Abstract
OBJECTIVE
To evaluate cervical changes and delivery at term during pregnancy in rats after various progestin treatments.
STUDY DESIGN
Pregnant rats were treated by various routes and vehicles with progesterone (P4), 17-alpha-hydroxyprogesterone caproate (17P), R5020 and RU-486. Delivery time was determined and cervical ripening assessed in vivo by collagen light-induced fluorescence (LIF).
RESULTS
The cervix is rigid in P4 injection, 17P and vaginal R5020 groups versus controls. Vaginal P4 had no effect. RU-486 treatment softened the cervix during preterm delivery. Only s.c. P4, R5020 (s.c. and vaginal) and topical P4 in sesame and fish oil inhibits delivery. Delivery is not changed by s.c. 17P, vaginal P4, oral P4 and topical P4 in Replens®.
CONCLUSION
Inhibition of cervical ripening and delivery by progestins depends upon many factors including their properties, route of administration and the vehicle. This study suggests why present treatments for preterm labor are not efficacious.
Keywords: Cervix, 17-alpha-hydroxyprogesterone caproate, preterm labor, progesterone, rats
Introduction
Preterm birth (less than 37 completed weeks of gestation) is one of the major problems and challenges in obstetrics. The frequency of preterm births is about 12–13% in the USA and 5–9% in many other developed countries.1,2 Despite all efforts to reduce the number of preterm births the problem is continuing to escalate: Since 1990 the percentage of births delivered preterm has risen more than 20 percent and is 36 percent higher since the early 1980s in the USA.3 Preterm birth is not only a major determinant of neonatal and infant morbidity, including neurodevelopmental handicaps, chronic respiratory problems, intraventricular hemorrhage, infection, retrolental fibroplasia, and necrotizing enterocolitis , but also the single most important cause of perinatal mortality in North America, Europe and particularly in undeveloped countries.4 Additionally, the neonatal and long-term health care costs of preterm infants impose a considerable economic strain both on individual families and on healthcare costs (> $26.2 billion in 2005 in the USA).5
Both uterine and cervical functions play important roles in the onset and progression of term and preterm labor and delivery. The cervix undergoes dramatic changes throughout pregnancy and parturition, a process that is termed cervical ripening. From a firm, rigid and closed state, that is protecting the special milieu of the fetus from the environment, to a soft and easy-to-open state, that is essential for successful vaginal delivery. The cervix is dominated by fibrous connective tissue that is composed of an extracellular matrix which consists mostly of collagen (70 % type I and ~ 30 % type III6) with elastin and proteoglycans and a cellular portion that consists of smooth muscles, fibroblasts, epithelium and blood vessels. Cervical ripening is an active biochemical process, which occurs independent of uterine contractions. Studies have shown that cervical ripening is associated with a strong reorganization of the extracellular matrix, especially collagen: Not only the concentration decreases by 30–70 % but there is also a switch from insoluble to more soluble collagen.6,7 Ripening of the cervix is an inflammatory like reaction with infiltration of leukocytes, increase of cytokines (interleukin (IL)- 1 and IL-8) and an increase in metalloproteinases.8, 9,10 This process also seems to be at least partially regulated by steroid hormones (in particular progesterone (P4) and estrogen), as antiprogestins successfully induce cervical ripening.11,12,13 Other hormones and mediators shown to be involved in cervical ripening are dihydrotestosterone14, prostaglandins15, and local mediators such as platelet-activating factor16 and nitric oxide.15 Various methods have been used to evaluate cervical ripening and effects of progestins, including cervical length.17,18 We have used light-induced fluorescence (LIF) of the cervix to estimate changes in cervical collagen and effects of treatments.16,19 However the biochemical mechanisms that are responsible for the remarkable changes in the cervix remain poorly understood.
P4 has also long been considered as a candidate to regulate uterine contractility and cervical function and consequently the onset and progression of labor. Early studies also discussed the potential benefit of 17-α-hydroxyprogesterone caproate (17P), a synthetic caproate ester of the naturally occurring metabolite of P4, for the treatment or prevention of preterm labor.20,21 The interest in these treatments was renewed by two trials using intramuscular injections of 17P or prophylactic vaginal P4 for the prevention of preterm labor that confirmed the previous studies by showing a reduction of recurrent preterm birth. 22,23 In a comment on those trials, Keirse initiated a controversial debate on this topic where he indicated that “critical analysis of the reports provides no convincing evidence that either one of these treatments is worth pursuing outside the context of controlled research…”.24 Recently a number of large randomized control trials using different formulations and routes of administration of progestins have been used to study their effects on reducing preterm delivery.17,18,25,26,27 Because of the differences in study populations and drug treatments it is unclear if one formulation or route of administration is more effective in reducing preterm birth. Pregnant rats are exquisitely sensitive to changes in P4 with preterm delivery or prolonged gestation when P4 levels are manipulated or P4 receptor antagonists are used.28 The aim of this study was to assess cervical changes throughout pregnancy in rats and the timing of term and preterm delivery after various progestin treatments given by different routes and vehicles in hope of identifying better treatment regimens.
Material and Methods
Animals
Nonpregnant and timed-pregnant Sprague-Dawley rats (200–250 g) from Charles-River Laboratories (Wilmington, MA, USA) were delivered to our animal care facilities on day 12 of gestation (day 1 being the day when a sperm plug was observed). The animals were housed separately, with free access to food and water and maintained on a constant 12-hour light-dark cycle. Control pregnant rats were spontaneously delivering on day 22 and 23 of gestation. For the measurements with the collascope the animals were anaesthetized (i.p. injection) with a combination of xylazine (Gemini, Burns Veterinary Supply Inc, Rockville Center, NY, USA) and ketamine HCl (Ketaset; Fort Dodge Laboratories Inc, Fort Dodge, IO, USA). The animals were randomly allocated to one of the groups and sacrificed by carbon dioxide inhalation on day 3, 5, 7 and 10 postpartum or on day 25 of pregnancy in the groups with delayed delivery. All procedures were approved by the Animal Care and Use Committee of the St. Joseph’s Hospital and Medical Center in Phoenix.
Treatments
Prior to any treatment LIF measurements were made in control rats throughout pregnancy and postpartum to estimate the LIF profile during gestation (see Figure 1). Pregnant rats (N = 6/ group) were treated (see Figure 2), when not otherwise mentioned (see Figure 3), from day 13 of pregnancy until delivery. Single daily treatments were performed at 8 a.m. and twice a day treatments at 8 a.m. and 8 p.m. All single injections (4 mg P4, 10 mg 17P, 2 mg R5020) were by the subcutaneous route (s.c.) in sesame oil (0.2 ml), which was also used for the controls. Vaginal gels were applied twice a day with a blunt ball-top needle deep into the vagina. Crinone® was used for the P4 vaginal group (we used equivalent volumes of Crinone® for 2–15 mg P4/treatment, all data presented show the results of the highest dose (total daily dose of 30 mg P4 = 1/3 of a applicator of 8% Crinone® that contains 90 mg P4). Another group was treated vaginally with P4 (30 mg) in 0.5 ml fish oil that was soaked in a cotton plug, inserted each day and removed prior to insertion of fresh one. For the vaginal R5020 group micronized R5020 (1 mg/ treatment) was mixed into 0.18 ml of Replens®. The control rats for the vaginal groups were treated with Replens® (0.18 ml/ treatment). For oral P4 treatments (15 mg, bid, vehicle sesame oil or H2O, volume 1 ml) gavage was used. For topical P4 treatment (15 mg, bid, P4 in 1 ml sesame oil, fish oil or in Replens®) the drug was applied on the back of animals that were shaved on day 13, 17 and 21. In some animals (N = 6) RU-486 (3 mg in 0.2 ml sesame oil) was injected s.c. once on day 16 of gestation.
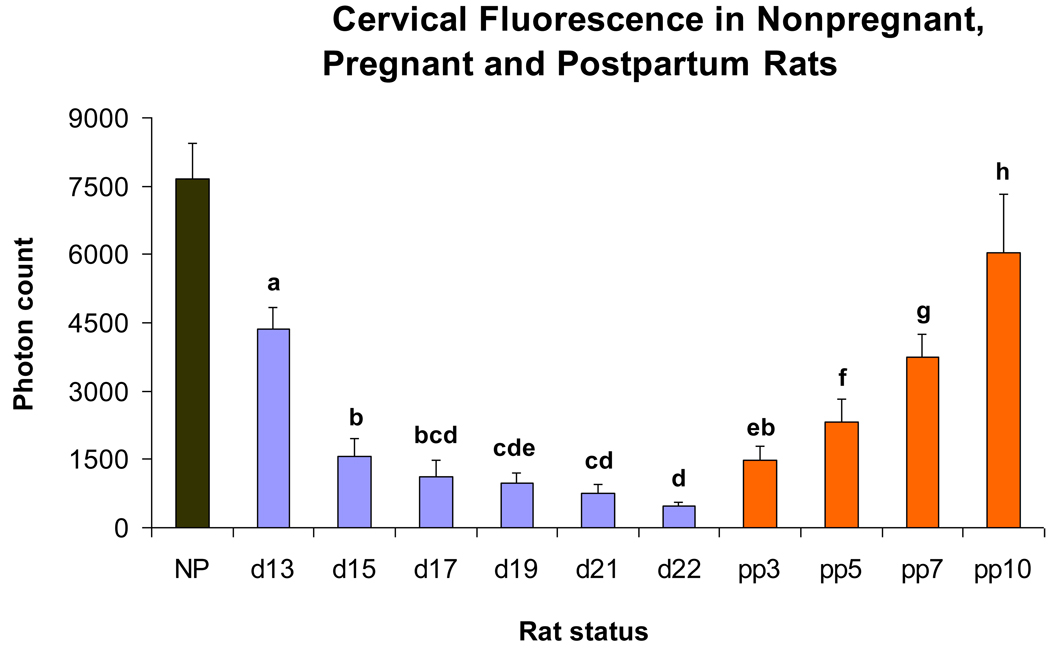
Figure 1.
Bar graphs showing means ± SD of cervical light-induced fluorescence (LIF) obtained in vivo from nonpregnant (N = 3), pregnant (d13, 15 and 17: N = 12/ group; d19 and d21: N = 11 / group; d22: N = 6) and postpartum rats (pp3, 5 and 7: N = 7; pp10: N = 6). Significant differences (P <0 .05) between groups are marked with different letters.
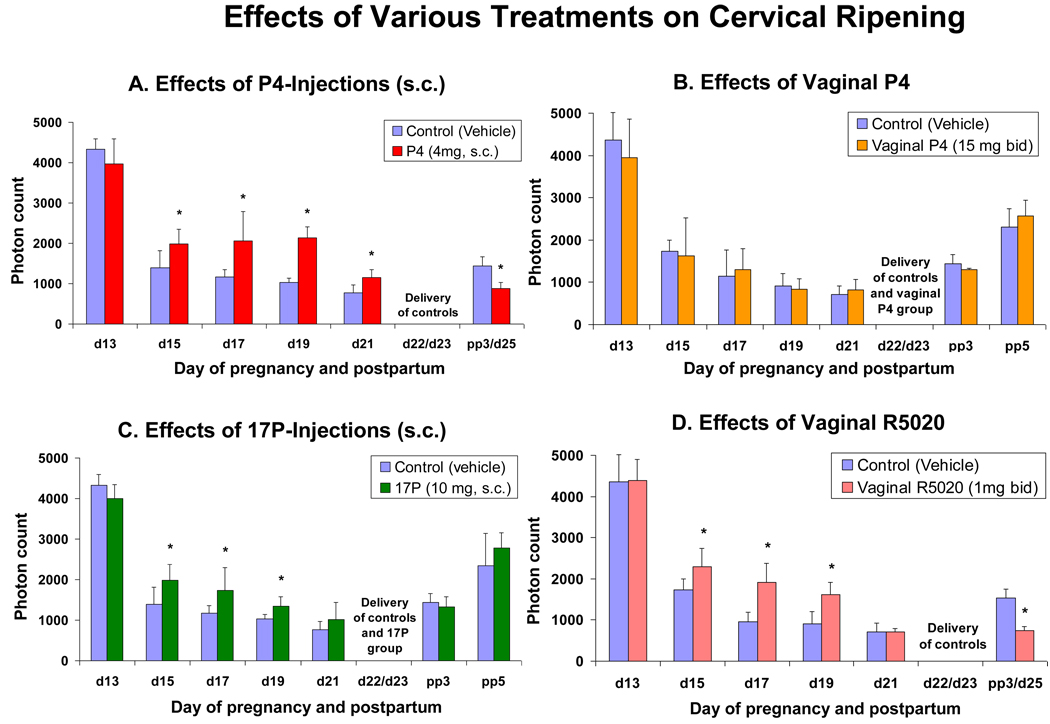
Figure 2.
Bar graphs showing means ± SD of cervical light-induced fluorescence (LIF) obtained in vivo from pregnant rats at different days of pregnancy and postpartum (N = 6/ group) treated with various progestins or vehicle. Figure 2A: Daily treatment with vehicle (controls) or P4 (4 mg, s.c.). Note that delivery is inhibited in the treatment group. Figure 2B: Twice a day treatment with vehicle (controls) or vaginal P4 (15 mg bid). Note that no significant differences are observed at any time between controls vs. treated animals. Figure 2C: Treatment daily with vehicle (controls) or 17P (10 mg, s.c.). Note that significant differences are only observed until day 19 of gestation. Figure 2D: Twice a day treatment with vehicle (controls) or vaginal R5020 (1 mg bid). Note that significant differences are observed only until day 19, but delivery is blocked in the treatment group. Asterisks indicate P < 0.05 compared with controls.
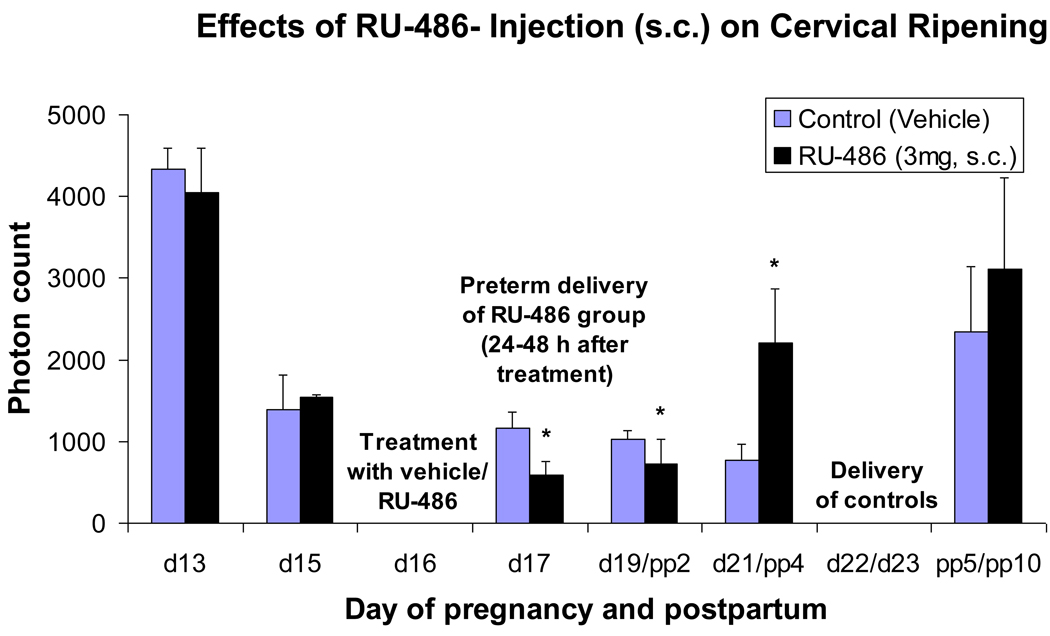
Figure 3.
Bar graphs showing means ± SD of cervical light-induced fluorescence (LIF) obtained in vivo from pregnant rats at different days of pregnancy and postpartum (N = 6/ group) treated once on day 16 with vehicle (controls) or RU-486 (3 mg s.c.). Asterisks indicate P <0 .05 compared with controls.
Reagents
Crystalline progesterone (used for oral and subcutaneous P4), RU-486, sesame oil and ethanol were purchased from Sigma (St Louis, MO, USA), micronized progesterone (used for topical P4 and vaginal P4 in cotton plug) from Spectrum Chemical MFG Corp. (Gardena, CA, USA), fish oil (concentrated omega-3 fatty acids) was obtained from General Nutrition Corp. (Pittsburgh, PA, USA), 17-alpha-hydroxyprogesterone caproate from MP Biomedicals (Solon, OH, USA) and promegestone (R5020) from Roussel Uclaf, France. P4, 17P, R5020 and RU-486 were dissolved in ethanol and then mixed with sesame or fish oil. Crinone® (micronized P4 in Replens®, a bioadhesive gel) was used for vaginal P4) and Replens® were gifts from Columbia Laboratories (Livingston, NJ, USA).
Assessment of cervical ripening
The amount of cervical collagen was evaluated in vivo (only in group s.c. P4, vaginal P4, s.c. 17P, vaginal R5020, s.c. RU-486) by measurement of the autofluorescent properties of cross-linked collagen with a new prototype of an instrument, termed collascope (Reproductive Research Technologies, Houston, TX, USA), as used previously with an earlier prototype.11,16,19 After insertion of a small speculum into the vagina of the anesthetized animal, the optical probe of the collascope was placed on the surface of the exocervix. The probe, which is connected to the main unit of the instrument by a fiberoptic cable, delivers not only excitation light (wavelength: 339 nm) onto the cervix but also carries the fluorescent light (mainly caused by pyridinoline cross-links of collagen with a maximum peak at 390 nm) back to the instrument to a CCD camera to display the full spectrum of fluorescence and analysis of the photons emitted by the cervix. In the current study, the exposure time for excitation was 100 msec. The average of 20 measurements of the detected fluorescent intensity (photon count) at 390 nm was used for each animal at any given time. Measurements of cervical light-induced fluorescence (LIF) were performed on nonpregnant animals once and in pregnant animals every other day starting at day 13 of gestation until delivery and on postpartum day 3 and/ or postpartum day 5 (see Figure 2 and Figure 3), and for some animals also on postpartum days 7 and 10 (see Figure 1 and Figure 3).
Determining the changes in delivery time
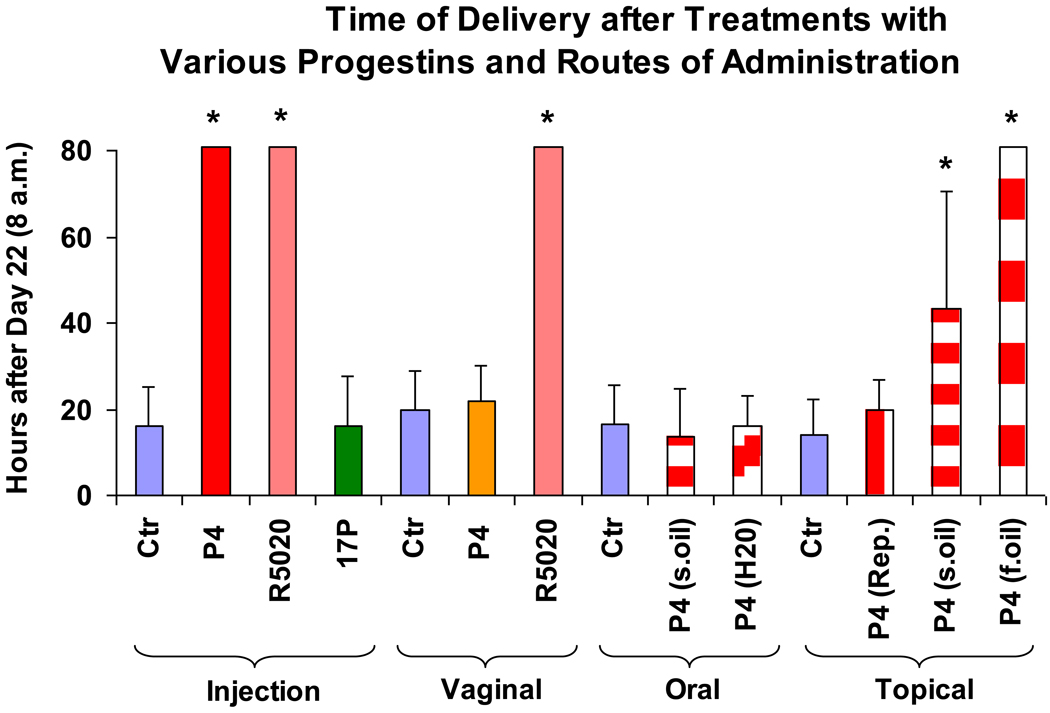
Times of delivery (see also Figure 4) of controls and various treatment groups were determined as hours after 8 a.m. of day 22 of gestation (Figure 5). The expulsion of one pup was defined as delivery.
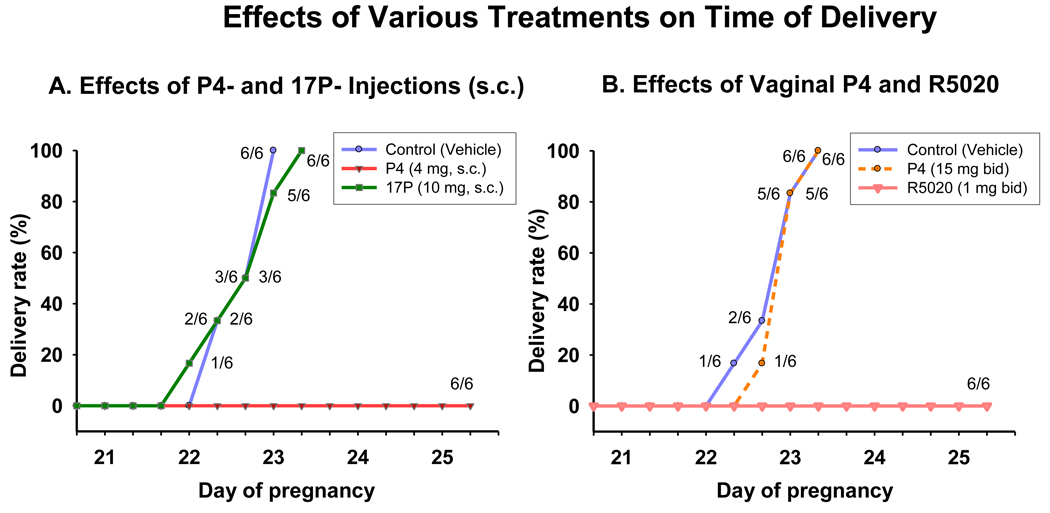
Figure 4.
Shown is the percent of animals delivering versus day of pregnancy after various treatments. Figure 4A: Delivery times after daily treatment with vehicle (s.c., controls), P4 (4 mg, s.c.) and 17P (10 mg, s.c.). Note that injections of P4 completely blocked delivery, whereas 17P had no significant effect on delaying term delivery (see also Figure 5). Figure 4B: Percent of animals delivering versus time of delivery following twice a day treatment with vaginal vehicle (controls), vaginal P4 (15 mg bid) and vaginal R5020 (1 mg bid). Note that vaginal R5020 completely blocked delivery, whereas vaginal P4 had no significant effect on delaying term delivery (P >0 .05 compared with controls, see also Figure 5).
Figure 5.
Shown is the time of delivery (= hours after 8 a.m. of day 22 of gestation) of pregnant rats treated with vehicles (controls) and various progestins by different routes of administration – injections (s.c.; daily): vehicle: sesame oil; P4 (4 mg); R5020 (2 mg); 17P (10 mg); vaginal (bid): vehicle: Replens®; P4 (15 mg, Crinone®); R5020 (1 mg); oral (bid): vehicle: sesame oil or H2O; P4 (15 mg); topical (bid): vehicle: Replens®, sesame oil or fish oil; P4 (15 mg). Rats with delayed parturition were sacrificed on day 25. Asterisks indicate P <0.05 compared with controls.
Statistical analyses
The cervical LIFs obtained at different times of gestation (Figure 1) were compared using one-way analysis of variance (ANOVA) and multiple pairwise comparison procedures (Holm-Sidak). Student’s t-test was used to compare the LIF results of a treatment group to its specific control group at any time in gestation and postpartum and also to determine the differences in delivery times (Figure 2, Figure 3 and Figure 5). A two-tailed probability value of P<0.05 was considered statistically significant.
Results
Collascope Measurements
LIF in nonpregnant, pregnant and postpartum rats
Measurements of cervical light-induced-fluorescence (LIF) in pregnant, non-treated animals show (Figure 1) a continuously decreasing photon count throughout pregnancy, reaching lowest values at term, and reversal postpartum. After significant (P<0.05) decrease from day 13 to day 15 LIF reaches a wider plateau of non-significant (P>0.05) decreases prior to delivery. LIF values progressively increase postpartum (P<0.05).
Effects of injections of P4 on LIF
LIF is significantly (P<0.05) higher in the P4 injection group compared with vehicle controls for any day of gestation (Figure 2A). There is no difference (P<0.05) between day 25 (delayed delivery) in the P4 injection group (and delivery is blocked see Figure 4A) compared to control animals at day 21 of gestation. LIF before treatment at day 13 shows no significant differences (P>0.05) between the treatment and the control group and this is similar for all treatment groups mentioned with other treatments (below, see Figure 2A–D and Figure 3).
Effects of vaginal P4 on LIF
There are no significant differences (P>0.05) between the vaginal P4 group and vehicle controls at any time in gestation (Figure 2B) and vaginal P4 failed to inhibit delivery (see figure 4B).
Effects of injections of 17P on LIF
LIF is significantly higher (P<0.05) in the 17P treated group (until day 19 only) compared with vehicle controls (Figure 2C).
Effects of vaginal R5020 on LIF
LIF is significantly higher (P<0.05) in the R5020 treated group (until day 19 only) compared with vehicle controls (Figure 2D).
Effects of a single injection of RU-486 on LIF
LIF is significantly lower (P<0.05) in the RU-486 treated group 24 and 72 hours after treatment compared with vehicle controls (Figure 3). LIF is higher in the RU-486 treated group 5 days after treatment compared with vehicle controls (P<0.05).
Effects of Treatments on Time of Delivery
Effects of injections of P4 and 17P on time of delivery
Injections of P4, but not 17P, completely block delivery (Figure 4A).
Effects of vaginal P4 and R5020 on time of delivery
Vaginal R5020, but not vaginal P4, completely block delivery (Figure 4B).
Effect of various progestins and routes of administration on time of delivery
Figure 5 summarizes the results of the different treatment groups on time of delivery and shows additional treatment groups. Animals treated as mentioned above. Other treatment groups followed the same design with treatments starting at day 13 of gestation until delivery. Additional treatment groups: Injections of R5020 also completely block delivery. Oral P4 suspended in sesame oil or H2O and vaginal P4 in cotton plug (results not shown) had no effect on time of delivery. However, topical P4 in sesame oil (partially) and in fish oil (completely), but not in Replens® prolongs delivery (P<0.05).
Effects of RU-486 on time of delivery
The P4 antagonist RU-486 (mifepristone) induced preterm delivery 24– 48 hours after injection (see Figure 3; 4 of 6 animals delivered after 24 hours and the remaining two animals delivered within 48 hours after treatment).
Comment
This study demonstrates that light-induced fluorescence can be useful to assess quantitative changes in cervical ripening in vivo. This has been shown previously using an older prototype of the collascope for rats, guinea pigs and humans.19,29,30 LIF is a direct result of the pyridinoline crosslinks between the collagen fibers and thus LIF measurements give estimates of the insoluble fraction of the collagen in the cervix. The collascope instrument is not only helpful to observe the regular changes throughout pregnancy and postpartum, but also indicates preterm cervical changes and the success of pharmacotherapy and interventions. As shown in this study progestins have the ability to delay cervical ripening and delivery in term pregnant rats. These effects depend critically on the choice of the progestin, vehicle and the route of administration.
There has been a long history of the concept to use progestins to prevent preterm birth. Several groups have recently investigated the effects of vaginal P4 to prevent preterm birth.18,23 In a multicenter study O’ Brien et al showed that daily intravaginal P4 had no effect on preventing recurrent preterm birth at various stages of gestation..25 This study seemed to contradict the earlier study by da Fonseca et al.23 In later analysis of the O’Brien study de Franco et al demonstrated that the rate of preterm birth at </= 32 weeks was significantly lower for those women with a cervical length < 28 mm receiving P4.18 The fact that vaginal P4 reduces the rate of preterm delivery in woman with a short cervix was confirmed by da Fonseca et al.26 In addition, in 2009 O’Brien et al in a secondary analysis of the original multinational study (published in 2007) showed significantly less cervical shortening in women with a history of spontaneous preterm birth or premature cervical shortening who were treated with daily vaginal P4 (90 mg) gel, compared to placebo.31 17P has also been used in early clinical trials to treat recurrent preterm birth by intramuscular injections.20,21 Facchinetti et al revealed the cervix as a potential target by demonstrating that the progressive shortening of the cervix is attenuated by treatment with 17P in patients hospitalized and treated for preterm labor.17 However Durnwald et al showed that 17P had no effect on the average weekly change in cervical length measurements over time in women at increased risk for prematurity who were treated compared to those without treatment.27 Thus the above studies and their conclusions raise questions about the ability of P4 and 17P to inhibit recurrent preterm labor and whether these progestins have effects on the cervix to prevent cervical ripening. It is not established which of the progestins and which route of administration is superior and there is controversy in the findings. Although P4 and 17P have been shown to reduce preterm labor rates slightly in some clinical studies these results are not supported by other trials. None of these treatments completely prevented preterm birth and many women were exposed to progestins whether they needed it or not.
As recommended in a current report of the American College of Obstetrics and Gynecology (ACOG) Committee and the Society for Maternal Fetal Medicine, P4 should only be used for prevention of preterm delivery in a “singleton pregnancy and a prior spontaneous preterm birth due to spontaneous preterm labor or premature rupture of membranes”.32 Still, the ideal P4 formulation is unidentified, as well as the dosage and route of administration.32 In addition treatment of acute preterm labor by progestins has yet to be extensively studied and management is limited to the use of tocolytics which suppress uterine contractility but do not reverse the labor process.
Treatment of preterm labor might be greatly improved if methods of administration, compounds, and vehicles were optimized and if we had a better understanding of how the progestins affect the uterus and cervix. This might also improve the use of progestins for other indications.
In this study injections of P4 show (Figure 2A) the longest effect on delaying cervical ripening in rats of all compounds used. This treatment even inhibited the progression of softening of the cervix in a stage of rat pregnancy, where the physiological P4-levels are still rising.33,34 Thus, higher P4 levels in midgestation of pregnancy in rat has an anti-ripening effect on the cervix. Exogenous P4, administered by subcutaneous injections also decelerate the consequences of the sharp withdrawal of P4 that occurs at day 19 of rat pregnancy .33,34 The consequences of blocking the P4 receptors during pregnancy with the P4 antagonist RU-486 results in termination of pregnancy in both rats and humans.28,35 RU-486-induced cervical ripening occurs after 24 hours followed by preterm delivery (Figure 3) and this confirms effects of antiprogestins on the cervix36 and indicates the importance of P4 in control of the cervix and maintaining pregnancy. We have previously shown that P4 can not prevent RU-486-induced delivery in rats, probably because of higher affinity of the P4 antagonist for the P4 receptor.28 Because of this the RU-486-treated model is not useful to test the effects of progestins on preterm birth as we have done in this study on term animals. However the present study does demonstrate significant differences in cervical ripening in early pregnancy with some progestin treatments and this may correspond to differences in cervical length estimated in some of the clinical studies of progestins.
As mentioned above, contrary to humans, there is a sharp P4 withdrawal at the end of pregnancy in rats, that is due to the demise of the corpus luteum mediated by the Prostaglandin F2α.37 There have been previous studies of the changes in cervical ripening and effects of progestins and antiprogestins in rats15 and in other species that, similar to humans, do not show a pronounced P4 withdrawal at the end of pregnancy.36 Other studies have also shown effects of progestins on cervical collagen content in mice after treatment with progestins.38
The present study shows that the intrinsic properties of the progestins dictate their affects on the cervix and myometrium. Thus, 17P and R5020 delayed cervical ripening but not at term immediately preceding delivery and s.c. and vaginal R5020 and parenteral P4 prevented delivery. Cervical ripening is only attenuated until day 19 of gestation with R5020 and 17P in contrast to injection of P4 which also prevents further ripening on day 21 (Figure 2A, 2C, 2D). This indicates that P4 might accomplish inhibitory effects on the cervix beyond the ability of R5020 and 17P and demonstrates how properties of the progestins are significant factors affecting action. Exactly how any progestin interacts to control the cervix or myometrial contractility is uncertain. Recent studies indicate a non-genomic mechanism of action of P4, but not 17P, on uterine membrane receptors.39 Different chemical structures and with this different binding affinities to receptors as well as unknown actions could explain why the intrinsic properties of the compounds are important.
Despite any of the treatments with progestins the cervix still manages to ripen at the end of pregnancy (Figures 2A–D). The fact that prophylactic parenteral P4 can only delay, but not completely inhibit cervical ripening indicates the involvement of other control pathways in the ripening process. Recent studies suggest a functional P4 withdrawal in human myometrium and cervix.40,41 This implies changes in ratios of receptors and total number of receptors. Additionally the involvement of membrane P4 receptors has to been taken in consideration. Whether this possible functional P4 withdrawal of receptors also exists in rodents has to be evaluated. Nevertheless a potential functional P4 withdrawal at the end of human pregnancy could initiate essential steps for completing successful delivery. Furthermore possible functional changes of the P4 receptors also in the rat cervix could explain the fact, that injections of P4 lose their ability to inhibit ripening at the end of gestation.
This study shows (Figure 5) that P4 delays delivery and its effect crucially depends on the route of administration and the selection of the vehicle. Only injected and topical but not vaginal (applied as a P4 gel or P4 oil in cotton plug) or oral P4 delayed term delivery. The vaginal route is thought to be the superior route of administrating P4 when focused effects on the uterus are desired.42 This proposed concept is not substantiated by this study
Following the concept of the liver first-pass effect after administration of oral drugs, de Ziegler et al43 established the term “uterine first-pass effect” to point out the minimized systemic, but optimized uterine exposure after transvaginal treatment with sex steroids. However, in our study vaginal P4, even at a very high dose (7.5 × the injected dose) had no effect on cervical ripening and on delivery. We conclude that this may be due to a slower uptake of the hormone by the vaginal route or poor release of P4 by Replens®. It is also possible that the multiple probing with the collascope instrument may have affects on the uptake of the drug, e.g. by removing the drug of the previous day’s treatments and with this preventing an accumulation of the gel in the vagina. However, this seems unlikely since the animals were probed every other day for LIF and the measurement of LIF only takes a few minutes prior to reapplication of P4. There are many studies of the uptake of P4 by the vaginal route and increases in plasma levels of the hormone, that established the phenomenon of the uterine first-pass effect, but most of these studies were accomplished on postmenopausal women.44,45 Richardson et al showed that the absorption of drugs across the vaginal epithelium is influenced by several physiologic factors, such as the properties of the epithelium and the volume, viscosity and pH of the vaginal fluid.46 Thus the uptake of vaginal administered drugs may differ in pregnancy due to changes in the vagina epithelium or other factors. In addition placement of compounds in specific areas of the cervix should be considered. We used a blunt ball-top needle syringe to ensure a deep placement of the drug to follow the recommendations of Cicinelli et al47 and avoided positioning of the gel in the outer portion of the vagina. In contrast to vaginal P4, vaginal R5020 delayed cervical ripening in pregnant rats. R5020, a 19-norprogesterone derivate, is a high affinity P4 receptor agonist.48 Possibly, the problems of low uptake of any progestin vaginally is compensated by R5020 with a higher potency. Oral P4 probably failed because of a low uptake and the liver first-pass effect.
The importance of the vehicle is demonstrated by the observations where topical P4 in fish oil completely, in sesame oil only partially and in Replens® not at all inhibits delivery (Figure 5). This indicates that Replens® may not release P4 as effectively as oil and this should be also evaluated for the vaginal route of administration. Nevertheless vaginal R5020 in Replens® was effective in preventing delivery but this is probably related to the high affinity to the P4 receptors.
It has been suggested that P4 regulates parturition through genomic actions via various proteins that are thought to be involved in controlling myometrial contractility.49,50 Our group has shown in a recent study that P4, at concentrations equivalent to those present in the placenta and uterus, inhibits spontaneous myometrial contractility by nongenomic mechanism.39 In this study we measured delivery and cervical ripening. Delivery at term or preterm is thought from many studies, including our own, to be due to these two main processes and involves both uterine muscle activity and changes in the cervical connective tissue. We demonstrate that delivery is completely inhibited by some progestin treatments (e.g. s.c. P4 and both s.c. and vaginal R5020), but cervical ripening is delayed but not entirely blocked during the final days of gestation (e.g. s.c. P4) or not significantly different from controls (e.g. vaginal R5020 ) on day 21. Therefore inhibition of delivery is not due to an unripe cervix, but must be due to inhibition of uterine contractions. On the otherhand 17P partly delays ripening, similar to s.c. P4, and is not significantly different from controls at day 21, like R5020, but does not block delivery at all. RU-486, the antiprogestin used in this study is well known to act both on the cervix and uterus to induce delivery by stimulation of uterine contractility and cervical ripening. Additionally the uterine contractility increases dramatically during spontaneous delivery at term and preterm after RU-486 treatment.51. 17P had no effect on delaying term delivery so we conclude that 17P is not an effective treatment for preventing birth. This supports the observations made previously showing that 17P has little effect to inhibit contractility.39,52
Parenteral and topical P4 treatment may be the preferred treatment to prevent preterm cervical ripening, inhibition of uterine contractions and delivery as it does during term delivery. Action of progestins on the uterus and cervix to inhibit delivery depends upon many factors including the properties of the compound, route of administration and the vehicle as demonstrated in this study. These elements should be critically evaluated in clinical studies with progestins to inhibit preterm delivery. In addition use of progestins for other indications (such as menstrual cramps, uterine and other cancers, osteoporosis, contraception, to oppose unwanted effects of estrogens, amenorrhea and abnormal uterine bleeding, infertility, endometriosis, etc.) might be greatly improved by the methods described in this paper.
Acknowledgments
This study was supported by NIH R01 HD037480 and the St. Joseph’s Foundation at St. Joseph’s Hospital and Medical Center, Phoenix, AZ
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 30th Annual Meeting of the Society for Maternal-Fetal Medicine, Chicago, IL, Feb. 2–6, 2010.
References
- 1.Goldenberg R, Culhane J, Iams J, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steer P. The epidemiology of preterm labour. BJOG. 2005;112 Suppl 1:1–3. doi: 10.1111/j.1471-0528.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- 3.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Mathews TJ. Births: Final Data for 2006. Natl Vital Stat Rep. 2009;57:1–104. [PubMed] [Google Scholar]
- 4.Berkowitz GS, Papiernik E. Epidemiology of preterm birth. Epidemiol Rev. 1993;15(2):414–443. doi: 10.1093/oxfordjournals.epirev.a036128. [DOI] [PubMed] [Google Scholar]
- 5.Behrman R, Butler A. Preterm Birth: Causes, Consequences, and Prevention. Washington, D.C: The National Academies Press; 2007. pp. 398–429. [PubMed] [Google Scholar]
- 6.Uldbjerg N, Ekman G, Malmström A, Olsson K, Ulmsten U. Ripening of the human uterine cervix related to changes in collagen, glycosaminoglycans, and collagenolytic activity. Am J Obstet Gynecol. 1983;147:662–666. doi: 10.1016/0002-9378(83)90446-5. [DOI] [PubMed] [Google Scholar]
- 7.Rechberger T, Uldbjerg N, Oxlund H. Connective tissue changes in the cervix during normal pregnancy and pregnancy complicated by cervical incompetence. Obstet Gynecol. 1988;71(4):563–567. [PubMed] [Google Scholar]
- 8.Chwalisz K, Benson M, Scholz P, Daum J, Beier HM, Hegele-Hartung C. Cervical ripening with the cytokines IL-8, IL-1β1-beta and TNFα in guinea-pigs. Hum Reprod. 1994;9:2173–2181. doi: 10.1093/oxfordjournals.humrep.a138413. [DOI] [PubMed] [Google Scholar]
- 9.Junqueira LC, Zugaib M, Montes GS, Toledo OM, Krisztan RM, Shigihara KM. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilation. Am J Obstet Gynecol. 1980;138:273–281. doi: 10.1016/0002-9378(80)90248-3. [DOI] [PubMed] [Google Scholar]
- 10.Osmers R, Rath W, Adelmann-Grill BC, Fittkow C, Kuloczik M, Szeverényi M, Tschesche H, Kuhn W. Origin of cervical collagenase during parturition. Am J Obstet Gynecol. 1992;166(5):1455–1460. doi: 10.1016/0002-9378(92)91619-l. [DOI] [PubMed] [Google Scholar]
- 11.Glassman W, Byam-Smith M, Garfield RE. Changes in rat cervical collagen during gestation and after antiprogesterone treatment as measured in vivo with light-induced autofluorescence. Am J Obstet Gynecol. 1995;173:1550–1556. doi: 10.1016/0002-9378(95)90648-7. [DOI] [PubMed] [Google Scholar]
- 12.Clark K, Ji H, Feltovich H, Janowski J, Carroll C, Chien EK. Mifepristone-induced cervical ripening: structural, biomechanical, and molecular events. Am J Obstet Gynecol. 2006;194:1391–1398. doi: 10.1016/j.ajog.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Cheah SH, Ng KH, Johgalingam VT, Ragavan M. The effects of oestradiol and relaxin on extensibility and collagen organization of the pregnant rat cervix. J Endocrinol. 1995;146:331–337. doi: 10.1677/joe.0.1460331. [DOI] [PubMed] [Google Scholar]
- 14.Ji H, Dailey TL, Long V, Chien EK. Androgen-regulated cervical ripening: a structural, biomechanical, and molecular analysis. Am J Obstet Gynecol. 2008;198(543):e1–e9. doi: 10.1016/j.ajog.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Shi L, Shi SQ, Saade GR, Chwalisz K, Garfield RE. Studies of cervical ripening in pregnant rats: effects of various treatments. Mol Hum Reprod. 2000;6:382–389. doi: 10.1093/molehr/6.4.382. [DOI] [PubMed] [Google Scholar]
- 16.Maul H, Shi L, Marx SG, Garfield RE, Saade GR. Local application of platelet-activating factor induces cervical ripening accompanied by infiltration of polymorphonuclear leukocytes in rats. Am J Obstet Gynecol. 2002;187:829–833. doi: 10.1067/mob.2002.126983. [DOI] [PubMed] [Google Scholar]
- 17.Facchinetti F, Paganelli S, Comitini G, Dante G, Volpe A. Cervical length changes during preterm cervical ripening: effects of 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2007;196(453):e1–e4. doi: 10.1016/j.ajog.2006.09.009. discussion 421. [DOI] [PubMed] [Google Scholar]
- 18.DeFranco EA, O'Brien JM, Adair CD, Lewis DF, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:697–705. doi: 10.1002/uog.5159. [DOI] [PubMed] [Google Scholar]
- 19.Fittkow CT, Shi SQ, Bytautiene E, Olson G, Saade GR, Garfield RE. Changes in light-induced fluorescence of cervical collagen in guinea pigs during gestation and after sodium nitroprusside treatment. J Perinat Med. 2001;29:535–543. doi: 10.1515/JPM.2001.074. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med. 1975;293:675–680. doi: 10.1056/NEJM197510022931401. [DOI] [PubMed] [Google Scholar]
- 21.Keirse MJ. Progestogen administration in pregnancy may prevent preterm delivery. Br J Obstet Gynaecol. 1990;97:149–154. doi: 10.1111/j.1471-0528.1990.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 22.Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 23.Da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–424. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 24.Keirse MJ. Progesterone and preterm: seventy years of "déjà vu" or "still to be seen"? Birth. 2004;31:230–235. doi: 10.1111/j.0730-7659.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:687–696. doi: 10.1002/uog.5158. [DOI] [PubMed] [Google Scholar]
- 26.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 27.Durnwald CP, Lynch CD, Walker H, Iams JD. The effect of treatment with 17 alpha-hydroxyprogesterone caproate on changes in cervical length over time. Am J Obstet Gynecol. 2009;201(410):e1–e5. doi: 10.1016/j.ajog.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garfield RE, Gasc JM, Baulieu EE. Effects of the antiprogesterone RU 486 on preterm birth in the rat. Am J Obstet Gynecol. 1987;157:1281–1285. doi: 10.1016/s0002-9378(87)80315-0. [DOI] [PubMed] [Google Scholar]
- 29.Fittkow CT, Maul H, Olson G, Martin E, MacKay LB, Saade GR, Garfield RE. Light-induced fluorescence of the human cervix decreases after prostaglandin application for induction of labor at term. Eur J Obstet Gynecol Reprod Biol. 2005;123:62–66. doi: 10.1016/j.ejogrb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Shi L, Shi SQ, Saade GR, Chwalisz K, Garfield RE. Changes in cervical resistance and collagen fluorescence during gestation in rats. J Perinat Med. 1999;27:188–194. doi: 10.1515/JPM.1999.026. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien JM, Defranco EA, Adair CD, Lewis DF, Hall DR, How H, Bsharat M, Creasy GW. Effect of progesterone on cervical shortening in women at risk for preterm birth: secondary analysis from a multinational, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2009;34:653–659. doi: 10.1002/uog.7338. [DOI] [PubMed] [Google Scholar]
- 32.Society for Maternal Fetal Medicine Publications Committee. ACOG Committee Opinion number 419 October 2008 (replaces no. 291, November 2003). Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112:963–965. doi: 10.1097/AOG.0b013e31818b1ff6. [DOI] [PubMed]
- 33.Garfield RE, Puri CP, Csapo AI. Endocrine, structural, and functional changes in the uterus during premature labor. Am J Obstet Gynecol. 1982;142:21–27. doi: 10.1016/s0002-9378(16)32279-7. [DOI] [PubMed] [Google Scholar]
- 34.Pepe GJ, Rothchild I. A comparative study of serum progesterone levels in pregnancy and in various types of pseudopregnancy in the rat. Endocrinology. 1974;95:275–279. doi: 10.1210/endo-95-1-275. [DOI] [PubMed] [Google Scholar]
- 35.Peyron R, Aubény E, Targosz V, Silvestre L, Renault M, Elkik F, Leclerc P, Ulmann A, Baulieu EE. Early termination of pregnancy with mifepristone (RU 486) and the orally active prostaglandin misoprostol. N Engl J Med. 1993;328:1509–1513. doi: 10.1056/NEJM199305273282101. [DOI] [PubMed] [Google Scholar]
- 36.Chwalisz K. The use of progesterone antagonists for cervical ripening and as an adjunct to labour and delivery. Hum Reprod. 1994;9 Suppl 1:131–161. doi: 10.1093/humrep/9.suppl_1.131. [DOI] [PubMed] [Google Scholar]
- 37.Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol. 2007;196:289–296. doi: 10.1016/j.ajog.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Yellon SM, Ebner CA, Elovitz MA. Medroxyprogesterone acetate modulates remodeling, immune cell census, and nerve fibers in the cervix of a mouse model for inflammation-induced preterm birth. Reprod Sci. 2009;16:257–264. doi: 10.1177/1933719108325757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruddock NK, Shi SQ, Jain S, Moore G, Hankins GD, Romero R, Garfield RE. Progesterone, but not 17-alpha-hydroxyprogesterone caproate, inhibits human myometrial contractions. Am J Obstet Gynecol. 2008;199(391):e1–e7. doi: 10.1016/j.ajog.2008.06.085. [DOI] [PubMed] [Google Scholar]
- 40.Stjernholm-Vladic Y, Wang H, Stygar D, Ekman G, Sahlin L. Differential regulation of the progesterone receptor A and B in the human uterine cervix at parturition. Gynecol Endocrinol. 2004;18(1):41–46. doi: 10.1080/09513590310001651777. [DOI] [PubMed] [Google Scholar]
- 41.Mesiano S. Myometrial progesterone responsiveness and the control of human parturition. J Soc Gynecol Investig. 2004;11:193–202. doi: 10.1016/j.jsgi.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Cicinelli E. Intravaginal oestrogen and progestin administration: advantages and disadvantages. Best Pract Res Clin Obstet Gynaecol. 2008;22(2):391–405. doi: 10.1016/j.bpobgyn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 43.De Ziegler D, Bulletti C, De Monstier B, et al. The first pass uterine effect. Ann N Ann N Y Acad SciY Acad Sci. 1997;828:291–299. doi: 10.1111/j.1749-6632.1997.tb48550.x. [DOI] [PubMed] [Google Scholar]
- 44.Cicinelli E, Cignarelli M, Sabatelli S, Romano F, Schonauer LM, Padovano R. Einer-Jensen N. Plasma concentrations of progesterone are higher in the uterine artery than in the radial artery after vaginal administration of micronized progesterone in an oil-based solution to postmenopausal women. Fertil Steril. 1998;69:471–473. doi: 10.1016/s0015-0282(97)00545-1. [DOI] [PubMed] [Google Scholar]
- 45.Levine H, Watson N. Comparison of the pharmacokinetics of crinone 8% administered vaginally versus Prometrium administered orally in postmenopausal women(3) Fertil Steril. 2000;73:516–521. doi: 10.1016/s0015-0282(99)00553-1. [DOI] [PubMed] [Google Scholar]
- 46.Richardson JL, Illum L. The vaginal route of peptide and protein drug delivery. Adv Drug Deliv Rev. 1992;8:341–366. [Google Scholar]
- 47.Cicinelli E, Di Naro E, De Ziegler D, et al. Placement of the vaginal 17b-estradiol tablets in the inner or outer one third of the vagina affects the preferential delivery of 17b-estradiol towards the uterus or peri-urethral areas, thereby modifying efficacy and endometrial safety. Am J Obstet Gynecol. 2003;189:55–58. doi: 10.1067/mob.2003.341. [DOI] [PubMed] [Google Scholar]
- 48.Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH. Classification and pharmacology of progestins. Maturitas. 2008;61:171–180. doi: 10.1016/j.maturitas.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 49.Garfield RE, Kannan MS, Daniel EE. Gap junction formation in myometrium: control by estrogens, progesterone, and prostaglandins. Am J Physiol. 1980;238:C81–C89. doi: 10.1152/ajpcell.1980.238.3.C81. [DOI] [PubMed] [Google Scholar]
- 50.Garfield RE, Saade G, Buhimschi C, Buhimschi I, Shi L, Shi SQ, Chwalisz K. Control and assessment of the uterus and cervix during pregnancy and labour. Hum Reprod Update. 1998;4:673–695. doi: 10.1093/humupd/4.5.673. [DOI] [PubMed] [Google Scholar]
- 51.Shi SQ, Maner WL, Mackay LB, Garfield RE. Identification of term and preterm labor in rats using artificial neural networks on uterine electromyography signals. Am J Obstet Gynecol. 2008;198(235):e1–e4. doi: 10.1016/j.ajog.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sexton DJ, O'Reilly MW, Friel AM, Morrison JJ. Functional effects of 17alpha-hydroxyprogesterone caproate (17P) on human myometrial contractility in vitro. Reprod Biol Endocrinol. 2004;2:80. doi: 10.1186/1477-7827-2-80. [DOI] [PMC free article] [PubMed] [Google Scholar]