Abstract
Malignant gliomas are the most common and lethal primary central nervous system cancer. Glioblastoma mutliforme (GBM), the most aggressive of these neoplasms, are generally lethal within two years of diagnosis due in part to the intense apoptosis resistance of its cancer cells, hence poor therapeutic response to conventional and targeted therapies. Twenty years of research has uncovered key genetic events involved in disease initiation and progression, foremost the Tp53 tumor suppressor that is mutated or deleted in 35% of GBM. The prime importance of p53 signaling for gliomapathogenesis is further evidenced by epistatic genetic events targeting additional pathway components including deletion of p14Arf (CDKN2A) and amplification of the p53-degrading ubiquitin ligases MDM2 and MDM4. Recent studies have identified and validated Bcl2-Like 12 (Bcl2L12) as a potent glioma oncoprotein with multiple strategic points in apoptosis regulatory networks, i.e. effector caspases and the p53 tumor suppressor. Bcl2L12 resides in both the cytoplasm and nucleus. In the cytoplasm, Bcl2L12 functions to inhibit caspases 3 and 7, in the nucleus, Bcl2L12 forms a complex with p53, modestly reduces p53 protein stability and prevents its binding to selected target gene promoters (e.g. p21, DR5, Noxa and PUMA), thereby inhibiting p53-directed transcriptomic changes upon DNA damage. Proteomic and multidimensional oncogenomic analyses confirmed a Bcl2L12-p53 signaling axis in GBM, as Bcl2L12 exhibited predominant genomic amplification, elevated mRNA and protein levels in GBM tumors with uncompromised p53 function. On the cell biological level, Bcl2L12 exerts robust inhibition of p53-dependent senescence and apoptosis processes in glioma cells. These multi-leveled studies establish Bcl2L12 as an important oncoprotein acting at the intersection of nuclear p53 and cytoplasmic caspase signaling and point to pharmacological disruption of the Bcl2L12:p53 complex as a promising novel therapeutic strategy for the enhanced treatment of GBM.
Key words: Bcl2L12, p53, glioblastoma multiforme (GBM), The Cancer Genome Atlas (TCGA) Project
Introduction
Neoplasms of glial cells represent the most common tumors in the central nervous system (CNS).1–3 They can be stratified into four different grades with distinct biological behaviors and clinical outcomes.4 GBM, or grade IV malignant glioma, represent the most prevalent and aggressive brain cancer with a highly progressive clinical course that culminates in death after only 1–2 years post diagnosis. GBM can manifest ‘de novo’ as primary GBM in older patients without any evidence of prior clinical disease or present as secondary GBM in younger patients evolving from progression of low-grade tumors.1–3 Aggressive surgical resection and advanced radiation and chemotherapy have minimally impacted the median survival of GBM patients; radiation, together with the alkylating agent temozolomide, is the current standard care and imparts only modestly on median survival.5
The feeble impact of anti-glioma drug regimens relates to many factors. In particular, there is imperfect surgical eradication due to the invasive nature of GBM throughout the brain, the presence of an intact blood-brain-barrier, which limits drug penetration at sites distant from the primary tumor, the high degree of inter- and intratumoral genomic heterogeneity and sustained genome instability providing opportunity for the development of additional resistance mechanisms. These factors, coupled with the presence of a therapy-resistant glioma stem population, have conspired to make GBM an incurable disease.
Recent astounding progress in functional genomics and genetic model systems has positioned the field to make meaningful advances. In the area of genomics, new genome scanning and computational technologies have resulted in the identification of numerous additional glioma oncogenes and tumor suppressors as novel, putative drug targets. Advances in RNAi and viral vector technology and a growing collection of cDNAs have greatly facilitated the validation of myriad genetic elements of interest. These rich genomic and functional validation efforts have provided a host of signature genetic events that define GBM subsets and enabled the construction of refined mouse models of human GBM that have deepened our understanding of how key glioma genes operate in disease pathogenesis. For example, multidimensional oncogenomics6 and sophisticated genetically engineered mouse models with concomitant CNS-specific deletion of p53 and Pten7,8 have produced penetrant high-grade malignant gliomas with clinical, pathological and molecular resemblance to primary GBM of the proneural subtype. This model afforded a system to define the cooperative interactions of p53 and Pten, revealing important roles in the regulation of Myc-directed stemness and differentiation. This model system and others, together with genomic profiles showing frequent inactivating mutations of Tp53 across multiple glioma grades,6, 9–11 establish p53 as a universal key tumor suppressor not only in low-grade astrocytoma and secondary GBM, but also in primary GBM tumors.
Functional genomics and subsequent cell biological validation efforts have identified a plethora of novel glioma oncoproteins and tumor suppressors that enhanced our knowledge of disease mechanisms. Here, we summarize our recent work that identified Bcl2L12, an atypical Bcl-2 family oncoprotein with potent cytoplasmic and nuclear anti-apoptotic activities, as an inhibitor of the tumor suppressive activity of p53.12 We review experimental paradigms to assess and quantify Bcl2L12-mediated p53 inhibition in glial cells, compare Bcl2L12's modus operandi to canonical Bcl-2 family proteins and discuss future anti-glioma strategies to disrupt the Bcl2L12:p53 complex that may enhance p53-dependent apoptosis, senescence and proliferative arrest.
Bcl2L12 is a Glioma Oncoprotein Governing the Apoptosis and Necrosis Balance in Glial Cells
Bcl2L12 is a proline-rich protein characterized by a C-terminal 14 amino acid sequence with significant homology to the BH (Bcl-2 Homology) 2 domain found in several members of the Bcl-2 family.13,14 Tissue microarray analysis validated robust Bcl2L12 protein expression in 96% of human primary GBM specimens with low or undetectable levels in cells of glial origin in normal brain surrounding tumor tissue or in low-grade astrocytoma.14 Enforced expression of Bcl2L12 in primary cortical astrocytes and transformed glioma cell lines enhanced cellular growth and in vivo tumorigenicity, conferred marked apoptosis resistance, yet engendered cellular necrosis and effected malignant transformation in cooperation with other glioma genes. On the biochemical level, Bcl2L12's oncogenic actions stemmed in part from its capacity to inhibit apoptosis by neutralizing effector caspase activity downstream of mitochondrial dysfunction and apoptosome activity. This caspase-inhibitory activity of cytoplasmic Bcl2L12 is associated with direct binding and inhibition of caspase-714 as well as with transcriptional up-regulation of the αB-crystallin gene, encoding a small heat shock protein, which potently inhibits caspase-315,16 (Fig. 1, right panel). This dual inhibition of effector caspase-3 and caspase-7 downstream of mitochondrial membrane disintegration is reminiscent of Inhibitor-of-Apoptosis (IAP) molecules.17,18 Intriguingly, while Bcl2L12 contributes to intense apoptosis resistance of GBM, the post-mitochondrial block at the level of effector caspases can shift GBM cells towards a necrotic fate. That is, under apoptosis-inducing conditions, mitochondrial dysfunction and extensive cytochrome c release impair oxidative phosphorylation and ATP production, rendering cells unable to maintain ion homeostasis and provoking cellular edema, dissolution of organelles and plasma membranes. Indeed, analysis of plasma membrane integrity and subcellular organelle morphology comprehensively demonstrated a pro-necrotic activity of Bcl2L12 in response to apoptotic stimulation supporting the general notion that apoptosis and non-apoptotic death paradigms are intertwined in GBM.14,15 By blocking apoptosis signaling at the post-mitochondrial level and thereby redirecting the death program to necrosis, the molecular profile of Bcl2L12 provides a rational explanation for a prime paradox in GBM—apoptosis resistance yet florid necrosis—and points to Bcl2L12 up-regulation as a key progression event in malignant glioma. Together, these observations support a model where Bcl2L12 up-regulation, together with hypoxic stresses and nutrient deprivation in the tumor microenvironment caused by vascular regression/occlusion and intravascular thrombosis, promote neurologically debilitating necrosis.
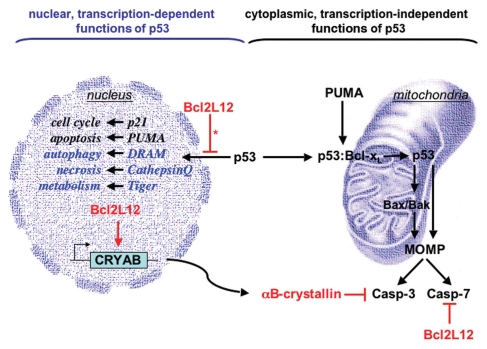
Figure 1.
Nuclear and cytoplasmic anti-apoptotic activities of Bcl2L12. Bcl2L12 inhibits p53's transactivational activity and consequently abrogates transcription of selective cell cycle and apoptosis modulators, such as p21 and PUMA (left, nuclear, transcription-dependent functions). Bcl2L12's impact on p53-insigated autophagy, necrosis and metabolism-related pathways (representative targets highlighted in blue) require further studies. In the cytosol (right panel), p53 has direct apoptogenic activities at the level of mitochondria. Here, PUMA can displace p53 from an inhibitory p53:Bcl-xL complex. Released p53 can act as a ‘BH3’-only activator of Bax/Bak to induce mitochondrial outer membrane permeabilization (MOMP) and subsequent caspase activation. Besides impacting p53, Bcl2L12 is a well-characterized inhibitor of postmitochondrial effector caspase activation, as it binds to and inhibits caspase-7 (Casp-7) and upregulates the small heat shock protein and caspase-3-specific inhibitor αB-crystallin (CRYAB). *, of note, Bcl2L12 selectively impacts p53 transcription and promoter occupancy.
While Bcl2L12's impact on effector caspases proved to be an important aspect of its oncogenicity, we considered the possibility that Bcl2L12 may have additional molecular functions. Specifically, Bcl2L12's nuclear localization and the capacity of other Bcl-2 family proteins (e.g., Bax, Bak, and Bcl-xL) to interact with and functionally impact p5319–23 prompted us to assess the potential for physical and functional Bcl2L12-p53 interactions.
Bcl2L12 Inhibits p53-Directed Replicative Senescence and DNA Damage-Instigated Apoptosis
Replicative senescence in serially passaged primary mouse and human cells is strongly dependent on a functional p53 pathway. Mouse embryonic fibroblast (MEF) culture have served as a murine model system to study the molecular regulators of senescence and have established the essentiality of either p53 or p19Arf deficiencies in bypassing passage-induced senescence and maintenance of indefinite proliferative potential.24,25 To assess whether Bcl2L12 can bypass p53-dependent senescence programs, Bcl2L12 was retrovirally transduced into low-passage wt MEFs. While vector control cultures senesced after 10–15 passages, Bcl2L12-expressing cultures showed unabated growth and attained an immortal phenotype in the absence p53 or p19Arf extinction.12
p53 has many distinct functional activities, including its prominent role in apoptosis signaling. In particular, p53 acts as a cellular sentinel for DNA damage and orchestrates complex pro-apoptotic responses via transcription-dependent and independent mechanisms (see below). In this cellular program, p53 can potently transactivate the expression of a variety of apoptosis inducers, such as death receptors and pro-apoptotic Bcl-2 family proteins, and directly facilitates cytochrome c release through physical interaction with several Bcl-2 family proteins and mitochondrial membranes.26 This knowledge prompted us to assess Bcl2L12's impact on p53-mediated apoptosis. We surveyed effector caspase activation in cortical astrocytes treated with the DNA-intercalating drugs doxorubicin and actinomycin D, and found robust inhibition of p53-dependent apoptosis in response to DNA damage. Together, these initial cell culture-based assays demonstrated that Bcl2L12 potently inhibited the cardinal p53-ochestrated biological processes of replicative senescence and apoptosis and pointed to p53 pathway inactivation as an additional dimension of Bcl2L12-directed gliomagenesis.12 How then does Bcl2L12 neutralize p53 activity?
Bcl2L12 and its Distant Relatives of the Bcl-2 Family Meet p53—Once a Black Sheep, Always a Black Sheep
p53 is a transcription factor that orchestrates the expression of more than 2500 target genes to restrain tumor growth.27 In recent years, this classical view of p53 action has been expanded significantly to encompass transcription-independent cyotplasmic interactions with Bcl-2 family proteins to regulate apoptosis. Specifically, transactivation-incompetent p53 mutants have been shown to promote apoptosis and suppress oncogene-induced cellular transformation of rodent fibroblasts and human osteosarcoma cells.28,29 Notably, p53-directed apoptosis can proceed in the absence of de novo RNA and protein synthesis,30 operate in enucleated cells,31 and can be triggered by p53-reactivating drugs under conditions of complete transcriptional or translational blockade.31,32 Importantly, fibroblasts derived from mice engineered with a transactivation-competent p53 knock-in allele that lacks domains essential for cytoplasmic functions can undergo senescence, but fail to undergo apoptosis.33
Mechanistically, p53 exerts its cytoplasmic and transcription-independent functions by intricately impacting mitochondrial physiology and canonical Bcl-2 family proteins as important regulators of mitochondrial membrane integrity. Anti-apoptotic members, such as prototypic Bcl-2 and Bcl-xL, inhibit mitochondrial outer membrane permeabilization (MOMP) by sequestering their pro-apoptotic relatives Bax and Bak to prevent their oligomerization into the outer mitochondrial leaflet and subsequent formation of MOMP-triggering supramolecular complexes. ‘Activator’ BH3-only proteins, such as Bid or Bad, bind to and directly activate Bax/Bak to induce cytochrome c release and subsequent caspase-9, -3 and -7 activation. ‘Enabler’ BH3-only proteins form complexes with anti-apoptotic Bcl-2/Bcl-xL to displace activator BH3-only proteins or even Bax or Bak themselves from an inhibitory interaction with Bcl-2/Bcl-xL.34
Chipuk et al.19,20 recently identified the p53-PUMA signaling axis as an important modulator of MOMP. In the absence of cellular stresses/oncogene activation, p53 is not stabilized and its low expression levels are insufficient to induce PUMA transcription and consequently apoptosis. In addition, the small quantities of cytoplasmic p53 are sequestered and incapacitated by Bcl-xL.22 Upon genotoxic stresses, nuclear p53 transcriptionally induces PUMA, which subsequently binds to Bcl-xL, thereby liberating p53 from a p53:Bcl-xL. Released p53 can then directly bind to and activate Bax.19,20 Alternatively, p53 may also function as an enabler that binds Bcl-2/Bcl-xL to release the Bax/Bak activator Bid22 or as an activator that forms a complex with Bak, thereby disrupting a Bak-inhibitory Bak:Mcl-1 heterodimer.21
Interestingly, nuclear magnetic resonance (NMR) together with molecular structure-function analyses identified the DNA-binding domain (DBD) of p53 as a critical binding interface for Bcl-2 proteins.22 Consequently, oncogenic hot-spot mutations affecting the DBD of p53 not only abrogate DNA binding and transactivational activity, but also hamper p53's direct apoptogenic role as a mitochondrial effector. Interestingly, cytoplasmic and nuclear functions of p53 are intertwined on different levels. The p53 transcriptional target Mdm2 is important to regulate its cytoplasmic localization through mono-ubiquitinylation35 and p53-induced PUMA can release Bax and/or p53 from inhibitory Bax/Bcl-xL and p53/Bcl-xL complexes,19 suggesting that p53's nuclear functions are essential for its activities in the cytoplasm.
Given such prominence of Bcl-2 family proteins in regulating p53 activity, we dissected the molecular mechanisms, by which Bcl2L12 compromises p53 function. Bcl2L12 is an atypical Bcl-2-like protein with only focal homology to canonical family members. The polypeptide contains a single Bcl-2 homology domain 2 (BH2), but lacks additional BH as well as transmembrane motifs and consequently is not inducibly and constitutively associated with intracellular, most importantly mitochondrial membranes.14 Reflecting its atypical domain structure and lack of phylogenetic resemblance to canonical Bcl-2 proteins, Bcl2L12 does not safeguard mitochondrial membrane integrity but instead, as noted above, inhibits postmitochondrial effector caspase activation in an IAP-like fashion (Fig. 1, right panel).14–16 Further reflecting such functional distinctiveness relative to prototypic Bcl-2 family proteins, Bcl2L12 limits p53 binding to selective target gene promoters and consequently reduces transcription of important p53 downstream effectors such as Puma, Noxa, DR5, p21 and cyclin G1, but not of Mdm2 (Fig. 1, left panel).12
The precise molecular mechanism of Bcl2L12's selective impact on the p53 transcriptome is not known. Intriguingly, the capacity of p53 to differentially impact target genes and selectively bind certain promoters is based on (1) the affinity of their p53 binding sites, (2) complex formation of p53 with cofactors, such as Mdm2/Mdm4, p63/p73, Bbp and ASPP proteins, and (3) the post-translational status of the p53 polypeptide.36 Future studies aiming to further characterize the Bcl2L12:p53 complex and define essential cofactors driving selective p53 binding to promoter elements will provide important clues for understanding how Bcl2L12 impact the p53 transcriptome. In addition, it will be imperative to understand in more detail, how Bcl2L12 controls p53 post-translational modifications to drive selective p53 promoter binding, in particular acetylation/deacetylation, methylation, sumoylation and neddylation. Here, additional studies should probe further for functional and physical interactions between Bcl2L12 and post-translational modifiers of p53 such as the histone acetyltransferases CBP/p300, p300/CBP associated factor (PCAF), Tip60, hMof, acetyltransferases of the MYST family, histone deacetylase complexes such as HDAC and Sir2α/Sirt1, the methyltransferases Set7/9, Smyd2, Set8/PR-Set7, the demethylase LSD1 and PRMT, and the neddylating enzyme FBXO11.36
Interestingly, Bcl2L12-driven cDNA complementation and RNAi loss-of-function studies in primary and transformed glial cells demonstrated that Bcl2L12 modestly impacts p53 protein stability. Enforced Bcl2L12 expression reduced p53 abundance with limited p53 posttranslational modifications (i.e. phosphorylation and acetylation), and stable Bcl2L12 protein knockdown triggered enhanced p53 induction with augmented acetylation and Ser/Thr-directed phosphorylation.12 We hypothesize that reduced p53 post-translational modifications, in particular N-terminally directed Ser/Thr phosphorylation in the setting of intact Mdm2 might contribute to enhanced p53 protein degradation. While multiple in vitro studies demonstrated that p53 can be phosphorylated by a broad range of kinases, such as ATM, ATR, DNA-PK and Chk1/2 [in particular at Ser15 (mouse Ser18) and Ser20 (mouse Ser23)] to block binding to and degradation by Mdm2, it is important to note, that in vivo studies with Ser18A/Ser23A knock-in mouse models revealed only minor impact of Ser18/23 phosphorylation on p53 stability and consequently argued against phosphorylation as a general requirement for p53 stabilization.36 Additional, more elaborate regulatory networks are likely operative to further regulate p53 stability. Such signaling pathways that are potentially impacted by Bcl2L12, include Mdm2 and its regulators HAUSP, the ribosomal proteins L5, 11 and 23, RASSF1A, Daxx, YY1, HLI98, and additional E3 ubiqutin ligases, such as Mdm4, COP1, Prih2 and Arf-BP1 that all can impact p53 protein stability via different modes of action.36 Future studies aim to analyze Bcl2L12's impact on these multi-faceted signaling circuits that regulate p53 protein abundance.
Relationship of Bcl2L12 Expression and p53 Status in the TCGA GBM Dataset
Beyond direct mutation/deletion of p53, the tumor suppressive function of p53 can be neutralized by the E3 ubiquitin ligase Mdm2 acting to limit p53 action through ubiquitinylation and subsequent proteosomal degradation,37 and the cell cycle inhibitor p14Arf that binds Mdm2 and prevents formation of a p53-degrading Mdm2:p53 complex.38–41 To genetically verify a p53-Mdm2-p14Arf signaling axis in GBM, several oncogenomic and immunohistochemical surveys assessed co-amplification and -expression of p53, Mdm2 and p14Arf in clinical samples. Specifically, studies in GBM39,42 and other cancer types, such as sarcoma43,44 demonstrated that Mdm2 amplification and TP53 mutation/deletion are mutually exclusive events. Additionally, p14Arf under- and Mdm2 over-expression are inversely correlated in neuroendocrine and non-small cell lung carcinoma with an Mdm2/p14Arf ratio > 1 indicative of a high-grade tumor phenotype.45 Such studies genetically verified that Mdm2 and p14Arf act in common pathway(s) to regulate p53 function. Mirroring and expanding these studies, TCGA-based oncogenomic and small-scale proteomic analyses of Bcl2L12 copy number alterations (CNA, seen as non-focal gain of chromosome 19q), mRNA and protein expression revealed higher Bcl2L12 levels in specimens with uncompromised p53 signaling and less robust chromosomal gains and expression in tumors with p53 pathway inactivation.12
Based on GBM's expression profiles and genetic aberrations, GBM tumors are subclassified into proneural, neural, classical and mesenchymal subtypes.46 Deletions and somatic mutations of p53 occur predominantly in the proneural subtype. In this subtype, Bcl2L12 expression was also anti-correlated with the presence of p53 mutation further supporting a Bcl2L12-p53 signaling axis.
Conclusions and Future Directions
Continued study of Bcl2L12 has revealed a rich network of molecular interactions that impact key glioma molecules, such as effector caspases and p53 and control the hallmark tumor biological features of apoptosis resistance and pro-necrotic tendencies.12,14–16 At the same time, many unanswered question remain, as to how Bcl2L12 expression is regulated, how it impacts BTSC biology, whether its CNS-specific deletion or transgenic expression in glioma-prone mouse strains impact gliomapathogensis in vivo, how it selectively neutralizes p53 targets, and whether it operates in controlling autophagic processes and MOMP-triggering apoptogenic functions.
The nuclear, transactivational activity of p53 represents one important facet of a rich spectrum of tumorsuppressive activities. In addition to transcriptional activation and repression of genes governing apoptosis, senescence and growth arrest, p53 also regulates autophagy, necrosis and mitochondrial membrane integrity. While a role for Bcl2L12 in autophagy and MOMP induction has not yet been established, a number of observations make these connections an intriguing possibility. Autophagy has diametrically opposing roles in cancer initiation/early progression and regulation of therapy responsiveness in established tumors. As a tumor suppressive mechanism, autophagy limits oxidative stress and chromosomal instability,47 but represents a survival mechanism in response to anti-neoplastic agents.48 Fascinatingly, p53 can induce and suppress autophagy: nuclear p53 can transactivate genes that induce autophagy (such as DRAM and sestrins-1 and-2), 49–51 while cytoplasmic p53 represses autophagy via inhibition of the AMP-dependent kinase (AMPK), a positive regulator of autophagy, and activation of mTOR as a negative regulator of autophagy.52 Given the selective impact of Bcl2L12 on p53 promoter occupancy and gene transactivation, it will be important to determine whether and how Bcl2L12 modulates p53-directed trancriptomic changes regulating the autophagic process. Specifically, ChIP and mRNA expression analyses may illuminate whether Bcl2L12 inhibits p53-mediated induction of the autophagy inducers DRAM and sestrins. Although Bcl2L12 and p53 preferentially co-localize in the cell nucleus, it remains to be seen whether or not cytoplasmic Bcl2L12 may impact p53's ability to inhibit AMPK and mTOR and perhaps modulate p53's propensity to disrupt outer mitochondrial membranes. In this context, it will be important to assess whether PUMA can displace p53 from a cytoplasmic Bcl2L12:p53 complex in analogy to the PUMA-p53-Bcl-xL interplay described above.
Recent studies implicated p53 in programmed necrosis, as DNA-damaged Bax/Bak-deficient cells underwent necrotic cell death via p53-induced cathepsin Q that cooperated with reactive oxygen species (ROS) to execute necrotic cell death.53 Given the importance of Bcl2L12 as a post-mitochondrial apoptosis modulator with potent anti-apoptotic and pro-necrogenic functions (see above), future studies aim to decipher how Bcl2L12 impacts p53-instigated programmed necrosis. Specifically, promoter occupancy and mRNA expression analyses will determine whether and how Bcl2L12 impacts the p53-cathepsin Q signaling axis: Does Bcl2L12 modulate p53 binding to the cathepsin Q promoter? Besides cathepsin Q, does Bcl2L12 impact other p53-induced genes implicated in programmed necrosis, such as the post-mitochondrial effector XIAP that could drive necrotic cell death by blocking effector caspase activation similar to Bcl2L12?
p53 is arguably the most important tumor suppressor and its reactivation in established murine tumors could transiently halt malignant growth and, depending on the tumor type, triggers apoptosis, growth arrest or senescence.54–56 Several therapeutic strategies have been develop to restore p53 function in glioma cell, including adenoviral gene therapy, p53-stabilizing small molecules (CP-31398, PRIMA-1, MIRA-1, Nutlins, RITA, geldamycine), and substances that induce mitochondrial translocation of p53 to trigger MOMP (CP-31398).57 Along similar lines, the above actions of Bcl2L12 on p53 function raises the possibility of therapies that disrupt this interaction either via Bcl2L12-targeting RNAi or small molecules that may disrupt the Bcl2L12:p53 complex.
Acknowledgements
A.H.S. was supported by NIH grant 5R00CA129172-04, a Zell and a Sidney Kimmel Scholar award. Grant support to R.A.D. derives from the Ben and Catherine Ivy Foundation, the Goldhirsh Foundation and NIH grant 5P01CA95616. R.A.D. is an American Cancer Society Research Professor and supported by the Robert A. and Renee E. Belfer Foundation Institute for Innovative Cancer Science. The results reviewed here are in part based upon data generated by The Cancer Genome Atlas pilot project established by the NCI and NHGRI. Information about TCGA and the investigators and institutions who constitute the TCGA research network can be found at cancergenome.nih.gov.
References
- 1.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 2.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, et al. Malignant glioma: Genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y, Parada LF. The molecular and genetic basis of neurological tumors. Nat Rev Cancer. 2002;2:616–626. doi: 10.1038/nrc866. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumors of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norden AD, Wen PY. Glioma therapy in adults. Neurologist. 2006;12:279–292. doi: 10.1097/01.nrl.0000250928.26044.47. [DOI] [PubMed] [Google Scholar]
- 6.The Cancer Genome Atlas Research Network, author. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, et al. Pten and p53 converge on c-Myc to control differentiation, self-renewal and transformation of normal and neoplastic stem cells in glioblastoma. Cold Spring Harb Symp Quant Biol. 2008;73:427–437. doi: 10.1101/sqb.2008.73.047. [DOI] [PubMed] [Google Scholar]
- 10.Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, et al. Genetic pathways to glioblastoma: A population-based study. Cancer Res. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 11.Fukushima T, Favereaux A, Huang H, Shimizu T, Yonekawa Y, Nakazato Y, et al. Genetic alterations in primary glioblastomas in Japan. J Neuropathol Exp Neurol. 2006;65:12–18. doi: 10.1097/01.jnen.0000196132.66464.96. [DOI] [PubMed] [Google Scholar]
- 12.Stegh AH, Brennan C, Mahoney JA, Forloney KL, Jenq HT, Luciano JP, et al. Glioma oncoprotein Bcl2L12 inhibits the p53 tumor suppressor. Genes Dev. 2010;24:2194–2204. doi: 10.1101/gad.1924710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scorilas A, Kyriakopoulou L, Yousef GM, Ashworth LK, Kwamie A, Diamandis EP. Molecular cloning, physical mapping and expression analysis of a novel gene, BCL2L12, encoding a proline-rich protein with a highly conserved BH2 domain of the Bcl-2 family. Genomics. 2001;72:217–221. doi: 10.1006/geno.2000.6455. [DOI] [PubMed] [Google Scholar]
- 14.Stegh AH, Kim H, Bachoo RM, Forloney KL, Zhang J, Schulze H, et al. Bcl2L12 inhibits post-mitochondrial apoptosis signaling in glioblastoma. Genes Dev. 2007;21:98–111. doi: 10.1101/gad.1480007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stegh AH, Chin L, Louis DN, DePinho RA. What drives intense apoptosis resistance and propensity for necrosis in glioblastoma? A role for Bcl2L12 as a multifunctional cell death regulator. Cell Cycle. 2008;7:2833–2839. doi: 10.4161/cc.7.18.6759. [DOI] [PubMed] [Google Scholar]
- 16.Stegh AH, Kesari S, Mahoney JE, Jenq HT, Forloney KL, Protopopov A, et al. Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc Natl Acad Sci USA. 2008;105:10703–10708. doi: 10.1073/pnas.0712034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: Why XIAP is the black sheep of the family. EMBO Rep. 2006;7:988–994. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvesen GS, Duckett CS. IAP proteins: Blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 19.Chipuk JE, Bouchier-Hayes L, Kuwana T, Newmeyer DD, Green DR. PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science. 2005;309:1732–1735. doi: 10.1126/science.1114297. [DOI] [PubMed] [Google Scholar]
- 20.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 21.Leu JI, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 2004;6:443–450. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- 22.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 23.Petros AM, Gunasekera A, Xu N, Olejniczak ET, Fesik SW. Defining the p53 DNA-binding domain/Bcl-x(L)-binding interface using NMR. FEBS Lett. 2004;559:171–174. doi: 10.1016/S0014-5793(04)00059-6. [DOI] [PubMed] [Google Scholar]
- 24.Lowe SW, Cepero E, Evan G. Intrinsic tumor suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 25.Sherr CJ, DePinho RA. Cellular senescence: mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 26.Green DR, Kroemer G. Cytoplasmic functions of the tumor suppressor p53. Nature. 2009;458:1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beckerman R, Prives C. Transcriptional regulation by p53. Cold Spring Harb Perspect Biol. 2010;2:a000935. doi: 10.1101/cshperspect.a000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kakudo Y, Shibata H, Otsuka K, Kato S, Ishioka C. Lack of correlation between p53-dependent transcriptional activity and the ability to induce apoptosis among 179 mutant p53s. Cancer Res. 2005;65:2108–2114. doi: 10.1158/0008-5472.CAN-04-2935. [DOI] [PubMed] [Google Scholar]
- 29.Haupt Y, Rowan S, Shaulian E, Vousden KH, Oren M. Induction of apoptosis in HeLa cells by trans-activation-deficient p53. Genes Dev. 1995;9:2170–2183. doi: 10.1101/gad.9.17.2170. [DOI] [PubMed] [Google Scholar]
- 30.Caelles C, Helmberg A, Karin M. p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature. 1994;370:220–223. doi: 10.1038/370220a0. [DOI] [PubMed] [Google Scholar]
- 31.Chipuk JE, Maurer U, Green DR, Schuler M. Pharmacologic activation of p53 elicits Bax-dependent apoptosis in the absence of transcription. Cancer Cell. 2003;4:371–381. doi: 10.1016/s1535-6108(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 32.Tang X, Zhu Y, Han L, Kim AL, Kopelovich L, Bickers DR, et al. CP-31398 restores mutant p53 tumor suppressor function and inhibits UVB-induced skin carcinogenesis in mice. J Clin Invest. 2007;117:3753–3764. doi: 10.1172/JCI32481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson TM, Meade K, Pathak N, Marques MR, Attardi LD. Knockin mice expressing a chimeric p53 protein reveal mechanistic differences in how p53 triggers apoptosis and senescence. Proc Natl Acad Sci USA. 2008;105:1215–1220. doi: 10.1073/pnas.0706764105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youle RJ, Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 35.Marchenko ND, Wolff S, Erster S, Becker K, Moll UM. Monoubiquitylation promotes mitochondrial p53 translocation. EMBO J. 2007;26:923–934. doi: 10.1038/sj.emboj.7601560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toledo F, Wahl GM. Regulating the p53 pathway: In vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–923. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- 38.Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 39.Pomerantz J, Schreiber-Agus N, Liegeois NJ, Silverman A, Alland L, Chin L, et al. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- 40.Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, et al. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. Embo J. 1998;17:5001–5014. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725–734. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]
- 42.Reifenberger G, Liu L, Ichimura K, Schmidt EE, Collins VP. Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res. 1993;53:2736–2739. [PubMed] [Google Scholar]
- 43.Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumor suppressor p53. Nature. 1993;362:857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 44.Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358:80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- 45.Eymin B, Gazzeri S, Brambilla C, Brambilla E. Mdm2 overexpression and p14(ARF) inactivation are two mutually exclusive events in primary human lung tumors. Oncogene. 2002;21:2750–2761. doi: 10.1038/sj.onc.1205359. [DOI] [PubMed] [Google Scholar]
- 46.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21:1367–1381. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morselli E, Galluzzi L, Kepp O, Vicencio JM, Criollo A, Maiuri MC, et al. Anti- and pro-tumor functions of autophagy. Biochim Biophys Acta. 2009;1793:1524–1532. doi: 10.1016/j.bbamcr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Crighton D, Wilkinson S, Ryan KM. DRAM links autophagy to p53 and programmed cell death. Autophagy. 2007;3:72–74. doi: 10.4161/auto.3438. [DOI] [PubMed] [Google Scholar]
- 50.Crighton D, Wilkinson S, O'Prey J, Syed N, Smith P, Harrison PR, et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 51.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D'Amelio M, et al. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol. 2008;10:676–687. doi: 10.1038/ncb1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tu HC, Ren D, Wang GX, Chen DY, Westergard TD, Kim H, et al. The p53-cathepsin axis cooperates with ROS to activate programmed necrotic death upon DNA damage. Proc Natl Acad Sci USA. 2009;106:1093–1098. doi: 10.1073/pnas.0808173106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martins CP, Brown-Swigart L, Evan GI. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell. 2006;127:1323–1334. doi: 10.1016/j.cell.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 55.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, et al. Restoration of p53 function leads to tumor regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 56.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumor clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: Drugging the p53 pathway. Nat Rev Cancer. 2009;9:862–873. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]