Abstract
Trophinin is an intrinsic membrane protein expressed in trophectoderm cells of embryos and in uterine epithelial cells. Trophinin potentially mediates apical cell adhesion at human embryo implantation sites through trophinin-trophinin binding in these two cell types. Trophinin-mediated cell adhesion activates trophectoderm cells for invasion, whereas the effect of adhesion on maternal side is not known. We show that addition of GWRQ peptide, a previously established peptide that mimics trophinin-mediated cell adhesion, to human endometrial epithelial cells expressing trophinin induces their apoptosis. FAS involvement was excluded, as GWRQ did not bind to FAS, and FAS knockdown did not alter GWRQ-induced apoptosis. Immunoblotting analyses of protein kinases revealed an elevation of PKC-δ protein in GWRQ-bound endometrial epithelial cells. In the absence of GWRQ, PKC-δ associated with trophinin and remained cytoplasmic, but after GWRQ binding to the trophinin extracellular domain, PKC-δ became tyrosine phosphorylated, dissociated from trophinin and entered the nucleus. In PKC-δ knockdown endometrial cells, GWRQ did not induce apoptosis. These results suggest that trophinin-mediated cell adhesion functions as a molecular switch to induce apoptosis through the PKC-δ pathway in endometrial epithelial cells. Thus, trophinin-mediated induction of apoptosis of endometrial epithelial cells, which function as a barrier to embryo invasion, allows trophoblast invasion of maternal tissue and embryo implantation in humans.
Key words: blastocyst, embryo implantation, apoptosis, cell adhesion, signal transduction
Introduction
Embryo implantation is a uniquely mammalian reproductive strategy and a process that varies significantly among mammalian species.1 Consequently, at least some mechanisms underlying embryo implantation are unique to humans.2–6 Trophinin is an intrinsic membrane protein expressed on apical plasma membranes in human trophoblastic cells and endometrial epithelial cells, which mediates homophilic cell adhesion at respective apical cell surfaces.7,8 Trophinin is not expressed in human endometrial epithelia throughout the hormonal cycle, except only those cells located close to the implanting blastocyst or the implantation site may express trophinin. Trophinin expression by endometrial epithelia is induced by human chorionic gonadotrophin (hCG) derived from trophoblastic cells of the implanting embryo.4,5,7
Previously, we defined the mechanism underlying activation of trophectoderm cells of the blastocyst, which is triggered by trophinin-mediated cell adhesion using human embryonal carcinoma cell line HT-H.9 The trophinin cytoplasmic domain forms a complex with bystin,10 which arrests the epidermal growth factor (EGF) family receptor tyrosine kinase ErbB4 at its cytoplasmic face. In this condition, when heparin-binding EGF-like growth factor (HB-EGF) binds to ErbB4 on the cell surface, ErbB4 autophosphorylation does not occur and the tyrosine kinase is not active. However, upon trophinin-mediated cell adhesion, trophinin releases bystin and ErbB4 is activated by autophosphorylation. Thus trophinin functions as a molecular switch transforming silent trophectoderm to an active trophoblast upon trophinin-mediated cell adhesion.2,9
Several reports suggest that endometrial epithelial cells undergo apoptosis upon adhesion of the blastocyst.11–14 We asked whether trophinin-mediated cell adhesion promotes apoptosis of human endometrial cells simultaneously with activation, invasion and proliferation of trophoblastic cells. The present study was undertaken to determine cytoplasmic events occurring following trophinin-mediated cell adhesion in human endometrial epithelial SNG-M cells, the line employed together with HT-H in our in vitro model of human embryo implantation.7 We show here that trophinin-mediated cell adhesion triggers an apoptotic signal in SNG-M cells through the PKC-δ pathway.
Results
Trophinin-mediated adhesion induces apoptosis of human endometrial epithelial cells.
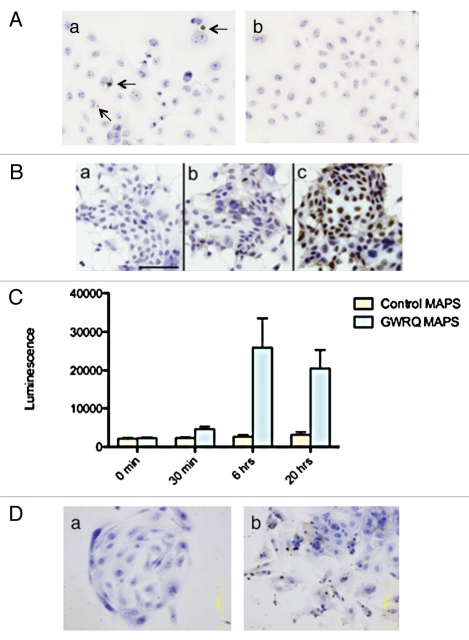
To investigate the reactions of human endometrial epithelial cells caused by trophinin-mediated cell adhesion, we employed SNG-M cells in an adhesion assay with human trophoblastic HT-H cells, as these cell types have been established as an in vitro model for mimicking the initial adhesion for human embryo implantation.2,7,9,15 HT-H cells grown as a monolayer were trypsinized and added to an SNG-M cell monolayer. As reported previously,7 HT-H cells immediately adhered to the upper surface of SNG-M cells. When cells were left in contact for 30 minutes, adherent HT-H cells did not spread on the SNG-M monolayer but remained morphologically distinct. HT-H cells then were removed mechanically by splashing medium on the SNG-M monolayer. Twenty-four hours later, an apoptag TUNEL analysis was performed on SNG-M cells, revealing that some SNG-M cells showed positive TUNEL signals (Fig. 1A, a). By contrast, SNG-M monolayer that received control A431 cells, which lack trophinin expression, showed no signs of apoptosis (Fig. 1A, b). As we reported previously,9 trophinin-mediated cell adhesion promotes proliferation and invasion of HT-H cells, phenotypes opposite to those indicating cell death. Therefore we conclude that dying cells are SNG-M cells, not HT-H cells.
Figure 1.
Trophinin-mediated apoptosis of human endometrial epithelial cells. (A) Either trophinin-positive human trophoblastic embryonic carcinoma HT-H cells (a) or control trophinin-negative A431 cells (b) were added to monolayers of human endometrial adenocarcinoma SNG-M cells. Thirty minutes later, cells were removed from the monolayer, and an apoptag TUNEL assay was performed after 24 hours. Arrows in (a) indicate apoptotic nuclei. (B) Human endometrial adenocarcinoma SNG-M cells were subjected to a TUNEL assay 24 hours after adding none (a), control-MAPS (b) or GWRQ-MAPS (c). In all parts, cells were counterstained by hematoxylin. (C) Caspase 3/7 activities were measured using the lysates of SNG-M cells treated with 10 µg/mL each control-MAPS or with GWRQ-MAPS for the indicated times. Each bar and error bar represents mean ± SEM of triplicate measurements. (D) Human endometrial epithelial primary cell cultures were treated with hCG and IL-1β for 6 hours (5) to induce trophinin expression. Cells were subjected to a TUNEL assay 24 hours after addition of control-MAPS (a) or GWRQ-MAPS (b). Scale bars indicate 100 µm.
We reported previously that tetrapeptide GWRQ binds to the trophinin extracellular domain and mimicks trophinin-mediated cell adhesion to induce cytoskeletal changes and tyrosine phosphorylations of cytoplasmic proteins in HT-H cells. When GWRQ was added to SNG-M monolayers, almost all SNG-M cells underwent apoptosis, whereas treatment of a control peptide did not result in apoptosis which were revealed by TUNEL (Fig. 1B), caspase 3/7 (Fig. 1C) and annexin V binding assay (Sup. Fig. 1). When GWRQ was added to A431 cells, which do not exhibit trophinin-mediated cell adhesion, A431 cells did not result in apoptosis (Sup. Fig. 2).
We also showed previously that primary cultured human endometrial epithelial cells from the human uterus express trophinin in pinopodes, the epithelial structures likely responsible for initial adhesion to trophectoderm, following culture in medium containing hCG and IL-1β.5 Trophinin-expressing primary cultured endometrial cells exhibited apical adhesion to HT-H cells.5 When GWRQ was added to trophinin-expressing primary cultured human endometrial epithelial cells, we observed apoptosis (Fig. 1D). These results strongly suggest that engaging trophinin on the cell surface elicits a signal that leads apoptosis of human endometrial epithelial cells.
GWRQ-binding to SNG-M causes no cytoplasmic protein phosphorylation.
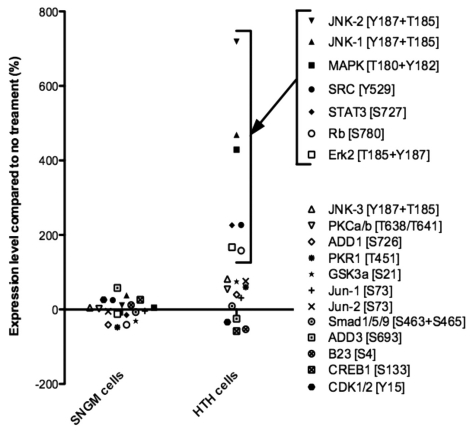
We have shown previously, by multiple western blot analyses, that trophinin-mediated cell adhesion mimicked by GWRQ increases protein phosphorylation in HT-H cells.9 When we analyzed SNG-M cells, GWRQ-treated SNG-M cells showed no sign of altered protein phosphorylation (Fig. 2). In trophoblastic HT-H cells, trophinin associates with ErbB4.9 SNG-M cells also express ErbB4; however, in contrast to HT-H cells, trophinin did not associate with ErbB4 in SNG-M cells (Fig. S3), suggesting that signal transduction downstream of trophinin differs in these two cell types.
Figure 2.
Summary of quantitative western blot analysis of phosphorylated proteins. Relative levels of phosphorylated proteins in SNG-M and HT-H cells after treatment with GWRQ-MAPS peptide for 30 minutes. Each point represents levels of phosphorylated protein, which either increased or decreased relative to levels seen in the absence of GWRQ-MAPS treatment. Phospho site-specific antibodies were used to detect each protein. The raw data are presented by Figure S5 and S6, Table S1 and S2.
Trophinin-mediated apoptosis is Fas-independent.
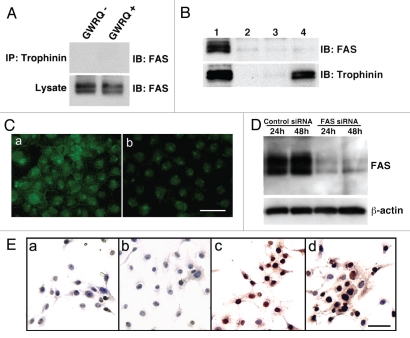
In many cell types, the Fas ligand induces apoptosis,16 and Fas-mediated apoptosis reportedly occurs in endometrial epithelial cells during human embryo implantation.12,17 We used three approaches to determine whether trophinin-mediated apoptosis requires Fas activity in endometrial epithelial cells. First, we examined interaction between trophinin and Fas proteins in SNG-M cells by co-immunoprecipitation and observed no interaction between trophinin and Fas in the presence or absence of GWRQ (Fig. 3A). Second, phage displaying GWRQ peptide bound to the SNG-M cell surface (Sup. Fig. 4) and were immunoprecipitated. Immunoblot analysis showed that GWRQ phage bound to trophinin, but not to Fas (Fig. 3B). Finally, Fas transcripts were knocked-down in SNG-M cells by siRNA (Fig. 3C and D), and the effect of GWRQ was investigated. We found that GWRQ induced apoptosis equally well in Fas knocked-down and control SNG-M cells (Fig. 3E). These results strongly suggest that GWRQ-induced apoptosis is independent of Fas.
Figure 3.
Relationship of Fas/FasL- and trophinin-mediated apoptosis of SNG-M cells. (A) Nagative association of trophinin with Fas in SNG-M cells treated with or without GWRQ. Immunoprecipitation of SNG-M cell lysates with anti-trophinin antibody followed by western blot for FAS shows a lack of association between trophinin and FAS. (B) Western blot of immunoprecipitates with anti-fd phage antibody for FAS (upper parts) or trophinin (lower parts). Lane 1, total cell lysate from untreated SNG-M cells; lanes 2–4, immunoprecipitates of SNG-M cell lysates prepared from GWRQ (lanes 2 and 4) or control (lane 3) phage-bound SNG-M cells using control rabbit IgG (lane 2) or rabbit anti-phage antibody (lanes 3 and 4). (C) Immunocytochemistry of SNG-M cells using an anti-FAS antibody. SNG-M cells were transfected by negative control (a) or FAS (b) siRNA. Scale bars indicate 50 mm. (D) Western blot for Fas of SNG-M cell lysates 24 hours and 48 hours after being transfected with negative control siRNA or FAS siRNA. (E) TUNEL assay of SNG-M cells treated with control (a and b) or GWRQ (c and d) peptide after transfection with control (a and c) or FAS (b and d) siRNA. Scale bars indicate 50 mm.
Trophinin occupancy leads nuclear translocation of PKC-δ.
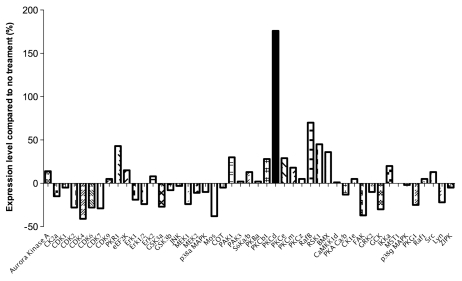
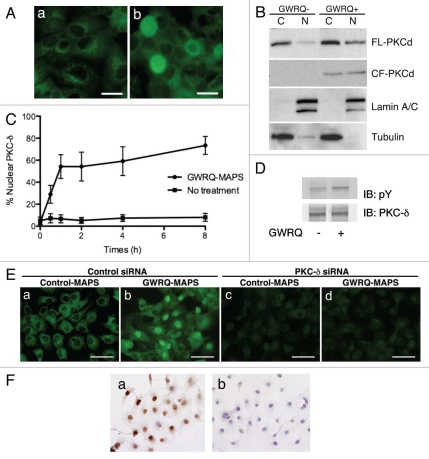
To obtain a clue for the downstream target of trophinin-mediated apoptosis in SNG-M cells, we compared the levels of protein kinases in SNG-M cells before and after GWRQ-binding on the cell surface. Quantitative western blot analysis of protein kinases in SNG-M cells revealed that PKC-δ levels were increased in GWRQ-treated cells (Fig. 4). In epithelial cells, PKC-δ is cleaved by caspase 3 to become the active apoptosis-inducing form;18 thus we hypothesized that PKC-δ is the potential mediator of GWRQ-induced apoptosis in SNG-M cells.
Figure 4.
Summary of quantitative western blot analysis of protein kinases in SNG-M cells before and after treatment with GWRQ-MAPS peptide for 30 minutes. The raw data are presented by Figure S7 and Table S3.
In the absence of GWRQ, we found that PKC-δ was cytoplasmic in SNG-M cells (Fig. 5A and B). By contrast, in the presence of GWRQ, some PKC-δ was seen in SNG-M cell nuclei (Fig. 5A and B). PKC-δ nuclear translocation began within 30 min of GWRQ treatment and reached a plateau at 1 hour, when approximately 60% of cells exhibited nuclear PKC-δ (Fig. 5C). A time course assay showed a gradual increase in caspase 3/7 activity over 6 hrs after GWRQ-treatment of SNG-M cells (Sup. Fig. 8). Thus nuclear translocation of PKC-δ precedes apoptosis.
Figure 5.
Involvement of PKC-δ in trophinin-mediated apoptosis in human endometrial epithelial SNG-M cells. (A) Immunocytochemistry of PKC-δ in SNG-M cells treated with (b) or without (a) GWRQ-MAPS for 30 minutes. Scale bars indicate 25 µm. (B) Western blot analysis of full-length (FL) PKC-δ and the caspase-3 cleaved form (CF) of PKC-δ distributed to either nuclear (N) or cytoplasmic (C) fractions prepared from SNG-M cells treated with or without GWRQ-MAPS peptide. Lamin and tubulin were included as a control for fractionation. (C) Time-dependency of nuclear translocation of PKC-δ in SNG-M cells cultured in media with or without GWRQ-MAPS. (D) Tyrosine phosphorylation of PKC-δ in SNG-M cells treated with or without GWRQ-MAPS for 30 min. (E) Immunocytochemistry of SNG-M cells by an anti-PKC-δ antibody. SNG-M cells transfected by control (a and b) or PKC-δ (c and d) siRNA were treated with GWRQ-MAPS (b and d) or with control peptide (a and c). Scale bars indicate 50 µm. (F) TUNEL assay of control (a) or PKC-δ (b) siRNA-transfected SNG-M cells treated with GWRQ peptide. A scale bar indicates 50 µm.
PKC-δ from SNG-M cells showed low levels tyrosine phosphate, whereas that from GWRQ-treated SNG-M cells showed higher levels of tyrosine phosphorylation (Fig. 5D). PKC-δ is reportedly tyrosine-phosphorylated prior to nuclear translocation.19 These results suggest that GWRQ binding to the extracellular domain of trophinin induces tyrosine phosphorylation of PKC-δ, which is required for translocation to the nucleus to induce caspase 3-activated apoptosis.18
To further analyze involvement of PKC-δ in GWRQ-induced apoptosis in SNG-M cells, PKC-δ was knocked-down by siRNA. Immunocytochemistry showed that PKC-δ-targeting siRNA efficiently reduced levels of endogenous PKC-δ protein (Fig. 5E). In PKC-δ knocked-down SNG-M cells, GWRQ did not induce apoptosis (Fig. 5F), supporting the hypothesis that trophinin-induced apoptosis in SNG-M cells is mediated through PKC-δ pathway.
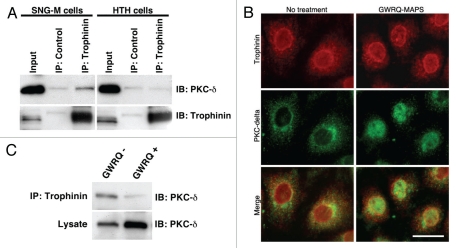
Finally, we determined whether trophinin associates with PKC-δ in SNG-M cells. Immunoprecipitation by anti-trophinin antibody followed by immunoblotting for PKC-δ showed that PKC-δ associates with trophinin in SNG-M cells in the absence of GWRQ (Fig. 6A and left parts). Human trophoblastic HT-H cells to which GWRQ binds but does not promote apoptosis exhibited no association of trophinin and PKC-δ (Fig. 6A and right parts). Double immunostaining of SNG-M cells for trophinin and PKC-δ showed that both proteins were largely co-localized in the cytoplasm and plasma membranes in the absence of GWRQ (Fig. 6B). In the presence of GWRQ, PKC-δ entered the nuclei, whereas trophinin's localization was unchanged. Finally, GWRQ binding to SNG-M cells resulted in decreased PKC-δ association with trophinin (Fig. 6C). These findings suggest that when GWRQ binds to the extracellular domain of trophinin in SNG-M cells, PKC-δ dissociates from the trophinin cytoplasmic domain and becomes tyrosine-phosphorylated, leading to nuclear translocation and caspase 3-activated apoptosis.
Figure 6.
Association of trophinin with PKC-δ in SNG-M cells. (A) Immunoprecipitation by anti-trophinin antibody followed by western blot for PKC-δ. Cell lysates of SNG-M cells and HT-H cells were immunoprecipitated using an anti-trophinin antibody, followed by western blot analysis for PKC-δ. (B) Double immunofluorescence microscopy of SNG-M cells treated with or without GWRQ-MAPS for trophinin or PKC-δ. A scale bar presents 25 µm. (C) Dissociation of PKC-δ from trophinin in GWRQ-treated SNG-M cells. Western blot analysis of immunoprecipitates using an anti-trophinin antibody from lysates of SNG-M cells treated with or without GWRQ-MAPS.
Discussion
We showed in this study that trophinin-mediated cell adhesion, mimicked by a trophinin-binding GWRQ peptide, promotes apoptosis of human endometrial epithelial cells in primary cultures and in an endometrial adenocarcinoma SNG-M cell line (Fig. 1). Previously, we showed that trophinin-mediated cell adhesion triggers a molecular switch for trophectoderm activation and invasion.2,9 This study also shows that trophinin functions as a switch, but by contrast to activation of trophectoderm cells, for apoptosis in endometrial epithelia. Given that the endometrial epithelial layer represents a barrier to the blastocyst to be implanted, it may require a mechanism for overcoming this barrier prior to embryo invasion. Indeed, the morphologies of human embryo implantation sites in vivo indicate a lack of endometrial epithelial layer at the boundary between invading trophectoderm cells and the maternal stroma,8,20,21 suggesting that epithelial layers are degenerated. Trophinin's apoptotic activity in endometrial epithelial cells is consistent with these observations and supports the hypothesis that trophinin functions in human embryo implantation.2–5,7,9
In the first trimester placenta, invasive extravillous cytotrophoblasts confront the endometrial glandular epithelia, initiating the steps required for blood vessel formation of the placenta.22 Trophinin expression by extravillous cytotrophoblasts (Sup. Fig. 9) suggests that it may function in these processes. In testicular and colon cancer, trophinin expression is associated with invasive activity of tumor cells.23–25 In the invasive trophoblast, interactions of trophinin with cytoplasmic signaling molecules may differ from those in silent trophectoderm cells described our previous study in reference 9, or from those occurring in endometrial epithelial cells described here.
Since human trophectoderm cells express Fas ligand, and endometrial epithelial cells express Fas, the Fas/FasL system has been proposed as mediating implantation-dependent apoptosis of endometrial cells.12,17 Fas/FasL activity is linked to activation of mitogen-activated protein kinases (MAPK) and c-Jun N-terminal kinase (JNK) in endometrial epithelial cells.14,17 Since trophinin-mediated apoptosis is independent of Fas (Fig. 3), it is likely that two apoptotic pathways, mediated by Fas/FasL or by trophinin, operate during human embryo implantation to ensure removal of endometrial epithelial layer.
The present study identifies PKC-δ as the mediator of trophinin-stimulated apoptosis. PKC-δ may be sequestered by trophinin before cell adhesion, whereas upon cell adhesion, PKC-δ is released from trophinin, phosphorylated and translocated to nuclei (Figs. 5 and 6). PKC-δ contains a nuclear localization signal near the C-terminus.26 It is known that in non-apoptotic cells, PKC-δ remains in the cytoplasm and binds to cytoplasmic proteins including the tyrosine kinase c-Abl,27 whereas in apoptotic cells PKC-δ enters the nucleus in response to various apoptotic agents.28–32 Nuclear PKC-δ undergoes caspase 3-mediated proteolysis and caspase-3-cleaved PKC-δ accumulates in the nucleus, binds DNA and inhibits DNA polymerase activity.33,34 Therefore, nuclear localization of PKC-δ is necessary and sufficient for apoptosis.18,28,34
When the human blastocyst approaches endometrial epithelial cells, trophinin is induced in response to high concentrations of hCG secreted by the blastocyst,5 which may trap PKC-δ near the plasma membrane, reducing cytoplasmic PKC-δ levels. Once trophinin-mediated cell adhesion occurs, PKC-δ is released from trophinin to the cytoplasm, allowing its translocation to the nucleus, where full-length PKC-δ may be cleaved to an active form by caspase 3 (Fig. 5B). Present study also suggest that trophinin-mediated cell adhesion induces the expression of PKC-δ (Fig. 4), which further facilitates apoptosis of endometrial epithelial cells.
Humans reproduce a limited number of offspring, requiring “selection” of optimal endometrial epithelial sites for implantation. Numerous mechanisms and factors govern the apposition and initial adhesion of the human embryo to endometrial epithelium: L-selectin/selectin ligand,6 ErbB4/HB-EGF,35–37 and trophinin7,9 all ensure strict quality control during the initial adhesion between trophectoderm and endometrial epithelia.3 Dual entries of endometrial epithelia via either Fas or trophinin activity may represent a redundant form of quality control in embryo implantation.
The mechanisms underlying human embryo implantation cannot be investigated directly. Therefore, the in vitro model employed in this and previous studies in reference 7 and 9, provides valuable information relevant to this intriguing method of reproduction at the molecular level. Further understanding of the mechanism of human embryo implantation will provide us with new diagnostic and therapeutic approaches to improve human reproductive health.
Materials and Methods
Antibodies and synthetic peptides.
GWRQ-MAPS, in which 8 linear tetra peptides of G (glycine), W (tryptophan), R (arginine) and Q (glutamine) residues9,38 were linked to an 8-branched lysine cluster (MAP), and control MAPS in which alanine was linked to an 8-branched lysine cluster, were synthesized by Genscript, as were GWRQ-C and QRWG-C (C, cysteine) peptides. A phage clone displaying GWRQ peptide was prepared as described in reference 9 and 38. A monoclonal anti-trophinin antibody (3–11, mouse IgM) was prepared as described in reference 4 and 8. This antibody was also purchased from Upstate Biotechnology. Rabbit anti-FAS and anti-Lamin A/C antibodies were from Cell Signaling. Rabbit anti-PKC delta antibody was from Santa Cruz Biotechnology. Rabbit anti-fd phage and anti-β-tubulin antibodies were purchased from Sigma. Alexa-Fluor 488-conjugated secondary antibodies (anti-mouse IgM and anti-rabbit IgG) were purchased from Molecular Probes.
Primary culture of human endometrial epithelial cells and cell lines.
Primary cultures of human endometrial epithelial cells were prepared as described in reference 5. Briefly, human endometrium obtained from normally cycling women undergoing hysterectomy for benign uterine disease was finely minced and digested with collagenase (Sigma, type 1A, 0.2 mg/ml) at 37°C for 30 min in DMEM/F12 medium supplemented with 10% fetal calf serum (FCS) and penicillin/streptomycin. After incubation, the cell suspension was passed through a sterile wire sieve. Epithelial and stromal cells obtained were resuspended in medium containing FCS. The cell suspension was placed onto 3 ml of Ficoll (Histopaque 1119, Sigma) and centrifuged at 400x g for 5 min. The interface containing an enriched fraction of endometrial cells was plated on 10 cm tissue culture dishes coated with gelatin in DME supplemented with 10% FCS. After incubation at 37°C for 1 hr, stromal cells adhered to the plastic dish were removed and non-adherent epithelial cells were transferred to a new gelatin-coated dish. Epithelial cells were cultured at 37°C for 3–4 days in a humidified incubator in DME supplemented with 10% FCS and 50 µg/mL primocin (Invitrogen). Epithelial cell purity was assessed by immunocytochemistry by anti-cytokeratin antibody. Human trophoblastic embryonal carcinoma HT-H and endometrial adenocarcinoma SNG-M lines,39,40 as well as assays of apical adhesion between these two cell types, have been described in reference 7.
Immunofluorescence microscopy.
SNG-M and primary endometrial epithelial cells were grown on glass coverslips placed in 3.5 cm culture dishes. Cells on coverslips were washed with PBS, fixed with 4% paraformaldehyde in PBS for 15 min, blocked with 4% (v/v) goat serum in PBS at room temperature for 15 min and incubated with diluted (1:200, 2 µg/mL) anti-trophinin antibody for 3 hours. After washing with PBS, cells were incubated with diluted (1:1,000) Alexa-Fluor 488-conjugated anti-mouse IgM antibodies for 1 hr. Immunofluorescence microscopy for FAS and PKC-δ was performed as described for trophinin. Phage binding to SNG-M cells was determined as follows. Phage solution containing 105 pfu suspended in 10% goat serum in PBS was added to fixed cells on coverslips. Ten minutes later, the phage solution was removed and cells were washed with PBS. Bound phage were reacted with diluted (1:100) rabbit anti-phage fd antibody followed by diluted (1:1,000) A488 conjugated anti-rabbit IgG antibody. To visualize nuclei, cells were covered with mounting medium containing 4′,6-diamidino-2-phenylindoleincubated (DAPI) (Vector Laboratories).
Western blot and immunoprecipitation.
SNG-M cells grown in 10 cm tissue culture plates were incubated with GWRQ peptide-displaying phage (1 × 108 pfu)9 for 15 min. Controls were undertaken using phage without inserts. After washing with cold PBS, cells were scraped using rubber policemen, collected by centrifugation and solubilized in 1% NP-40 in PBS containing a protease inhibitor cocktail (Roche Diagnostics). Cell lysates were incubated with rabbit anti-phage fd antibody or with control rabbit IgG, and immune complexes were collected by protein A agarose beads (Sigma). Immunoprecipitates were boiled in SDS sample buffer, resolved on SDS-polyacrylamide gels and transferred to a polyvinyldifluoride (PVDF) membrane. After blocking, PVDF membrane was incubated in PBS containing diluted (1:100) anti-trophinin antibody (3–11, mouse IgM) at room temperature for 60 min. After washing with PBS containing 0.02% Tween 20, membranes were reacted with diluted peroxidase-conjugated goat anti-mouse Ig antibody for 60 min. Immunoreactive bands were visualized using ECL reagent followed by exposure to X-ray film. Immunoblotting for FAS and PKC-δelta was carried out as described for trophinin. Dilutions of first antibodies were: anti-β-tubulin (1:1,000), anti-Lamin A/C (1:1,000), anti-FAS (1:1,000) and anti-PKC d (1:1,000).
Subcellular fractionation.
Cytoplasmic and nuclear fractions were prepared as described in reference 41 and 42. Briefly, SNG-M cells grown in 10 cm dishes were scraped with a rubber policeman and washed with PBS. Cells were suspended in 2 ml 10 mM Tris/HCl, pH 7.4, 10 mM NaCl and 1.5 mM MgCl2 buffer, kept on ice for 10 min and homogenized with a tight-fitting glass Dounce homogenizer (Wheaton Science Products). Homogenization was verified by phase-contrast microscopy. Protease inhibitor paramethylsulfofluoride and phosphatase inhibitor cocktails 1 and 2 (Sigma) were added, and homogenates were centrifuged at 1,000x g for 3 min at 4°C. The pellet served as the nuclear fraction. Supernatant were centrifuged at 2,000x g for 30 min at 4°C, and the resultant supernatant was collected as the cytoplasmic fraction.
Apoptosis assays.
SNG-M cells on glass coverslips were subjected to the TdT-mediated dUTP nick-end labeling (TUNEL) assay using the ApopTag peroxidase kit (Chemicon) and 3,3-diaminobenzidine (DAB) served as the peroxidase substrate. An Annexin V assay was carried out using Annexin-V-FLUOS staining kit (Roche), according to the manufacturer's instructions. Caspase 3/7 activity assay was performed using Caspase 3/7 Glo assay reagents (Promega).
FAS and PKC-δ SiRNAs.
A pool of target-specific 20–25 nucleotide siRNAs for respective Fas (h, sc-29311) and PKC-δ (h, sc-36253) were purchased from Santa Cruz Biotechnology. Silencer control no. 1 (Ambion) served as the negative control. SNG-M cells were transfected with siRNA using X-treme reagent (Roche, Indianapolis, IN). Briefly, 50 µl of Opti-MEM (Invitrogen) was mixed with 2.5 µl of X-treme (Roche). Then 5 µl siRNA (50 µM in water) was diluted with 50 µl of Opti-MEM. Diluted siRNA and X-treme were then combined and added to SNG-M cells on glass coverslips. Thirty minutes later, medium containing 10% FCS was added. Twenty four hours later, medium with or without GWRQ-MAPS (10 µg/mL) was added, and cells were cultured another 24 h before assessing apoptosis.
Multiple western blot analysis.
Monolayer SNG-M cells grown in 10 cm tissue culture dishes were incubated in DME containing 1 mM Na3VO4 for 30 min. Cells were then stimulated at 37°C for 30 min with or without GWRQ-MAPS (10 µg/ml). Cells were harvested by scraping with rubber policeman and cell lysates were prepared according to the Kinexus protocol. Quantitative western blot analysis for phosphorylated proteins and protein kinases was carried out by Kinexus.
Acknowledgements
The authors thank Drs. Guy Salvesen and Ze'ev Ronai for their critical reading the manuscript, Dr. Elise Lamar for her excellent editing and Merryl Mathew for technical assistance. This study has been supported by the grant DoD prostate cancer research grant W81XWH040917.
Supplementary Material
References
- 1.Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, et al. Embryo implantation. Developmental Biology. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda MN, Sugihara K. Signal transduction in human embryo implantation. Cell Cycle. 2007;6:1153–1156. doi: 10.4161/cc.6.10.4266. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda MN, Sugihara K. An integrated view of L-selectin and trophinin function in human embryo implantation. J Obstet Gynaecol Res. 2008;34:129–136. doi: 10.1111/j.1447-0756.2008.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama J, Aoki D, Suga T, Akama TO, Ishizone S, Yamaguchi H, et al. Implantation-Dependent Expression of Trophinin by Maternal Fallopian Tube Epithelia during Tubal Pregnancies: Possible Role of Human Chorionic Gonadotrophin on Ectopic Pregnancy. Am J Pathol. 2003;163:2211–2219. doi: 10.1016/S0002-9440(10)63579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugihara K, Kabir-Salmani M, Byrne J, Wolf DP, Lessey B, Iwashita M, et al. Induction of trophinin in human endometrial surface epithelia by CGbeta and IL-1beta. FEBS Lett. 2008;582:197–202. doi: 10.1016/j.febslet.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, et al. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299:405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda MN, Sato T, Nakayama J, Klier G, Mikami M, Aoki D, et al. Trophinin and tastin, a novel cell adhesion molecule complex with potential involvement in embryo implantation. Genes Dev. 1995;9:1199–1210. doi: 10.1101/gad.9.10.1199. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki N, Nakayama J, Shih IM, Aoki D, Nozawa S, Fukuda MN. Expression of trophinin, tastin and bystin by trophoblast and endometrial cells in human placenta. Biol Reprod. 1999;60:621–627. doi: 10.1095/biolreprod60.3.621. [DOI] [PubMed] [Google Scholar]
- 9.Sugihara K, Sugiyama D, Byrne J, Wolf DP, Lowitz KP, Kobayashi Y, et al. Trophoblast cell activation by trophinin ligation is implicated in human embryo implantation. Proc Natl Acad Sci USA. 2007;104:3799–3804. doi: 10.1073/pnas.0611516104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki N, Zara J, Sato T, Ong E, Bakhiet N, Oshima RG, et al. A novel cytoplasmic protein, bystin, interacts with trophinin, tastin and cytokeratin, and may be involved in trophinin mediated cell adhesion between trophoblast and endometrial epithelial cells. Proceedings of the National Academy of Sciences, USA. 1998;95:5027–5032. doi: 10.1073/pnas.95.9.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welsh AO. Uterine cell death during implantation and early placentation. Microsc Res Tech. 1993;25:223–245. doi: 10.1002/jemt.1070250305. [DOI] [PubMed] [Google Scholar]
- 12.Galan A, O'Connor JE, Valbuena D, Herrer R, Remohi J, Pampfer S, et al. The human blastocyst regulates endometrial epithelial apoptosis in embryonic adhesion. Biol Reprod. 2000;63:430–439. doi: 10.1093/biolreprod/63.2.430. [DOI] [PubMed] [Google Scholar]
- 13.Simon C, Dominguez F, Remohi J, Pellicer A. Embryo effects in human implantation: embryonic regulation of endometrial molecules in human implantation. Ann N Y Acad Sci. 2001;943:1–16. doi: 10.1111/j.1749-6632.2001.tb03785.x. [DOI] [PubMed] [Google Scholar]
- 14.Li HY, Chang SP, Yuan CC, Chao HT, Ng HT, Sung YJ. Induction of p38 mitogen-activated protein kinase-mediated apoptosis is involved in outgrowth of trophoblast cells on endometrial epithelial cells in a model of human trophoblast-endometrial interactions. Biol Reprod. 2003;69:1515–1524. doi: 10.1095/biolreprod.103.015669. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda MN, Nozawa S. Trophinin, tastin and bystin: a complex mediating unique attachment between trophoblastic and endometrial epithelial cells at their respective apical cell membranes. Semin Reprod Endocrinol. 1999;17:229–234. doi: 10.1055/s-2007-1016230. [DOI] [PubMed] [Google Scholar]
- 16.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 17.Hsu WL, Chen YH, Chao KC, Chang SP, Tsui KH, Li HY, et al. Anti-fas activating antibody enhances trophoblast outgrowth on endometrial epithelial cells by induction of P38 MAPK/JNK-mediated apoptosis. Placenta. 2008;29:338–346. doi: 10.1016/j.placenta.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Reyland ME. Protein kinase Cdelta and apoptosis. Biochem Soc Trans. 2007;35:1001–1004. doi: 10.1042/BST0351001. [DOI] [PubMed] [Google Scholar]
- 19.Humphries MJ, Ohm AM, Schaack J, Adwan TS, Reyland ME. Tyrosine phosphorylation regulates nuclear translocation of PKCdelta. Oncogene. 2008;27:3045–3053. doi: 10.1038/sj.onc.1210967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hertig AT, Rock J. Searching for early fertilized human ova. Gynecol Invest. 1973;4:121–139. doi: 10.1159/000301716. [DOI] [PubMed] [Google Scholar]
- 21.Enders AC. Cytotrophoblast invasion of the endometrium in the human and macaque early villous stage of implantation. Trophoblast Res. 1997;10:83–95. [Google Scholar]
- 22.Moser G, Gauster M, Orendi K, Glasner A, Theuerkauf R, Huppertz B. Endoglandular trophoblast, an alternative route of trophoblast invasion? Analysis with novel confrontation co-culture models. Hum Reprod. 25:1127–1136. doi: 10.1093/humrep/deq035. [DOI] [PubMed] [Google Scholar]
- 23.Hatakeyama S, Ohyama C, Minagawa S, Inoue T, Kakinuma H, Kyan A, et al. Functional correlation of trophinin expression with the malignancy of testicular germ cell tumor. Cancer Res. 2004;64:4257–4262. doi: 10.1158/0008-5472.CAN-04-0732. [DOI] [PubMed] [Google Scholar]
- 24.Harada O, Suga T, Suzuki T, Nakamoto K, Kobayashi M, Nomiyama T, et al. The role of trophinin, an adhesion molecule unique to human trophoblasts, in progression of colorectal cancer. Int J Cancer. 2007;121:1072–1078. doi: 10.1002/ijc.22821. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda MN, Sugihara K, Nakayama J. Trophinin: what embryo implantation teaches us about human cancer. Cancer Biol Ther. 2008;7:1165. doi: 10.4161/cbt.7.8.6696. [DOI] [PubMed] [Google Scholar]
- 26.Martelli AM, Faenza I, Billi AM, Fala F, Cocco L, Manzoli L. Nuclear protein kinase C isoforms: key players in multiple cell functions? Histol Histopathol. 2003;18:1301–1312. doi: 10.14670/HH-18.1301. [DOI] [PubMed] [Google Scholar]
- 27.Qi X, Mochly-Rosen D. The PKCdelta-Abl complex communicates ER stress to the mitochondria—an essential step in subsequent apoptosis. J Cell Sci. 2008;121:804–813. doi: 10.1242/jcs.024653. [DOI] [PubMed] [Google Scholar]
- 28.DeVries-Seimon TA, Ohm AM, Humphries MJ, Reyland ME. Induction of apoptosis is driven by nuclear retention of protein kinase Cdelta. J Biol Chem. 2007;282:22307–22314. doi: 10.1074/jbc.M703661200. [DOI] [PubMed] [Google Scholar]
- 29.Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase cdelta. Apoptosis. 2003;8:19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- 30.Basu A, Adkins B, Basu C. Downregulation of caspase-2 by rottlerin via protein kinase C-delta-independent pathway. Cancer Res. 2008;68:2795–2802. doi: 10.1158/0008-5472.CAN-07-6244. [DOI] [PubMed] [Google Scholar]
- 31.Yuan ZM, Utsugisawa T, Ishiko T, Nakada S, Huang Y, Kharbanda S, et al. Activation of protein kinase Cdelta by the c-Abl tyrosine kinase in response to ionizing radiation. Oncogene. 1998;16:1643–1648. doi: 10.1038/sj.onc.1201698. [DOI] [PubMed] [Google Scholar]
- 32.Scheel-Toellner D, Pilling D, Akbar AN, Hardie D, Lombardi G, Salmon M, et al. Inhibition of T cell apoptosis by IFNbeta rapidly reverses nuclear translocation of protein kinase C-delta. Eur J Immunol. 1999;29:2603–2612. doi: 10.1002/(SICI)1521-4141(199908)29:08<2603::AID-IMMU2603>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 33.Ghayur T, Hugunin M, Talanian RV, Ratnofsky S, Quinlan C, Emoto Y, et al. Proteolytic activation of protein kinase Cdelta by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson DN, Foster DA. The enigmatic protein kinase Cdelta: complex roles in cell proliferation and survival. Faseb J. 2004;18:627–636. doi: 10.1096/fj.03-0979rev. [DOI] [PubMed] [Google Scholar]
- 35.Lessey BA, Gui Y, Apparao KB, Young SL, Mulholland J. Regulated expression of heparin-binding EGF-like growth factor (HB-EGF) in the human endometrium: a potential paracrine role during implantation. Mol Reprod Dev. 2002;62:446–455. doi: 10.1002/mrd.10129. [DOI] [PubMed] [Google Scholar]
- 36.Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst implantation. Dev Genet. 1997;21:102–108. doi: 10.1002/(SICI)1520-6408(1997)21:1<102::AID-DVG12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Stavreus-Evers A, Aghajanova L, Brismar H, Eriksson H, Landgren BM, Hovatta O. Co-existence of heparin-binding epidermal growth factor-like growth factor and pinopodes in human endometrium at the time of implantation. Mol Hum Reprod. 2002;8:765–769. doi: 10.1093/molehr/8.8.765. [DOI] [PubMed] [Google Scholar]
- 38.Hatakeyama S, Sugihara K, Lee SH, Nadano D, Nakayama J, Ohyama C, et al. Enhancement of human sperm motility by trophinin binding peptide. J Urol. 2008;180:767–771. doi: 10.1016/j.juro.2008.03.185. [DOI] [PubMed] [Google Scholar]
- 39.Izhar M, Siebert P, Oshima RG, DeWolf WC, Fukuda MN. Trophoblastic differentiation of human teratocarcinoma cell line HT-H. Developmental Biology. 1986;116:510–518. doi: 10.1016/0012-1606(86)90151-x. [DOI] [PubMed] [Google Scholar]
- 40.Ishiwata I, Nozawa S, Inoue T, Okumura H. Development and characterization of established cell lines from primary and metastatic regions of human endometrial adenocarcinoma. Cancer Res. 1977;37:1777–1785. [PubMed] [Google Scholar]
- 41.Pederson T. Cells: a Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 1998. [Google Scholar]
- 42.Miyoshi M, Okajima T, Matsuda T, Fukuda MN, Nadano D. Bystin in human cancer cells: intracellular localization and function in ribosome biogenesis. Biochem J. 2007;404:373–381. doi: 10.1042/BJ20061597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.