined 125 patients with LV dysfunction and ejection fraction of 35% or lower. Fourier analysis converts regional myocardial counts into a continuous thickening function, allowing resolution of phase of onset of myocardial thickening. The SD of LV phase distribution (phase SD) and histogram bandwidth describe LV phase dispersion as a measure of dyssynchrony. Heart failure (HF) patients with perfusion abnormalities ities have higher degrees of dyssynchrony measured by median phase SD (45.5° vs 27.7°, P < .0001) and bandwidth (117.0° vs 73.0°, P = .0006). HF patients with prolonged QRS durations have higher degrees of dyssynchrony measured by median phase SD (54.1° vs 34.7°, P < .0001) and bandwidth (136.5° vs 99.0°, P = .0005). Mild to moderate correlations exist between QRS duration and phase analysis indices of phase SD (r = 0.50) and bandwidth (r = 0.40). Mechanical dyssynchrony (phase SD >43°) was 43.2%.
Conclusions
HF patients with perfusion abnormalities or prolonged QRS durations QRS durations have higher degrees of mechanical dyssynchrony. Gated SPECT myocardial perfusion imaging can quantify myocardial function, perfusion, and dyssynchrony and may help in evaluating patients for cardiac resynchronization therapy.
Keywords: Mechanical dyssynchrony, single photon emission computed tomography imaging, left ventricular dysfunction, cardiac resynchronization therapy, heart failure
Heart failure (HF) affects more than 5 million in the United States. Approximately 550,000 new cases diagnosed annually, and acute decompensated HF accounts for over 1 million hospital admissions each year.1-3
Cardiac resynchronization therapy (CRT) is approved for the treatment of patients with advanced HF symptoms, ejection fraction of 35% or lower, and prolonged QRS duration. Several studies have shown improvements in quality of life, functional class, exercise capacity, and ejection fraction for patients who received CRT in addition to optimal medical therapy.4-7 Two recent studies have documented that CRT provides benefits beyond symptomatic improvement and have shown mortality benefits for patients undergoing CRT.8,9
Approximately 30% of patients who meet the QRS criteria do not benefit from CRT.10 Electrical dyssynchrony as determined by QRS duration may not reflect the degree of left ventricular (LV) mechanical chrony present, nor is it necessarily the best predict or patient response to CRT.11-13 Therefore efforts have been made to improve patient selection for CRT by precisely defining LV mechanical dyssynchrony. These efforts have previously focused on advanced echocardiographic techniques.14
A novel method to quantify LV mechanical dyssynchrony has recently been reported using phase analysis of gated single photon emission computed tomography (SPECT) myocardial perfusion imaging.15-17 Our study is the first to use the phase analysis technique to compare the degree of mechanical dyssynchrony in patients with LV dysfunction with and without myocardial perfusion abnormalities, to evaluate the relation between myocardial perfusion and mechanical dyssynchrony, to evaluate the relation between the degree of electrical dyssynchrony as measured by QRS duration and the degree of SPECT-derived mechanical dyssynchrony, and to describe the prevalence of mechanical dyssynchrony as measured by the phase analysis in patients with significant LV dysfunction.
METHODS
Patient Selection
We retrospectively examined 125 consecutive patients with LV dysfunction and ejection fraction of 35% or lower. All patients were referred for routine gated SPECT myocardial perfusion imaging at Duke University Medical Center (Durham, NC).
HF patients with myocardial perfusion abnormalities were defined as having an ejection fraction of 35% or lower with evidence of perfusion abnormalities on resting SPECT myocardial perfusion imaging. Patients were also classified as having perfusion abnormalities if there were perfusion abnormalities present only in the stress portion of the gated SPECT myocardial perfusion imaging if subsequent cardiac catheterization demonstrated significant coronary artery disease in a minimum of 1 major epicardial coronary artery. Significant coronary artery disease was defined as an obstruction of 50% or greater in an epicardial coronary artery.
Dyssynchrony Evaluation Using Phase Analysis of Gated SPECT Perfusion Imaging
Each patient enrolled underwent standard electrocardiography-gated SPECT myocardial perfusion imaging for clinical indications. Data were acquired at 8 frames per cardiac cycle. The short-axis data sets were generated by Butterworth filtering followed by filtered backprojection reconstruction and oblique reorientation via standard protocols. The gated poststress imaging was used for the phase analysis. Resting images were not gated at the time of image acquisition and were not available for use in the dyssynchrony determinations.
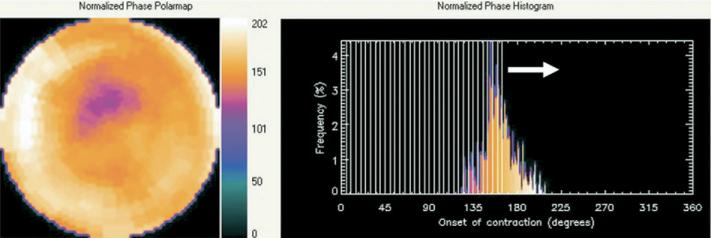
A method to extract amplitude (representing the degree of systolic wall thickening) and phase (representing the timing of the onset of mechanical contraction) from regional LV count changes obtained during gated SPECT myocardial perfusion imaging has been previously reported. The method has been incorporated into a clinical application to quantify dyssynchrony, and a comprehensive description of the phase analysis method has recently been presented.15 In brief, 3-dimensional count distributions are extracted from each of the 8 LV short-axis data sets and submitted to Fourier phase analysis. The analysis applies 1-dimensional fast Fourier transform to the count variation over time of each voxel to calculate the phase of the first Fourier harmonics. Then, the analysis generates a 3-dimensional phase distribution describing the timing of the LV regional onset of mechanical contraction as a function of degrees, with the 360° range representing the entire length of the R-R interval. Once the phase distribution is generated, it is displayed on the polar map as well as in histogram format. An example of the phase histogram is shown in Figure 1. The x-axis represents the timing of 1 cardiac cycle (R-R interval) in degrees. The y-axis represents the percent of myocardium that demonstrated the onset of mechanical contraction during any particular time of the cardiac cycle.
Figure 1.
Representative phase histogram. The x-axis represents the timing of 1 cardiac cycle (R-R interval) in degrees. The y-axis represents the percent of myocardium demonstrating the onset of mechanical contraction during any particular phase of the cardiac cycle. The color maps have 256 levels, with the minimum level corresponding to black and the maximum level corresponding to white. The normalized phase polar map and histogram are displayed with 0° as black and the maximum phase as white.
Two quantitative indices are calculated from the phase distribution to describe the phase dispersion of the LV regional onset of mechanical contraction to quantify dyssynchrony. Phase SD is the SD of the phase distribution. Histogram bandwidth represents the duration of the cardiac cycle, during which 95% of the myocardium initiates contraction. A higher phase SD or bandwidth corresponds to increased dyssynchrony. The software has been implemented in the Emory Cardiac Toolbox (Syntermed, Atlanta, Ga) for analysis of gated SPECT myocardial perfusion imaging.
Evaluation of Mechanical Dyssynchrony in Patients with LV Dysfunction
We compared the distributions of the phase analysis results in HF patients with and without myocardial perfusion abnormalities and in HF patients with and without prolonged QRS durations. The degree of perfusion abnormalities on resting SPECT perfusion imaging was quantified by use of the summed rest perfusion score. The degree of myocardial ischemia on SPECT perfusion imaging was quantified by use of the summed difference perfusion score.
Statistical Methods
Baseline characteristics and SPECT imaging phase indices were examined. The Wilcoxon rank sum test was used to determine whether there were any significant differences in the distribution of the 4 quantitative phase analysis indices describing the degree of mechanical dyssynchrony between the cohorts studied. Results presented include mean values ± SD and medians (25th and 75th percentiles) for each cohort of patients studied. Correlation coefficients were used to examine the relation between myocardial perfusion defects as measured by the summed rest score, between myocardial ischemia as measured by the summed difference score, and between QRS duration and the degree of mechanical dyssynchrony as quantified by use of the phase analysis indices.
RESULTS
Baseline characteristics are shown in Table 1. Of the 125 patients with LV dysfunction, 98 had myocardial perfusion abnormalities, 77 had QRS durations of less than 120 milliseconds, and 48 had QRS durations of 120 milliseconds or greater. The median age was 65 years, and 71.2% of patients were men. The median ejection fraction was 26%, and the median end-systolic volume was 146.0 mL.
Table 1.
Baseline characteristics
| Demographics | LV dysfunction (N = 125) |
|---|---|
| Age [median (25th percentile, 75th percentile)] (y) | 65 (53, 74) |
| Male | 71.2 |
| Race | |
| White | 48.8 |
| Black | 47.2 |
| Other | 4.0 |
| Medical comorbidities | |
| CAD | 71.2 |
| CABG | 35.2 |
| Diabetes | 49.6 |
| Hypertension | 84.0 |
| Chronic renal insufficiency | 40.0 |
| ECG data | |
| QRS duration [median (25th percentile, 75th percentile)] (ms) | 106 (92, 140) |
| Sinus rhythm | 75.2 |
| Paced rhythm | 15.2 |
| Atrial fibrillation | 8.8 |
| LBBB | 11.2 |
| RBBB | 3.2 |
| IVCD | 18.4 |
| Gated SPECT data [median (25th percentile, 75th percentile)] | |
| EF (%) | 26 (21, 31) |
| End-diastolic volume (mL) | 217 (173, 269) |
| End-systolic volume (mL) | 146 (106, 190) |
| LV mass (g) | 206 (175, 231) |
Values are expressed as percentages, unless indicated otherwise. CAD, Coronary artery disease; CABG, coronary artery bypass graft; ECG, electrocardiogram; LBBB, left bundle branch block; RBBB, right bundle branch block; IVCD, intraventricular conduction defect; EF, ejection fraction.
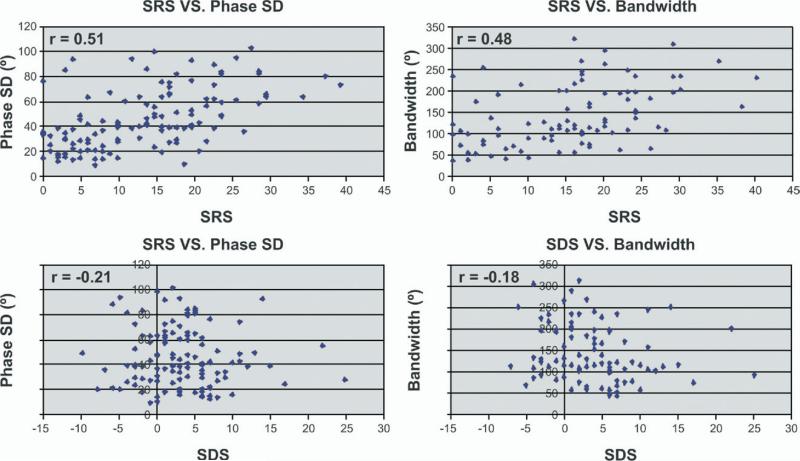
Table 2 evaluates the relation between myocardial perfusion abnormalities and mechanical dyssynchrony as quantified by the phase analysis indices. HF patients with myocardial perfusion abnormalities showed higher levels of mechanical dyssynchrony than patients without perfusion abnormalities as measured by the median phase SD (45.5° vs 27.8°, P < .0001) and bandwidth (117.0° vs 73.0°, P = .0006). There were moderate correlations between the sum rest score and the degree of mechanical dyssynchrony as measured by the phase SD and bandwidth in patients with myocardial perfusion abnormalities (correlation coeffi cients, 0.51 and 0.48, respectively) (Figure 2). In patients with myocardial perfusion abnormalities, there were weak correlations between the degree of myocardial ischemia and the degree of mechanical dyssynchrony as described by phase SD (r = –0.21) and bandwidth (r = –0.18).
Table 2.
Phase indices in patients with LV dysfunction with or without myocardial perfusion abnormalities
| With perfusion abnormalities (n = 98) | Without perfusion abnormalities (n = 27) | Correlation coefficient |
|||
|---|---|---|---|---|---|
| P value* | SRS† | SDS† | |||
| Phase SD (°) | |||||
| Mean ± SD | 49.3 ± 24.3 | 28.0 ± 13.3 | <.0001 | 0.51 | –0.21 |
| Median (25th percentile, 75th percentile) | 45.5 (30.0, 65.6) | 27.7 (18.9, 36.9) | |||
| Bandwidth (°) | |||||
| Mean ± SD | 138.7 ± 71.2 | 88.9 ± 45.8 | .0006 | 0.48 | –0.18 |
| Median (25th percentile, 75th percentile) | 117.0 (83.0, 197.0) | 73.0 (59.0, 110.0) | |||
SRS, Summed rest score; SDS, summed difference score.
P value comparing phase analysis indices in subjects with and without myocardial perfusion abnormalities.
Correlation coefficients between SRS and SSS and the individual phase analysis indices.
Figure 2.
Correlation of mechanical dyssynchrony as measured by phase SD and bandwidth with resting myocardial perfusion abnormalities (summed rest score [SRS]) and myocardial ischemia (summed difference score [SDS]). The correlation between SRS and phase SD (r = 0.51) and bandwidth (r = 0.48) is shown in the upper two plots. The correlation between SDS and phase SD (r = –0.21) and bandwidth (r = –0.18) is shown in the lower two plots.
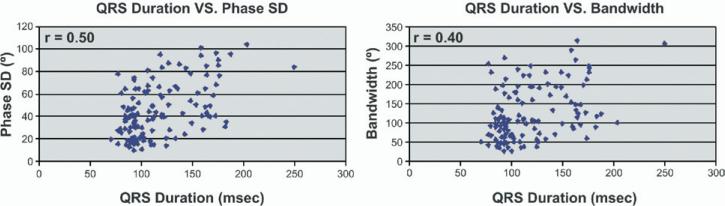
The comparisons of the phase analysis indices in patients with LV dysfunction and prolonged QRS durations with the phase analysis indices in patients with LV dysfunction and normal QRS durations are presented in Table 3. Patients with prolonged QRS durations had, on average, higher levels of mechanical dyssynchrony as described by median phase SD (54.1° vs 34.7°, P < .0001) and bandwidth (136.5° vs 99.0°, P = .0005) than patients with normal QRS durations. However, there were only mild to moderate correlations between QRS duration and the degree of mechanical dyssynchrony as described by phase SD (r = 0.50) and bandwidth (r = 0.40) (Figure 3).
Table 3.
Comparison of phase indices in patients with LV dysfunction with and without QRS prolongation
| QRS <120 ms (n = 77) | QRS ≥120 ms (n = 48) | P value | |
|---|---|---|---|
| Phase SD (°) | |||
| Mean ± SD | 36.9 ± 19.9 | 57.2 ± 24.8 | <.0001 |
| Median (25th percentile, 75th percentile) | 34.7 (19.3, 47.5) | 54.1 (38.3, 82.2) | |
| Bandwidth (°) | |||
| Mean ± SD | 111.2 ± 62.7 | 154.8 ± 72.0 | .0005 |
| Median (25th percentile, 75th percentile) | 99.0 (62.0, 141.0) | 136.5 (100.5, 203.5) |
Figure 3.
Comparison of electrical dyssynchrony as measured by QRS duration and mechanical dyssynchrony as measured by phase analysis indices in patients with LV dysfunction. The correlation between QRS duration and phase SD and bandwidth were 0.50 and 0.40, respectively.
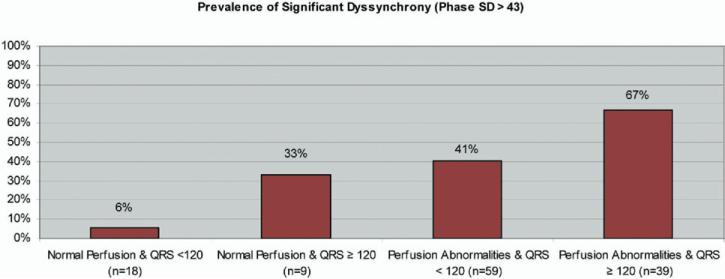
Figure 4 shows the prevalence of significant mechanical dyssynchrony (phase SD >43°) in the cohort of HF patients studied with and without myocardial perfusion abnormalities stratified by QRS duration. Patients with myocardial perfusion abnormalities have a higher prevalence of dyssynchrony than patients with normal myocardial perfusion (51.0% vs 14.8%). Similarly, there is a higher prevalence of dyssynchrony in HF patients with prolonged QRS durations than in patients with QRS durations of less than 120 milliseconds (60.4% vs 32.5%).
Figure 4.
Prevalence of significant dyssynchrony (phase SD >43°) in HF patients with or without myocardial perfusion abnormalities stratified by QRS duration.
DISCUSSION
HF patients with an ejection fraction of 35% or lower with advanced symptoms and prolonged QRS duration are eligible to receive CRT with the use of a biventricular pacemaker. For this group of patients, improvements in quality of life, functional class, exercise capacity, ejection fraction, and mortality rate have been shown.4-9 However, a significant percentage of patients who meet these eligibility requirements do not improve with CRT.10 Therefore efforts have been made to more accurately measure dyssynchrony in the hopes of improving patient selection for CRT. The majority of these efforts have previously focused on advanced echocardiographic techniques.14
This study presents data using a novel method to quantify LV mechanical dyssynchrony by use of phase analysis of gated SPECT imaging. The validation of the phase analysis began in studies describing normal databases of the phase indices, demonstrating a correlation with tissue Doppler echocardiography, and demonstrating abnormal phase indices in patients with LV dysfunction and in patients with normal LV function who had conduction delays.15-17 Our study differs from those studies by comprehensively evaluating mechanical dyssynchrony in a separate, large cohort of patients with higher degrees of LV dysfunction. This study is the first to describe the relation between myocardial perfusion and mechanical dyssynchrony as quantified by SPECT imaging. Similarly, this study is the first to describe the relation between electrical dyssynchrony as measured by QRS duration and SPECT-derived mechanical dyssynchrony indices, and this study is the first to begin to quantify the prevalence of mechanical dyssynchrony in patients with significant LV dysfunction with and without QRS prolongation.
The use of gated SPECT myocardial perfusion imaging has several potential advantages in the evaluation of dyssynchrony in patients with LV dysfunction. The technique is automated and takes less than 1 minute to perform. This is in contrast to currently used echocardiographic techniques that require significant offline data processing and analysis and have varying degrees of reproducibility.14 Second, the addition of a dyssynchrony evaluation in patients having gated SPECT perfusion imaging performed for other routine indications would be very cost-effective and could potentially obviate the need for additional diagnostic testing. Finally, concomitant myocardial perfusion information may be helpful in predicting patient response to CRT.18-21
Several studies have shown an inverse relation between the degree of myocardial perfusion abnormalities and response to CRT. The degree of viability as measured by fluorine 18 fluorodeoxyglucose SPECT perfusion imaging and the degree of myocardial perfusion abnormalities demonstrated on thallium 201 or technetium 99m SPECT perfusion imaging has been shown to predict patient response to CRT.18,19 In addition, 2 studies using delayed-enhancement cardiac magnetic resonance imaging have shown a relation between myocardial scar tissue and patient response to CRT.20,21 Specifically, patients with posterolateral scar tissue seen on delayed-enhancement magnetic resonance imaging were much less likely to respond to CRT than patients without posterolateral scar (14% vs 81%).
This study is the first to describe the effect of myocardial perfusion abnormalities on the quantification of mechanical dyssynchrony using the phase analysis of gated SPECT imaging. We showed increased levels of dyssynchrony among HF patients with myocardial perfusion abnormalities compared with patients without myocardial perfusion abnormalities. This has important implications. Higher levels of dyssynchrony have been shown to predict response to CRT; at the same time, higher levels of myocardial perfusion abnormalities have been shown to prevent response to CRT.18-23 Given that patients with myocardial perfusion abnormalities also have higher degrees of mechanical dyssynchrony as measured by gated SPECT imaging, a direct relation between the degree of dyssynchrony and the likelihood of response to CRT in HF patients cannot be established. For example, patients with significant myocardial perfusion abnormalities may not respond to CRT irrespective of the degree of dyssynchrony present.
Henneman et al23 recently showed that a phase SD of 43° demonstrated a sensitivity and specificity of 74% for predicting response to CRT in a pilot study of 42 patients. The degree of myocardial perfusion abnormalities was not reported. The relative importance of the degree of dyssynchrony as measured by phase analysis of gated SPECT perfusion imaging and the degree or location of myocardial perfusion abnormalities with regard to the likelihood of patient response to CRT is not known. A model to predict patient response to CRT that uses concomitant dyssynchrony information and myocardial perfusion information may eventually prove superior to models using dyssynchrony information alone and may improve on the ability of gated SPECT imaging to predict response to CRT.
There are 2 groups of patients who could potentially derive significant benefits from more precise measurement of dyssynchrony and improved selection of patients for CRT. The first comprises patients currently eligible for CRT who do not benefit from the procedure. These patients could be spared from the risks associated with invasive implantation of CRT devices. Reynolds et al22 reported a 10.5% acute complication rate among Medicare patients who received CRT devices in 2003. This included a 1.1% risk of death during the initial hospitalization for CRT. An even higher 28% acute complication rate was associated with CRT implantation and a 46% 6-month complication rate was reported in the MIRACLE ICD (Multicenter InSync ICD [implantable cardioverter defibrillator] Randomized Clinical Evaluation) trial.6 In addition, hospital costs associated with CRT implantation are approximately $42,000.22 More accurate selection of patients for CRT will allow for more appropriate allocation of these health care resources.
The second group of patients who could potentially derive significant benefits from more precise measurement of dyssynchrony and improved selection of patients for CRT are those who are not currently eligible for CRT but who have significant mechanical dyssynchrony. This could include patients with normal QRS durations, patients with New York Heart Association (NYHA) class II symptoms, and patients with mild reductions in LV systolic function.
Previous echocardiographic studies have shown a weak correlation between the degree of electrical dyssynchrony as measured by QRS duration and the degree of mechanical dyssynchrony as measured by tissue Doppler echocardiography.24 Our study demonstrates that although patients with prolonged QRS durations have, on average, higher degrees of dyssynchrony as measured by the phase analysis, there is a weak correlation between mechanical dyssynchrony and QRS duration. We showed that 32.5% of patients with normal QRS durations have a phase SD of at least 43°. These data support the hypothesis that electrical dyssynchrony does not adequately describe the degree of mechanical dyssynchrony present in HF patients with LV dysfunction. Therefore QRS duration may not represent the optimal measure of dyssynchrony for selection of patients for CRT.
Two small studies have evaluated the use of CRT in patients with normal QRS durations and have shown benefits in clinical status and ventricular reverse remodeling in these patients.25,26 In addition, a small series of patients with mild NYHA class II symptoms were shown to have similar levels of baseline dyssynchrony when compared with patients with NYHA class III/IV symptoms, and they derived similar improvements in LV ejection fraction and end-systolic volume with CRT.27 Similarly, patients with an ejection fraction between 35% and 45% were shown to derive improvements in ventricular size, ventricular function, and functional class similar to patients with an ejection fraction of 35% or lower in a prospective evaluation of 15 patients.28 Larger outcomes studies need to be completed to define the role of CRT in patients not currently eligible for CRT, which includes patients with narrow QRS durations, mild symptoms, or mildly reduced ventricular function.
Alternative nuclear techniques to quantify dyssynchrony include gated blood pool imaging and the use of SPECT or traditional techniques.29,30 These techniques offer the potential advantage of increased count rates. Limitations include the lack of availability of concomitantly obtained myocardial perfusion data, which may be important in selecting patients for CRT as previously discussed. In addition, SPECT myocardial perfusion imaging has more widespread use in evaluating patients with LV dysfunction. Additional comparison studies with outcomes will be needed to guide recommendations on future use of these technologies.
There are limitations to our study. Although our study describes the relation between myocardial perfusion abnormalities and the quantification of dyssynchrony, it does not define a model using concomitant perfusion information with dyssynchrony information to predict response to CRT. Outcomes data that are not available at this time are needed for that determination. Second, the dyssynchrony evaluations in this study were done on poststress gated SPECT imaging. The majority of studies evaluating dyssynchrony have been performed using resting imaging by use of echocardiographic or SPECT techniques. This may have affected the results of dyssynchrony determinations, as the relation between poststress and resting phase analysis results is not known. However, the time delay between stress and perfusion imaging in this study, as well as the lack of significant relationships demonstrated between the degree of ischemia and the degree of dyssynchrony, likely mitigates these potential effects. Third, the study represents dyssynchrony determinations on SPECT studies acquired at 8 frames per cardiac cycle. This implies a limited temporal resolution to the technique. However, because of the use of the Fourier transform, the actual temporal resolution is significantly higher. A simulation study has recently reported that the actual temporal resolution approximates 1/64th of the cardiac cycle for clinically relevant count distributions.31 Lastly, our study included a minority of patients who had paced rhythms. Given the retrospective nature of the study, the pacing type was not available for analysis. The effect on the analyses in this article is not known.
Despite these limitations, we believe that our study further builds on the data available on the use of phase analysis of gated SPECT imaging to quantify dyssynchrony. Important contributions include defining the relation between myocardial perfusion and dyssynchrony and between mechanical and electrical dyssynchrony in patients with significant LV dysfunction. Furthermore, we began to define the prevalence of significant dyssynchrony in various cohorts of patients with LV dysfunction.
In summary, gated SPECT imaging has the ability to simultaneously quantify LV dyssynchrony, myocardial perfusion, and LV function. A model incorporating dyssynchrony data as measured by the quantitative phase analysis indices in conjunction with myocardial perfusion and functional data may improve the discriminatory ability of gated SPECT imaging for the selection of patients for CRT.
Acknowledgment
Dr Garcia has ownership interest in and serves as a consultant/advisory board member to Syntermed and receives royalties from the sale of clinical software used as part of this research. The other authors have indicated they have no financial conflicts of interest.
This study was funded by a research grant from the Medtronic-Duke Strategic Alliance, and Dr Trimble is the primary investigator.
References
- 1.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: A report from the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2005;46:1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association [January 7, 2008];Heart Disease and stroke statistics: 2005 update. Available from: URL: http://www.americanheart.org/downloadable/heart/1105390918119HDSStats2005Update.pdf.
- 3.O'Connell JB, Bristow MR. Economic impact of heart failure in the United States: Time for a different approach. J Heart Lung Transplant. 1994;13:S107–12. [PubMed] [Google Scholar]
- 4.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 5.Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42:1454–9. doi: 10.1016/s0735-1097(03)01042-8. [DOI] [PubMed] [Google Scholar]
- 6.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: The MIRACLE ICD Trial. JAMA. 2003;289:2685–94. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 7.McAlister F, Ezekowitz J, Wiebe N, Rowe B, Spooner C, Crumley E, et al. Cardiac resynchronization therapy for congestive heart failure. Evid Rep Technol Assess (Summ) 2004;106:1–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, DeMarco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 9.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 10.Leclercq C, Kass DA. Retiming the failing heart: Principles and current clinical status of cardiac resynchronization. J Am Coll Cardiol. 2002;39:194–201. doi: 10.1016/s0735-1097(01)01747-8. [DOI] [PubMed] [Google Scholar]
- 11.Leclercq C, Faris O, Tunin R, Johnson J, Kato R, Evans F, et al. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation. 2002;106:1760–3. doi: 10.1161/01.cir.0000035037.11968.5c. [DOI] [PubMed] [Google Scholar]
- 12.Achilli A, Sassara M, Ficili S, Pontillo D, Achilli P, Alessi C, et al. Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J Am Coll Cardiol. 2003;42:2117–24. doi: 10.1016/j.jacc.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Brecker SJ, Xiao HB, Sparrow J, Gibson DG. Effects of dual-chamber pacing with short atrioventricular delay in dilated cardiomyopathy. Lancet. 1992;340:1308–12. doi: 10.1016/0140-6736(92)92492-x. [DOI] [PubMed] [Google Scholar]
- 14.Bax JJ, Abraham T, Barold SS, Breithardt OA, Fung JW, Garrigue S, et al. Cardiac resynchronization therapy: Part 1—issues before device implantation. J Am Coll Cardiol. 2005;46:2153–67. doi: 10.1016/j.jacc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: Development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol. 2005;12:687–95. doi: 10.1016/j.nuclcard.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 16.Trimble M, Borges-Neto S, Smallheiser S, Chen J, Honeycutt EF, Shaw LK, et al. Evaluation of left ventricular mechanical dyssynchrony as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging in patients with left ventricular dysfunction and conduction disturbances. J Nucl Cardiol. 2007;14:298–307. doi: 10.1016/j.nuclcard.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 17.Henneman MM, Chen J, Ypenburg C, Dibbets P, Bleeker GB, Boersma E, et al. Phase analysis of gated myocardial perfusion single-photon emission computed tomography compared with tissue Doppler imaging for the assessment of left ventricular dyssynchrony. J Am Coll Cardiol. 2007;49:1708–14. doi: 10.1016/j.jacc.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 18.Ypenburg C, Schalij M, Bleeker GB, Steendijk P, Boersma E, Dibbets-Schneider P, et al. Extent of viability to predict response to cardiac resynchronization therapy in ischemic heart failure patients. J Nucl Med. 2006;47:1565–70. [PubMed] [Google Scholar]
- 19.Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J. 2007;153:105–12. doi: 10.1016/j.ahj.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Bleeker GB, Kaandorp TA, Lamb HJ, Boersma E, Steendijk P, de Roos A, et al. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113:969–76. doi: 10.1161/CIRCULATIONAHA.105.543678. [DOI] [PubMed] [Google Scholar]
- 21.White JA, Yee R, Yuan X, Krahn A, Skanes A, Parker M, et al. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol. 2006;48:1953–60. doi: 10.1016/j.jacc.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds MR, Cohen DJ, Kugelmass AD, Brown PP, Becker ER, Culler SD, et al. The frequency and incremental cost of major complications among Medicare beneficiaries receiving implantable cardioverter-defibrillators. J Am Coll Cardiol. 2006;47:2493–7. doi: 10.1016/j.jacc.2006.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henneman M, Chen J, Dibbets-Schneider P, Stokkel MP, Bleeker GB, Ypenburg C, et al. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J Nucl Med. 2007;48:1104–11. doi: 10.2967/jnumed.107.039925. [DOI] [PubMed] [Google Scholar]
- 24.Bleeker GB, Schalij MJ, Molhoek SG, Verwey HF, Holman ER, Boersma E, et al. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004;15:544–9. doi: 10.1046/j.1540-8167.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- 25.Bleeker GB, Holman ER, Steendijk P, Boersma E, van der Wall EE, Schalij MJ, et al. Cardiac resynchronization therapy in patients with a narrow QRS complex. J Am Coll Cardiol. 2006;48:2243–50. doi: 10.1016/j.jacc.2006.07.067. [DOI] [PubMed] [Google Scholar]
- 26.Yu CM, Chan YS, Zhang Q, Yip GW, Chan CK, Kum LC, et al. Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J Am Coll Cardiol. 2006;48:2251–7. doi: 10.1016/j.jacc.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 27.Bleeker GB, Schalij MJ, Holman ER, Steendijk P, van der Wall EE, Bax JJ. Cardiac resynchronization therapy in patients with systolic left ventricular dysfunction and symptoms of mild heart failure secondary to ischemic or nonischemic cardiomyopathy. Am J Cardiol. 2006;98:230–5. doi: 10.1016/j.amjcard.2006.01.080. [DOI] [PubMed] [Google Scholar]
- 28.Fung JW, Zhang Q, Yip GW, Chan JY, Chan HC, Yu CM. Effect of cardiac resynchronization therapy in patients with moderate left ventricular systolic dysfunction and wide QRS complex: A prospective study. J Cardiovasc Electrophysiol. 2006;17:1288–92. doi: 10.1111/j.1540-8167.2006.00612.x. [DOI] [PubMed] [Google Scholar]
- 29.O'Connell JW, Schreck C, Moles M, Badwar N, DeMarco T, Olgin J, et al. A unique method by which to quantitate synchrony with equilibrium radionuclide angiography. J Nucl Cardiol. 2005;2:441–50. doi: 10.1016/j.nuclcard.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Harel F, Finnerty V, Gregoire J, Thibault B, Khairy P. Comparison of left ventricular contraction homogeneity index using SPECT gated blood pool imaging and planar phase analysis. J Nucl Cardiol. 2008;15:10–2. doi: 10.1016/j.nuclcard.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Faber TL, Cooke CD, Garcia EV. Temporal resolution of multiharmonic phase analysis of ECG-gated myocardial perfusion SPECT studies. J Nucl Cardiol. 2008;15:383–91. doi: 10.1016/j.nuclcard.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]