Abstract
Background
Plasma total cholesterol is known to be decreasing while obesity is increasing, but information on recent population trends of high-density lipoprotein cholesterol (HDL-C) and triglycerides (TG) is sparse.
Methods
Plasma total cholesterol, HDL-C, and TG, measured by the same methods at the three most recently-completed examinations of Framingham Offspring Study participants (from 1991 to 2001), were compared in 1666 participants without prevalent cardiovascular disease, lipid or hormone replacement therapy (56% men; mean age, 53 and 60 years at first and last examination). Changes in age- and multivariable-adjusted mean lipid levels were related to changes in BMI.
Results
Over the three examinations, comparing earliest to most recent exam, HDL-C was significantly increased (multivariable-adjusted means, 44.4 and 46.6 mg/dL in men; 56.9 and 60.1 mg/dL in women; P for trend, <0.0001 in both sexes), whereas TG levels were decreased (144.5 and 134.1 mg/dL in men; 122.3 and 112.3 mg/dL in women; P for trend, 0.0041 in men, <0.0001 in women). Over the same time interval, BMI increased (27.8 and 28.5 kg/m2 in men; 27.0 and 27.6 kg/m2 in women; P for trend <0.0001 in men, 0.0011 in women). There was an inverse relation between changes in BMI and magnitude of dyslipidemia, i.e. individuals with least increase in BMI had most favorable changes in HDL-C and TG.
Conclusions
During a 10-year period of recent exams in the Framingham Heart Study there has been a decrease in dyslipidemia with an increase in HDL-C and decrease in TG despite an overall increase in BMI.
INTRODUCTION
The prevalence of obesity has increased dramatically over the past several decades in the United States, and throughout the world.1–3 There is also evidence of an increasing prevalence of the metabolic syndrome,4,5 a condition closely linked to obesity and associated with a clustering of cardiovascular disease risk factors including hypertension, glucose intolerance and a dyslipidemia characterized by high triglycerides (TG) and low HDL-cholesterol (HDL-C).6 Alone, or in the context of the metabolic syndrome, dyslipidemia is widely recognized as a cardiovascular risk factor that is associated with an increased likelihood of atherosclerosis.7
In the light of increasing obesity, it is surprising that the most recent report on lipid trends from the cross-sectional National Health and Nutrition Examination Survey (NHANES) reported that neither plasma levels of HDL-C nor TG showed significant changes from the examination in 1988–1994 to the most recent examination in 1999–2002.8 Over this same period NHANES reported that low-density lipoprotein cholesterol (LDL-C) and total cholesterol declined; a trend first noted about 30 years earlier.9 In contrast to the cross-sectional analysis performed by NHANES, the Framingham Heart Study, a large community-based sample of men and women, conducts longitudinal follow-up examinations in the same individuals approximately every four years. In the Framingham Study cohort, the prevalence of obesity increased with about 7% in men and 6% in women during the observed period,10 which corresponds well with the increase observed in non-Hispanic whites in NHANES.2 Sequential examinations in the Framingham Study provide an opportunity to examine lipid trends in the same individuals over time and to put changes in lipid values in the context of increasing obesity.
This analysis was undertaken to coincide with the period of the last report from NHANES to examine longitudinal trends in HDL-C, TG, and total cholesterol in the Framingham offspring cohort, a second-generation sample of Framingham men and women in their middle age.
METHODS
Study Sample
The Framingham Offspring Study was initiated in 1971, and the design and participant selection criteria have been described previously.11 Participants who attended each of the fifth (1991–94), sixth (1995–98), and seventh (1998–2001) examination cycles were eligible for the present study (N=3158). These examination cycles were selected a priori to roughly correspond to the period encompassed by the last two NHANES examinations (from 1988–2002). At each Framingham examination, the participants underwent a standardized medical history by a physician, a physical examination including blood pressure measurement and anthropometry, and blood sampling after an overnight fast. Participants were excluded from the current investigation if they had missing lipid data (N=56), or other covariates (N=78) at any of the three examinations. Further, we also excluded individuals with prevalent cardiovascular disease (CVD; N=403), individuals prescribed lipid therapy (N=458), or hormone replacement therapy (N=497) at any of the three examinations. Thus, the primary analyses were performed in a sample of 1666 individuals (929 men and 737 women). In secondary analyses, we analyzed trends of lipid concentrations in a larger sample, including individuals with prevalent CVD, lipid therapy, or hormone replacement therapy (N=3024 individuals; 1410 men and 1614 women). The study protocol was approved by Boston University Medical Center Institutional Review Board, and all participants provided written informed consent.
Lipid Measurements and Definitions of Covariates
The methods for measurements of lipid levels were the same for all three examinations and all testing was performed by the Framingham Heart Study laboratory that, throughout this period, participated in both Centers for Disease Control (CDC) and College of American Pathologists (CAP) programs for these lipid measurements. Venous blood samples were collected in tubes containing EDTA and plasma was separated by centrifugation. Plasma total cholesterol and triglycerides were measured using automated enzymatic assay procedures.12 HDL-C was measured after dextran sulfate-Mg2+ precipitation.13 LDL-C concentrations were estimated using the Friedewald formula.14 In order to exclude instrument drift or other assay-related changes over time as an explanation for changes in lipid levels, we repeated all lipid measurements from each of the three examinations in a subsample of 54 individuals prior to the start of this analysis. These individuals were selected to obtain samples across the range of values of each of the three lipid measures at the fifth examination cycle. The selection of samples was done blinded to lipid data at subsequent examinations. Re-measurements were done using the same methods as had originally been employed when these samples were collected.
Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Cigarette smoking was defined by self-reported cigarette use in the year preceding the Heart Study examination. Alcohol intake was defined by self-report of number of drinks per week. Use of lipid-modifying drugs, anti-hypertensive drugs, and hormone replacement therapy was also defined based on self-report. Dietary information on consumption of total calories during the previous year was collected at each exam using a 126-item food frequency questionnaire.15
Statistical Methods
The lipid measures of primary interest for the trend analyses were total cholesterol, HDL-C, and TG. Due to well-established sex differences in HDL-C levels, all analyses were performed separately in men and women.
In all analyses of lipid trends across examinations, we used generalized estimating equations to account for correlated observations. We constructed two sets of models: age-adjusted (adjusting for age and examination cycle), and multivariable-adjusted (adjusting for age, examination cycle, BMI, smoking, alcohol consumption, anti-hypertensive treatment, and total cholesterol [in analyses of HDL-C and TG] using covariates from the examination in question). In analyses of the larger sample including individuals with prevalent CVD, lipid therapy, or hormone replacement therapy, we performed additional adjustments for lipid therapy and hormone replacement therapy. The cumulative distribution functions of multivariable-adjusted levels of total cholesterol, HDL-C and TG levels at the three examinations were plotted separately for men and women. In secondary analyses, we redid all multivariable models with waist circumference instead of BMI.
In addition to analyzing the trends in lipid levels as continuous variables (with trend tests of age- and multivariable-adjusted mean levels across examinations), we also compared the following proportions across the examinations using pooled logistic regressions: proportions of participants using lipid-lowering medication; proportions of participants with low LDL-C (<130 mg/dL), borderline LDL-C (130–160 mg/dl), and high LDL-C (≥160 mg/dl); proportions of participants with high TG (>150 mg/dl); and proportions with low HDL-C (<40 mg/dl in men; <50 mg/dl in women). These comparisons were done in age- and multivariable-adjusted analyses (adjusting for the covariates defined above), and the difference in proportions across examination cycles were compared by trend tests.
To relate trends in lipids to changes in weight, we also performed age- and multivariable-adjusted analyses of the trends in BMI over the three exams. All the above analyses were repeated in a larger sample including individuals with prevalent CVD, lipid therapy, or hormone replacement therapy at any of the three examinations.
In order to explore the conjoint changes in HDL-C and TG, we calculated the age-adjusted pair-wise Spearman correlations between the change in HDL-C and TG between examinations 5 and 7 (delta HDL-C and delta TG) for men and women, separately. Also, we categorized all individuals into four groups by the pattern of change in HDL-C and TG between examinations 5 and 7 (any increase in both HDL-C and TG; any increase in HDL-C and any decrease in TG; any decrease in HDL-C and any increase in TG; and any decrease in both HDL-C and TG). Then, we calculated the age- and multivariable-adjusted mean changes between examination 5 and 7 in lipid levels, fasting glucose, and BMI in these four categories. Individuals without any change in either HDL-C or TG were excluded from these analyses (69 men, 40 women). In an attempt to explain the observed lipid trends, we examined changes in dietary intake of total calories between examinations 5 and 7. Two-sided p-values of <0.05 were considered statistically significant. All analyses were performed using SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
Re-measurement of plasma cholesterol, TG, and HDL-C concentrations in a subset of participants with plasma available at all 3 exam cycles showed excellent correlations with original measurements over a broad range of values for each lipid fraction, at each exam cycle. For the comparison of re-measured lipids with original values from the earliest exam cycle of this series of cycles (Exam 5) correlation coefficients were 0.985 for cholesterol, 0.997 for TG, and 0.948 for HDL-C, all significant at P <0.0001 (Supplementary Figure). The average intra-assay coefficients of variation when comparing the original measurements with these re-measurements were: for total cholesterol, 5% (examination 5), 3% (examination 6), and 3% (examination 7); for HDL-C, 7% (examination 5), 5% (examination 6), and 4% (examination 7); and for TG, 6% (examination 5), 3% (examination 6), and 4% (examination 7).
The clinical characteristics of the study sample at each of the three examinations are shown in Table 1.
Table 1.
Clinical Characteristics at Examination by Sex at Cycle 5, 6 and 7
| Men (N=929) | Women (N=737) | |||||
|---|---|---|---|---|---|---|
| Exam 5 | Exam 6 | Exam 7 | Exam 5 | Exam 6 | Exam 7 | |
| Age, years | 53±10 | 57±10 | 60±9 | 54±11 | 58±11 | 61±11 |
| Systolic blood pressure, mm Hg | 127±16 | 128±17 | 127±18 | 122±20 | 125±20 | 125±20 |
| Diastolic blood pressure, mm Hg | 77±10 | 78±9 | 77±9 | 72±10 | 73±9 | 72±9 |
| Anti-hypertensive treatment, % | 12 | 21 | 26 | 13 | 19 | 24 |
| Fasting glucose, mg/dL | 100±20 | 104±22 | 105±25 | 96±23 | 99±23 | 100±24 |
| Impaired fasting glucose, % | 37 | 48 | 51 | 24 | 31 | 36 |
| Diabetes treatment, % | 1 | 3 | 5 | 2 | 2 | 3 |
| Body mass index, kg/m2 | 27.9±4.1 | 28.3±4.3 | 28.5±4.6 | 26.8±5.7 | 27.4±5.7 | 27.7±5.8 |
| Waist circumference, inches | 39±4 | 40±4 | 40±4 | 34±6 | 37±6 | 38±6 |
| Smoking, % | 18 | 15 | 13 | 18 | 15 | 13 |
| Alcohol intake, drinks/week | 7.6±9.2 | 7.4±9.0 | 7.7±9.6 | 3.4±5.6 | 3.1±5.2 | 3.4±5.6 |
| Total cholesterol, mg/dL | 195.9±31.9 | 197.4±33.5 | 198.0±33.2 | 200.7±35.5 | 206.1±37.2 | 209.7±36.3 |
| LDL-C, mg/dL | 122.9±30.1 | 125.5±31.6 | 124.2±31.4 | 120.6±31.4 | 125.8±32.7 | 126.0±32.2 |
| HDL-C, mg/dL | 44.5±11.7 | 44.7±12.6 | 46.6±13.2 | 57.0±14.8 | 57.3±15.4 | 60.1±16.4 |
| Triglycerides, mg/dL | 142.3±92.9 | 135.8±102.0 | 136.0±93.8 | 115.4±62.0 | 114.7±62.4 | 118.2±67.0 |
Values are unadjusted means±standard deviations or proportions.
Abbreviations: NA, not applicable; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
Trends in Lipid Levels, Fasting Glucose, and BMI between 1991 and 2001
Total cholesterol levels did not change over the three examinations in either sex (Table 2). In contrast, HDL-C levels increased significantly over the examination cycles, also when adjusting for potential confounders including total cholesterol levels. This was accompanied by decreases of TG, which also were significant in age- and multivariable-adjusted models. These trends were essentially identical when using log-transformed TG in the models (data not shown).
Table 2.
Age- and Multivariable-Adjusted Mean Levels* of Lipids, Fasting Glucose and Body Mass Index By Examination Cycle
| Men (N=929) | Women (N=737) | |||||||
|---|---|---|---|---|---|---|---|---|
| Exam 5 | Exam 6 | Exam 7 | P for Trend | Exam 5 | Exam 6 | Exam 7 | P for Trend | |
| Age-adjusted analyses† | ||||||||
| Total cholesterol mg/dL | 196.1 (194.0–198.3) | 197.4 (195.2–199.6) | 197.8 (195.5–200.1) | 0.13 | 204.2 (201.6–206.7) | 205.7 (203.1–208.3) | 206.6 (204.0–209.2) | 0.064 |
| HDL-C mg/dL | 44.8 (43.9–45.6) | 44.7 (43.9–45.5) | 46.4 (45.5–47.2) | <0.0001 | 57.1 (55.9–58.2) | 57.3 (56.2–58.4) | 60.0 (58.8–61.2) | <0.0001 |
| TG mg/dL | 141.6 (135.6–147.7) | 135.9 (129.3–142.5) | 136.6 (129.7–143.5) | 0.18 | 120.3 (115.5–125.1) | 114.2 (109.9–118.6) | 113.9 (109.1–118.7) | 0.012 |
| Fasting glucose mg/dL | 100.6 (99.1–102.1) | 103.6 (102.1–105.0) | 104.2 (102.8–105.7) | <0.0001 | 97.6 (95.8–99.4) | 99.1 (97.4–100.8) | 99.1 (97.5–100.8) | 0.14 |
| BMI kg/m2 | 27.8 (27.6–28.1) | 28.3 (28.0–28.6) | 28.5 (28.2–28.9) | <0.0001 | 27.0 (26.6–27.5) | 27.4 (27.0–27.8) | 27.6 (27.1–28.0) | 0.0006 |
| Multivariable-adjusted analyses§ | ||||||||
| Total cholesterol mg/dL | 196.2 (194.1–198.4) | 197.5 (195.3–199.6) | 197.6 (195.3–199.9) | 0.22 | 204.5 (201.9–207.0) | 205.7 (203.1–208.3) | 206.3 (203.7–208.9) | 0.15 |
| HDL-C mg/dL | 44.4 (43.6–45.1) | 44.8 (44.0–45.6) | 46.6 (45.8–47.5) | <0.0001 | 56.9 (55.8–58.0) | 57.4 (56.4–58.4) | 60.1 (59.1–61.2) | <0.0001 |
| TG mg/dL | 144.5 (138.5–150.6) | 135.4 (129.1–141.7) | 134.1 (127.7–140.6) | 0.0041 | 122.3 (117.8–126.8) | 113.9 (110.0–117.8) | 112.3 (108.3–116.3) | <0.0001 |
| Fasting glucose mg/dL | 101.4 (100.1–102.8) | 103.5 (102.3–104.7) | 103.5 (102.3–104.7) | 0.0056 | 98.3 (96.8–99.8) | 99.1 (97.7–100.4) | 98.4 (97.2–99.7) | 0.93 |
| BMI kg/m2 | 27.8 (27.6–28.1) | 28.3 (28.0–28.6) | 28.5(28.2–28.9) | <0.0001 | 27.0(26.6–27.5) | 27.4(27.0–27.8) | 27.6(27.1–28.0) | 0.0011 |
Values are age- and multivariable-adjusted means (95% confidence intervals) from generalized estimating equations to account for correlated observations.
Adjusted for age and examination cycle.
Adjusted for age, examination cycle, body mass index, active smoking, alcohol consumption, anti-hypertensive treatment, and total cholesterol (in analyses of HDL-C and TG), using covariates from the examination in question.
Abbreviations: HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; BMI, body mass index.
In this same time period BMI significantly increased, in age-adjusted as well as in multivariable-adjusted models (Table 2). Fasting glucose levels increased in men, but not in women.
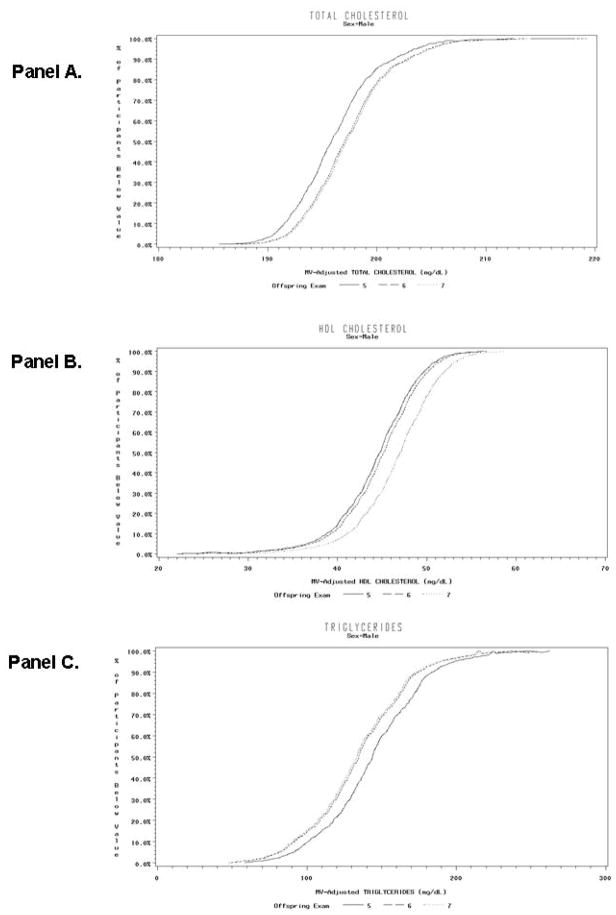
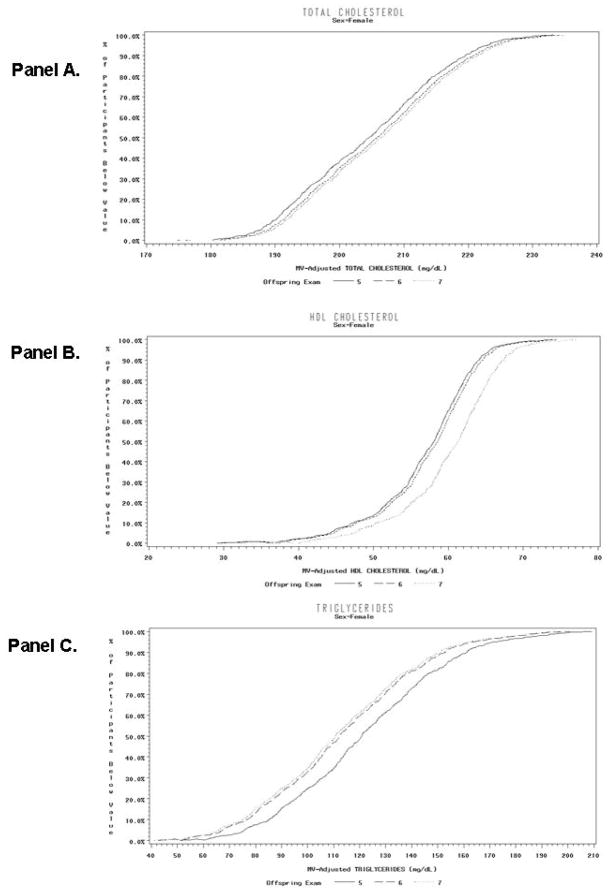
The cumulative distribution functions of multivariable-adjusted levels of total cholesterol, HDL-C, and TG for the three examinations are shown in Figure 1 (men), and Figure 2 (women). In general, these curves show a relatively similar sized shift in the distribution of each of these lipid fractions throughout the range of measured values from the earliest exam (Offspring 5) to the most recent (Offspring 7) with the distribution of values at the intermediate exam (Offspring 6) somewhat variable in relation to the earliest and most recent exam.
Figure 1.
Cumulative Distribution Functions of Multivariable-Adjusted Levels of Total Cholesterol (Panel A), High-Density Lipoprotein Cholesterol (Panel B), and Triglycerides (Panel C) at Examination 5, 6 and 7 in men not treated with lipid drugs and without cardiovascular disease.
Figure 2.
Cumulative Distribution Functions of Multivariable-Adjusted Levels of Total Cholesterol (Panel A), High-Density Lipoprotein Cholesterol (Panel B), and Triglycerides (Panel C) at Examination 5, 6 and 7 in women not treated with lipid drugs or hormone-replacement therapy and without cardiovascular disease.
In a larger sample including individuals with prevalent CVD, lipid therapy or hormone replacement therapy at any of the three examinations, there were also significant and substantial increases in HDL-C and decreases in TG over time in both men and women, in age- and multivariable-adjusted analyses (Supplementary Table 1). BMI significantly increased over the same time period, and in contrast to the primary analysis, there were significant decreases in total cholesterol levels in this larger sample.
Trends in Proportions of Individuals in Lipid Categories
Consistent with analyses of continuous variables, both the proportions of individuals with low HDL-C and the proportions of individuals with high TG decreased significantly in age- and multivariable-adjusted analyses in both men and women (Table 3). However, the categories of LDL-C levels did not change significantly over the three examinations.
Table 3.
Proportions of Participants in Lipid-Related Categories* By Examination Cycle
| Men (N=929) | Women (N=737) | |||||||
|---|---|---|---|---|---|---|---|---|
| Exam 5 | Exam 6 | Exam 7 | P for Trend | Exam 5 | Exam 6 | Exam 7 | P for Trend | |
| Age-adjusted analyses† | ||||||||
| Low LDL-C (<130 mg/dL), % | 59.2 | 57.3 | 59.4 | 0.89 | 61.7 | 57.3 | 59.8 | 0.40 |
| Borderline LDL-C (130-160 mg/dL), % | 30.8 | 29.0 | 28.3 | 0.24 | 26.3 | 29.4 | 27.7 | 0.54 |
| High LDL-C (>160 mg/dL), % | 10.0 | 13.8 | 12.2 | 0.10 | 11.3 | 12.8 | 12.1 | 0.62 |
| Low HDL-C (<40 mg/dL in men, <50 mg/dL in women),% | 37.4 | 36.6 | 33.2 | 0.019 | 34.5 | 31.6 | 26.2 | <0.0001 |
| High TG (>150 mg/dl), % | 32.0 | 29.0 | 31.0 | 0.57 | 22.0 | 20.2 | 18.6 | 0.055 |
| Multivariable-adjusted analyses‡ | ||||||||
| Low LDL-C (<130 mg/dL), % | 59.3 | 57.3 | 59.4 | 0.99 | 61.5 | 57.3 | 60.2 | 0.55 |
| Borderline LDL-C (130-160 mg/dL), % | 30.6 | 28.9 | 28.4 | 0.30 | 26.4 | 29.3 | 27.4 | 0.67 |
| High LDL-C (>160 mg/dL), % | 9.9 | 13.7 | 12.3 | 0.082 | 11.2 | 12.7 | 12.0 | 0.62 |
| Low HDL-C (<40 mg/dL in men, <50 mg/dL in women),% | 37.7 | 35.3 | 31.2 | 0.0007 | 33.4 | 29.3 | 23.7 | <0.0001 |
| High TG (>150 mg/dl), % | 32.0 | 27.0 | 28.1 | 0.045 | 19.6 | 16.6 | 14.7 | 0.0038 |
Values are age- and multivariable-adjusted proportions from pooled logistic regressions including examination cycle to account for correlated observations.
Adjusted for age and examination cycle.
Adjusted for age, examination cycle, body mass index, active smoking, alcohol consumption, anti-hypertensive treatment, and total cholesterol (in analyses of HDL-C and TG), using covariates from the examination in question.
Abbreviations: LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol, TG, triglycerides
In the larger sample including individuals with prevalent CVD, lipid therapy or hormone replacement therapy at any of the three examinations, the proportions of individuals with low HDL-C or high TG changed in the same manner as in the primary analysis (Supplementary Table 2). Also, in this sample the proportions of individuals on prescribed lipid therapy increased significantly in age- and multivariable-adjusted analyses in both men and women, and the categories of LDL-C levels changed significantly over the three examination periods.
Conjoint Changes in HDL-C and TG between 1991 and 2001
The age-adjusted Spearman correlations between change in HDL-C and change in TG (delta HDL-C and delta TG) from examination 5 to examination 7 were −0.35 in men and −0.31 in women, (P<0.0001 for both correlations). When study participants were grouped into four categories by the pattern of change in HDL-C and TG between examinations 5 and 7, the largest category of change for both men and women, consisted of participants with an increase in HDL-C and a decrease in TG (Table 4). Furthermore, this category had the smallest increases of BMI between examination 5 and 7 in both men and women in multivariable-adjusted analyses (P<0.005 for a comparison of the group with a decrease in TG and increase in HDL-C with the three other groups, except in women with an increase in both HDL-C and TG where P=0.035).
Table 4.
Age- and Multivariable-Adjusted Mean Changes* of Lipids, Fasting Glucose and Body Mass Index Between Examination Cycle 5 and 7 by Pattern of Change† in High-Density Lipoprotein Cholesterol and Triglycerides
| Men (N=860) | Women (N=697) | |||||||
|---|---|---|---|---|---|---|---|---|
| ↑ HDL-C ↑ TG (N=167) | ↑ HDL-C ↓ TG (N=339) | ↓ HDL-C ↑ TG (N=215) | ↓ HDL-C ↓ TG (N=139) | ↑ HDL-C ↑ TG (N=188) | ↑ HDL-C ↓ TG (N=263) | ↓ HDL-C ↑ TG (N=164) | ↓ HDL-C ↓ TG (N=82) | |
| Age-adjusted analyses‡ | ||||||||
| Change in total cholesterol, mg/dL | 19.3 (15.8–22.8) | −1.7 (−4.2 – 0.7) | 4.0 (0.9–7.0) | −14.0 (−17.8 – −10.2) | 21.8 (18.1–25.5) | 5.2 (2.1–8.4) | 8.1 (4.1–12.1) | −8.3 (−13.9 – −2.6) |
| Change in HDL-C, mg/dL | 6.1 (5.1–7.0) | 8.4 (7.8–9.1) | −6.0 (−6.8 – −5.2) | −5.6 (−6.7 – −4.6) | 7.5 (6.6–8.5) | 9.6 (8.8–10.4) | −7.2 (−8.2 – −6.2) | −6.7 (−8.3 – −5.5) |
| Change in TG §, mg/dL | 30.4 (20.4–40.5) | −56.8 (−63.8 – −50.0) | 61.5 (52.7–70.4) | −37.2 (−48.2 – −26.2) | 29.8 (23.8–35.8) | −37.3 (−42.4 – −32.2) | 49.4 (42.9–55.8) | −25.7 (−34.8 – −16.6) |
| Change in fasting glucose, mg/dL | 7.6 (4.3–10.9) | 3.9 (1.6–6.3) | 8.8 (5.9–11.7) | 3.4 (−0.2 – 7.0) | 6.0 (2.6–9.3) | 0.5 (−2.3 – 3.4) | 6.7 (3.1–10.3) | 3.3 (−1.8 – 8.4) |
| Change in BMI, kg/m2 | 0.7 (0.4–1.0) | 0.0 (−0.2 – 0.2) | 1.5 (1.2–1.7) | 0.8 (0.4–1.1) | 0.8 (0.5–1.2) | 0.2 (−0.1 – 0.5) | 1.8 (1.4–2.2) | 1.3 (0.7–1.8) |
| Multivariable-adjusted analyses§ | ||||||||
| Change in total cholesterol, mg/dL | 17.9 (14.7–21.1) | −0.9 (−3.2 – 1.4) | 3.9 (1.0–6.7) | −14.1 (−17.7 – −10.6) | 20.8 (17.3–24.4) | 5.3 (2.3–8.3) | 8.9 (5.1–12.7) | −7.8 (−13.2 – −2.4) |
| Change in HDL-C, mg/dL | 5.9 (5.0–6.9) | 8.4 (7.8–9.1) | −5.9 (−6.8 – −5.1) | −5.5 (−6.6 – −4.5) | 7.5 (6.6–8.5) | 9.6 (8.8–10.4) | −7.2 (−8.2 – −6.2) | −6.9 (−8.4 – −5.5) |
| Change in TG§, mg/dL | 29.3 (19.2–39.3) | −55.9 (−63.0 – −48.8) | 61.1 (52.3–70.0) | −37.2 (−48.2 – −26.2) | 29.4 (23.4–35.5) | −36.9 (−42.0 – −31.9) | 49.3 (42.8–55.7) | −25.9 (−35.0 – −16.8) |
| Change in fasting glucose, mg/dL | 7.5 (4.3–10.8) | 3.7 (1.4–6.0) | 9.1 (6.3–12.0) | 3.7 (0.1–7.2) | 5.7 (2.3–9.1) | 0.6 (−2.3 – 3.4) | 6.7 (3.1–10.3) | 3.4 (−1.4 – 8.9) |
| Change in BMI, kg/m2 | 0.7 (0.4–1.0) | 0.0 (−0.2 – 0.2) | 1.5 (1.2–1.7) | 0.8 (0.4–1.1) | 0.8 (0.4–1.1) | 0.3 (−0.1 – 0.6) | 1.9 (1.5–2.2) | 1.2 (0.7–1.8) |
Values are age- and multivariable-adjusted mean changes (95% confidence intervals) between examination 5 and 7.
All individuals were categorized into four groups by the pattern of change in HDL-C and TG between examination 5 and 7 (increase in both HDL-C and TG; increase in HDL-C and decrease in TG; decrease in HDL-D and increase in TG; and decrease in both HDL-C and TG).
Adjusted for age and examination cycle.
Adjusted for age, examination cycle, body mass index, active smoking, alcohol consumption, anti-hypertensive treatment, and total cholesterol (in analyses of HDL-C and TG). All covariates were collected at examination cycle 5.
Abbreviations: HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; BMI, body mass index.
A comparison of data from diet questionnaires at examination 5 and 7 did not show any significant changes in caloric intake between the various lipid groups. Although in multivariate-adjusted analysis there was a larger but non-significant decrease in calorie intake in men with a decrease in TG and increase in HDL-C compared to an index group with both a decrease in TG and HDL-C, this change was not significant (P=0.64) and virtually no differences were apparent in women.
In secondary analyses where we repeated all models with waist circumference instead of BMI, the reciprocal trends of HDL-C and TG were almost identical as in the main analyses (data not shown).
DISCUSSION
Principal Findings
We have found in the Framingham Heart Study (Offspring) population a substantial reduction in recent years in plasma TG coupled with an increase in HDL-C concentrations. This change in TG and HDL-C was observed over the relatively brief period of the last 3 completed Framingham exams (from 1991 to 2001) in both men and women; throughout a broad range of TG and HDL-C values; was independent of use of lipid-modifying drugs or hormone therapy; and, most notably, occurred in the presence of generally increasing levels of BMI. These results are in sharp contrast to results from recent broad cross-sectional population surveys in the United States 9,16 which have included individuals prescribed lipid therapy, and which have not found any significant changes in plasma TG or HDL-C.
Although overall there was an increase in HDL-C and decrease in TG in the Framingham population, it is apparent with the categorization of the population by patterns of change in TG and HDL-C (shown in Table 4) that there was appreciable heterogeneity in the change of these combined lipid fractions. In the largest segment of the population shown in Table 4 (39% of men and 38% of women) there was, notably, a decrease in TG coupled with an increase in HDL-C. In this group there was also the smallest increase in BMI. Thus, irrespective of the BMI increase in the population as a whole, the most favorable change in TG and HDL-C in Framingham was associated with the least increase in BMI. Consequently, although a population-wide increase in weight did occur in the Framingham Study during the period of these lipid changes, this increase was largely in association with increases in TG and/or decreases in HDL-C. Although we attempted to determine if a change in diet calories could be detected from food frequency questionnaires 15 that might be correlated with the differences in BMI and lipid changes, no clear differences between these subgroups could be detected. However, we acknowledge that such post-hoc analyses are secondary, and should only be considered hypothesis generating.
Comparison with Prior Studies
To our knowledge, no community-based studies have reported a recent population-wide increase in blood HDL-C associated with a decrease in TG levels. In the recent reports from the NHANES 3 or Minnesota Heart Survey 16, the authors did not report significant changes of HDL-C or TG in their primary analyses. The NHANES analysis 3 however, did show a slight increases of age-adjusted HDL-C in women (from 53.8 mg/dL in 1976–1980 to 55.9 mg/dL in 1999–2002), but not in men. Further, they found a borderline significant increase in TG levels when restricting their sample to adults aged 20 to 74 years. In the report from Minnesota Heart Survey 16 there was no information regarding TG levels, and HDL-C levels were found to be unchanged both in men and women, in all age categories. In further contrast to the results of the NHANES and Minnesota cross-sectional surveys, which have reported decreasing values of total blood cholesterol concentrations in United States adults without excluding individuals treated with lipid drugs, we did not find a change in total cholesterol in our sequentially examined Framingham Offspring cohort that was not treated with lipid drugs.
We believe that the primary reason other population surveys may not have detected the kind of changes in TG and HDL-C that we report is likely due to the cross-sectional design of these other studies, compounded, perhaps, by a failure to separate subgroups of individuals by patterns of lipid change. Some of the differences in results may also be attributed to differences in laboratory methods between exams or a failure to adjust analyses for potentially confounding other therapy. However, we also recognize that differences in results between repeated cross-sectional surveys and sequential population examinations may also be related to differences in dealing with the effects of age which in cross-sectional analysis entails “age-matching” and in a sequential study requires adjusting for age. We further recognize that the changes observed in sequential population examinations (like Framingham), and unlike cross-sectional studies (like NHANES), could be influenced not just by the changing age of a population but by previous examinations that, in particular, might provide an impetus for participants to adopt more favorable lifestyle measures (like decreased smoking or increased physical activity).
Potential Mechanism for the Observed Changes in Lipid Levels and Body Weight
At present, we have no certain explanation for what can be viewed as a potentially beneficial, recent change in blood lipid levels in a fairly large segment of the Framingham Heart Study population. The change we have observed in which TG and HDL-C are inversely related would strongly imply that there is a physiologic explanation that is responsible for an initial reduction in TG that is accompanied by a causally related increase in HDL-C. Blood levels of TG and HDL-C are well-known to be reciprocally related and are changed, principally through the action of the TG hydrolase, lipoprotein lipase. This enzyme catalyzes the hydrolysis of plasma TG-rich lipoproteins that results in not only smaller TG-rich particles but an increase in HDL particles that are newly created from the excess of polar lipids and apoproteins that originate from the surface of the TG-rich lipoproteins 17,18. Indeed, animal studies suggest that with acute inhibition of lipoprotein lipase and a reduction in blood HDL-C levels as much as 50% of the concentration of HDL-C in the blood may be the result of the catabolism of TG-rich lipoproteins 19.
We think it is possible that a decrease in plasma TG coupled with an increase in HDL-C in the Framingham population may be the result of more active and complete hydrolysis of TG-rich lipoproteins. If so, why this may have occurred is not known, but NHANES diet surveys suggest recent large changes in patterns of food consumption in the United States,20 characterized by an increase in carbohydrates, a reduction in total fats, and especially a reduction in saturated fat. We think it is possible that a relative reduction in dietary saturated fat could result in plasma triglyceride-rich lipoprotein particles with both phospholipids and triglycerides that have relatively more unsaturated acyl chains in most recent exams than in previous exams. TG-rich lipoproteins with more unsaturated acyl groups are likely to be more readily hydrolyzed than more saturated acylglycerides 21,22, and consequently result in increased formation of HDL.
Strengths and Limitations
The strengths of our study include the large, community-based sample consisting of men and women with repeated and standardized lipid measurements, allowing analyses of intra-individual changes, the re-measurement of lipid levels to exclude assay-related changes over time, and the detailed information on potential confounders. Nevertheless, there are also several limitations of our study. As any study of trends restricted to the same individuals, there may be a survivor basis that would more likely show a more favorable than unfavorable change in lipids. Additionally, we cannot entirely rule out the possibility that knowledge of the lipid levels could have induced lifestyle changes or medication use in the participants that may have affected the lipid trends in this longitudinal cohort study, i.e., a healthy cohort effect. Furthermore, our study sample consisted predominantly of middle-aged whites of European descent, limiting the generalizability of our findings to other age groups and ethnicities. We did not have uniform information about physical activity at each examination cycle, which might be relevant to changes in TG and HDL-C. However, the results we have obtained were robust upon adjustment for BMI, that is known to have an effect on TG and HDL-C levels and might be expected to (inversely) reflect the extent of exercise. Finally, our dietary information was obtained from a food frequency questionnaire, estimating the kinds of foods consumed over a one-year period. We recognize that although we found no differences in calories consumed between various lipid groups, small changes in the categories of dietary nutrients which might influence plasma lipids might not be detected by dietary (recall) histories.
Conclusions
In our longitudinal study of a large community-based population during the period from 1991 to 2001, we observed decreasing dyslipidemia characterized by increasing HDL-C levels coupled with decreasing TG. Over this same time period, BMI generally increased, but increased less in those with the most favorable change in TG and HDL-C. Our observation is not easily explained but may relate to diet changes, especially to changes in the kinds of fat that may influence the turnover of TG-rich lipoproteins. Additional studies are warranted to confirm our findings and to elucidate the mechanisms underlying these potentially favorable trends.
Supplementary Material
Acknowledgments
Sources of Support: This work was supported through the Swedish Heart-Lung Foundation (Dr Ingelsson) and National Institute of Health/National Heart, Lung & Blood Institute Contract N01-HC-25195.
Role of the Funding Source
The funding sources had no role in the study design, analyses, or drafting of the manuscript. The NHLBI reviews all manuscripts submitted for publication but it was not involved in the decision to publish.
Footnotes
Author Contributions:
Dr. Robins had full access to all ofthedata in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ingelsson, Massaro, D’Agostino, Vasan, Robins
Acquisition of data: Sutherland, Levy, Jacques, Vasan, Robins
Analysis and interpretation of data: Ingelsson, Massaro, Jacques, D’Agostino, Vasan, Robins
Drafting of the manuscript: Ingelsson, Robins
Critical revision of the manuscript for important intellectual content: Ingelsson, Massaro, Sutherland, Levy, Jacques, D’Agostino, Vasan, Robins
Statistical expertise: Massaro, D’Agostino
Administrative, technical, or material support: D’Agostino, Robins
Study supervision: D’Agostino, Vasan, Robins
Conflicts of Interest Statement
None of the authors have any conflicts of interest to disclose.
References
- 1.Eckel RH, York DA, Rossner S, et al. Prevention Conference VII: Obesity, a worldwide epidemic related to heart disease and stroke: executive summary. Circulation. 2004;110:2968–2975. doi: 10.1161/01.CIR.0000140086.88453.9A. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 3.Okosun IS, Chandra KM, Boev A, et al. Abdominal adiposity in U.S. adults: prevalence and trends, 1960–2000. Prev Med. 2004;39:197–206. doi: 10.1016/j.ypmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 5.Kraja AT, Borecki IB, North K, et al. Longitudinal and age trends of metabolic syndrome and its risk factors: The Family Heart Study. Nutr Metab (Lond) 2006;3:41. doi: 10.1186/1743-7075-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 7.Bamba V, Rader DJ. Obesity and atherogenic dyslipidemia. Gastroenterology. 2007;132:2181–2190. doi: 10.1053/j.gastro.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 8.Carroll MD, Lacher DA, Sorlie PD, et al. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA. 2005;294:1773–1781. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]
- 9.Johnson CL, Rifkind BM, Sempos CT, et al. Declining serum total cholesterol levels among US adults. JAMA. 1993;269:3002–3008. [PubMed] [Google Scholar]
- 10.Parikh NI, Pencina MJ, Wang TJ, et al. Increasing trends in incidence of overweight and obesity over 5 decades. Am J Med. 2007;120:242–250. doi: 10.1016/j.amjmed.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 12.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987;166:1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- 13.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 15.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 16.Arnett DK, Jacobs DR, Jr, Luepker RV, Blackburn H, Armstrong C, Claas SA. Twenty-year trends in serum cholesterol, hypercholesterolemia, and cholesterol medication use: the Minnesota Heart Survey, 1980–1982 to 2000–2002. Circulation. 2005;112:3884–3891. doi: 10.1161/CIRCULATIONAHA.105.549857. [DOI] [PubMed] [Google Scholar]
- 17.Redgrave TG, Small DM. Quantitation of the transfer of surface phospholipid of chylomicrons to the high density lipoprotein fraction during the catabolism of chylomicrons in the rat. J Clin Invest. 1979;64:162–171. doi: 10.1172/JCI109435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tall AR, Green PH, Glickman RM, Riley JW. Metabolic fate of chylomicron phospholipids and apoproteins in the rat. J Clin Invest. 1979;64:977–989. doi: 10.1172/JCI109564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behr SR, Patsch JR, Forte T, Bensadoun A. Plasma lipoprotein changes resulting from immunologically blocked lipolysis. J Lipid Res. 1981;22:443–451. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report: Trends in Intake of Energy and Macronutrients – United States, 1971–2000. 2004 Feb 6;53(4):80–82. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5304a3.htm. [PubMed]
- 21.Botham KM, Avella M, Cantafora A, Bravo E. The lipolysis of chylomicrons derived from different dietary fats by lipoprotein lipase in vitro. Biochim Biophys Acta. 1997;1349:257–263. doi: 10.1016/s0005-2760(97)00134-3. [DOI] [PubMed] [Google Scholar]
- 22.Clark SB, Derksen A. Phosphatidylcholine composition of emulsions influences triacylglycerol lipolysis and clearance from plasma. Biochim Biophys Acta. 1987;920:37–46. doi: 10.1016/0005-2760(87)90308-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.