Abstract
Background and aim
Early detection enables the possibility for interventions to reduce the future burden of COPD. The Danish National Board of Health recommends that individuals >35 years with tobacco/occupational exposure, and at least 1 respiratory symptom should be offered a spirometry to facilitate early detection of COPD. The aim, therefore, was to provide evidence for the feasibility and impact of doing spirometry in this target population.
Methods
Participating general practitioners (GPs) (n = 335; 10% of the Danish GPs) recruited consecutively, subjects with >35 years exposure, no previous diagnosis of obstructive lung disease, and at least 1 of the following symptoms: cough, dyspnea, wheezing, sputum, or recurrent respiratory infection. Data on age, smoking status, pack-years, body mass index (BMI), dyspnea score (Medical Research Council, MRC), and pre-bronchodilator spirometry (FEV1, FEV1% predicted, FEV1/FVC) were obtained.
Results
A total of 3.095 (51% females) subjects was included: mean age 58 years, BMI 26.3, and 31.5 pack-years. The majority of subjects (88%) reported MRC score 1 or 2. FEV1/FVC-ratio ≤ 0.7 was found in 34.8% of the subjects; the prevalence of airway obstruction increased with age and decreased with increasing BMI, and was higher in men and current smokers. According to the level of FEV1, 79% of the subjects with airway obstruction had mild to moderate COPD.
Conclusions
More than one-third of the recruited subjects had airway obstruction (FEV1/ FVC < 0.7). Early detection of COPD appears to be feasible through offering spirometry to adults with tobacco/occupational exposure and at least 1 respiratory symptom.
Keywords: COPD, spirometry, general practice, airway obstruction, screening
Introduction
COPD is a common disease, which causes substantially impaired quality of life and increased risk of premature death. Tobacco smoking is the most important risk factor for COPD in up to 90% of patients in Western societies, although environmental, occupational, and genetic factors also interact and play a role.1–3
In most cases COPD has its roots decades before the onset of symptoms and runs an insidious course over years, often with an undiagnosed initial phase.1,4,5 COPD is frequently diagnosed at a relatively late stage of the disease, probably because early symptoms of the disease are subtle and unrecognized due to individual perception and interpretation.5 Early symptoms of the disease may not be evident, although airflow obstruction is present on spirometry. Patients suffering from more severe COPD have substantial limitations in activities of daily living, leading to poor health-related quality of life and disability. Early recognition of cases may therefore have the potential to reduce the future burden of COPD for both morbidity and mortality.5
In Denmark, as in any country, general practitioners and family physicians (GPs) are on the frontline for prevention and early diagnosis of COPD. The GPs should therefore be encouraged to take the lead in implementing spirometry, as well as the recommendations of the updated treatment guidelines. In keeping with this, the Danish National Board of Health recommends that smokers/ex-smokers and individuals with relevant occupational exposure over 35 years with at least 1 respiratory symptom should be offered spirometry to facilitate early detection of COPD.6 This approach was recently adopted in the COPD guidelines generated by the Danish Society for General Practice.7
The aim of this general practice-based study was to investigate the feasibility for implementing the recommendation of spirometry in the defined risk population.
Material and methods
Denmark has approximately 3600 GPs, covering a population of 5.4 million. We aimed on a voluntary basis to include a representative sample comprising at least 200 GPs from all over Denmark. Written information about the project, as well as the invitation to participate, was distributed to all the interested GPs by the sponsoring companies’ local representatives. Each of the participating GPs was expected to screen at least 30 consecutive subjects who attended the practice and fulfilled the eligibility criteria.
Subjects were eligible for the study provided they fulfilled the following inclusion criteria: 1) age ≥ 35 years; 2) smoker/ ex-smoker or relevant occupational exposure; 3) ≥one respiratory symptom (dyspnea, cough, wheeze, sputum, and/ or recurrent chest infections); and not the following exclusion criteria: 1) unable to perform spirometry; 2) previous diagnosis of obstructive lung disease (asthma and/or COPD) or other chronic respiratory disease.
The case record form (CRF) consisted of 2 parts: 1) a questionnaire filled in by the participant for age, gender, height, body weight, smoking status, daily tobacco consumption, years of smoking, airway symptoms (cough, dyspnea, wheezing, sputum, and recurrent lower airway infection), and severity of dyspnea (graded according to Medical Research Council, MRC;8 2) data obtained by the GP in his/her own practice on spirometry (FEV1, expressed in absolute value and as a percentage of the predicted value, FVC, and FEV1/ FVC ratio), severity of COPD (graded according to GOLD9), the participants’ wishes in relation to smoking cessation (including need for nicotine replacement therapy or prescribed medication), and the final decision whether or not further intervention is indicated. All data from the individual CRF were entered into a consolidated web-based database; and on registration the body mass index (BMI), FEV1/FVC ratio, severity of COPD (based on FEV1% predicted), and number of pack-years was automatically calculated. Consultants from the sponsoring companies performed quality control of the CRFs.
Data analysis
Data from individual participant’s records were analyzed focusing on the defined outcome variables; only data for participants with complete CRFs (95.2%) were included in the analyses. The FEV1 was expressed as the absolute value and also as a percentage of the predicted value,10 and the FEV1/FVC ratio as the absolute value. Predictors of an FEV1/FVC ratio < 0.7 were identified by means of multiple logistic regressions with predictor variables for gender, age, BMI, and smoking status. Quantitative predictor variables were included using smoothing splines, and tested for possible nonlinearities. In all the statistical analyses a significance level of 5% was adopted and significance of predictor variables was tested by means of the likelihood ratio test. The results were interpreted terms of odds ratios and visualized using nomograms. The statistical software R11 was used for the statistical analyses. The TOP project was accepted by the Danish College of General Practitioners.
According to the EFPIA code,12 and the Danish Association of the Pharmaceutical Industry (LiF),13 the present study was a noninterventional trial, and approval from the scientific ethical committee and the Danish Medicines Agency were not mandatory, but they were given all relevant study information. The study was approved by the Danish Data Protection Agency.
Results
A total of 335 GPs participated in the study, and 3095 individuals (49% males) with complete data were included in the analyses. A total of 65% of the study population (62% males and 67% females) were current smokers, and 3% were never smokers. Approximately 50% of the current smokers stated that they were not interested in quitting smoking, and no difference was observed between smokers with and without airway obstruction. Further characteristics of the enrolled subjects are given in Table 1.
Table 1.
Characteristics of the enrolled subjects
| Males (n = 1503) | Females (n = 1594) | |
|---|---|---|
| Age (years) | 58 (36–90) | 57 (36–88) |
| BMI (kg/m2) | 27 (15–49) | 26 (14–55) |
| Pack-years (n) | 38 (0.5–175) | 29 (0.1–172)* |
Note: P < 0.05.
Abbreviation: BMI, body mass index.
The most common reported respiratory symptoms were dyspnea (66%) and cough (63%), whereas wheeze, sputum, and recurrent respiratory infection were reported by 21%, 31%, and 14%, respectively. The majority of subjects (88%) reported dyspnea score 1 and 2, whereas 12% of the enrolled subjects reported dyspnea of such severity (MRC 3 to 5) that they fulfilled the criteria for referral to a hospital-based rehabilitation program. Almost half of the individuals (45%) reported only 1 respiratory symptom, whereas 28% reported 2 symptoms.
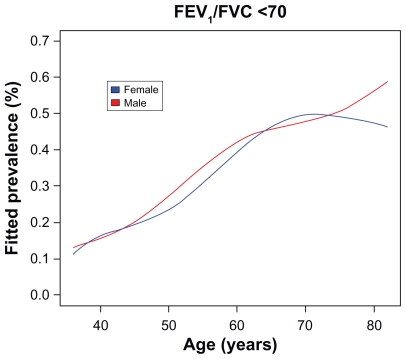
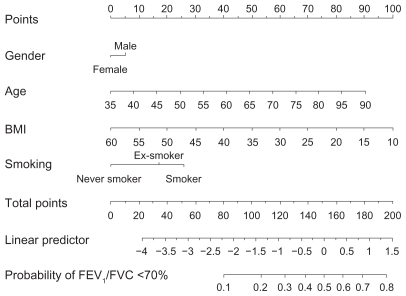
Airway obstruction, defined as a FEV1/FVC ratio ≤ 0.7, was found in 34.8% (n = 1079) of the enrolled subjects. The prevalence of airway obstruction was increasing with age, but airway obstruction was also found in a substantial proportion of the subjects in the younger age groups (Figure 1). In the analysis, gender, age, BMI, and smoking status were all found to be significant predictors of a FEV1/FVC ratio ≤ 0.7. The prevalence of airway obstruction was higher for increasing age and decreasing BMI. Furthermore, the prevalence was also higher in men than in women, and in smokers than in ex-smokers and never smokers. The 95% confidence intervals for odds ratios of these risk factors are given in Table 2. A nomogram for the analysis obtained by the multiple regression model is given in Figure 2. From the nomogram, individual predictions can be obtained by adding the partial effect on each risk factor and using the sum to calculate the risk of having a FEV1/FVC ratio < 0.7; furthermore, the relative importance of risk factors can be seen directly.
Figure 1.
Estimated age and gender specific prevalence of FEV1/FVC ratio ≤ 70%.
Table 2.
Distribution of FEV1/FVC < 70 stratified on gender, smoking status, age, and BMI
| Risk factor | FEV1/FVC < 70 N (%) | FEV1/FVC ≥ 70 N (%) | 95% CI for OR | P-value |
|---|---|---|---|---|
| Gender | ||||
| Female | 527 (33.1) | 1063 (66.9) | ||
| Male | 551 (36.6) | 954 (63.4) | [1.01–1.38] | 0.039 |
| Smoking | 1290 (64.5) | |||
| Smoker | 713 (35.5) | |||
| Ex-smoker | 343 (34.6) | 647 (65.4) | [0.64–0.91] | 0.002 |
| Never smoker | 22 (21.5) | 80 (78.5) | [0.27–0.74] | 0.001 |
| Age (years) | ||||
| 36–48 | 144 (17.9) | 657 (82.1) | ||
| 49–58 | 262 (31.6) | 566 (68.4) | ||
| 59–66 | 312 (41.7) | 436 (58.3) | ||
| 67–90 | 360 (50.0) | 358 (50.0) | [2.20–2.84] | <0.001 |
| BMI (kg/m2) | ||||
| 14.4–23.1 | 327 (42.2) | 447 (57.8) | ||
| 23.2–25.6 | 301 (39.3) | 464 (60.7) | ||
| 25.7–28.7 | 231 (30.1) | 536 (69.9) | ||
| 28.8–55.0 | 219 (27.7) | 570 (72.3) | [0.64–0.78] | <0.001 |
Notes: The grouping of age and body mass index (BMI) is based on quartiles. The 95% confidence intervals (CI) and P-values for odds ratios (OR) were obtained from multivariate logistic regression. Female smokers were set as reference levels for the OR. The OR for age and BMI correspond to an increase in interquartile range.
Figure 2.
Nomogram used for predicting the probability of FEV1/FVC ≤ 70%. The model reported in Table 2 has been used in constructing the nomogram.
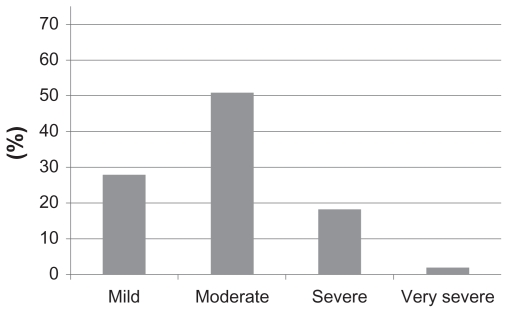
Further analysis of the data for the individuals with airway obstruction (n = 1078) showed that the level of FEV1% predicted in 28% and 51%, respectively, fulfilled the criteria for mild and moderate COPD (Figure 3).
Figure 3.
Distribution of enrolled subjects with airway obstruction (FEV1/FVC ≤ 70%) (n = 1078) according to severity of COPD (based on FEV1% predicted).
Discussion
Denmark has approximately 3600 GPs, covering a population of 5.4 million, and we aimed to include, on a voluntary basis, at least 200 GPs in the study from all over Denmark. However, the general interest for participating in the study exceeded our expectations, and a total of 335 GPs (almost 10% of the Danish GPs) agreed to participate, and included 3095 subjects in the study. As expected, not all of the enrolled GPs contributed subjects to the study sample, just as some of the participating GPs did not manage to enrol 30 subjects, but eventually more than 220 GPs contributed subjects with complete data for the study sample.
The present study demonstrated clearly that early detection of COPD is feasible in general practice by offering spirometry to individuals with relevant exposure and airway symptoms. Furthermore, the observed prevalence of airway obstruction was disturbingly high, but fortunately the majority of the subjects identified with airway obstruction appeared, as judged by the level of FEV1% predicted, to have mild to moderate COPD. Using this case finding approach may therefore provide an opportunity for early intervention aimed at improving prognosis.
Compared with previous reports from the Lung Health study14 and the study by Zielinski and Bednarek,15 the present study revealed a much higher prevalence of airway obstruction (>30%), although the subjects included in the present study did not have a higher mean cumulative life-long tobacco exposure. Furthermore, studies by Miravitlles et al16 and Hansen et al17 have likewise reported a much lower prevalence of COPD, 10% and 12%, respectively. These differences may be explained by differences in recruitment of subjects such as age, life-time tobacco exposure (pack-years), marketed type of cigarettes, genetics, differences in air pollution and environmental exposure, and/or pattern and availability of health care utilization. The case-finding design of offering spirometry to individuals with relevant exposure and at least 1 respiratory symptom used in the present study proved to be more cost-effective in general practice compared with previous reports.18
A ratio of FEV1 to FVC of less < 0.7 when measured after the administration of an inhaled bronchodilator is the spirometric criterion to define COPD,1,9 whereas no symptoms are needed. In the present study only prebronchodilator spirometry was performed, and some, most likely only a minority of the individuals identified with airway obstruction may have a post-bronchodilator FEV1/FVC ratio within the reference values. However, doing post-bronchodilator spirometry was beyond the scope of the present study. Furthermore, the validity of the present findings for the diagnosis of COPD is supported by the current UK National Institute for Clinical Excellence19 COPD guidance in which pre-bronchodilator spirometry values are used to diagnose and stage COPD.19,20
Previous studies suggest that using a fixed ratio for FEV1/ FVC as the diagnostic criterion may overestimate the prevalence of COPD in the elderly and underestimate the prevalence in younger subjects.5,16 It has, therefore, in the Spanish/Latin America guidelines20 been suggested to use the lower limit of normal, ie, the bottom 5% of the normal distribution of FEV1/FVC in a healthy reference population, for the diagnosis of COPD. In keeping with most published COPD guidelines, we decided to use the fixed FEV1/FVC ratio, not least because this appears to be easier to handle as the diagnostic criterion in general practice.
In conclusion, the present study showed that early detection of COPD in general practice by offering spirometry to individuals with relevant exposure and airway symptoms is not only feasible, but identified a high proportion of individuals with airway obstruction. Furthermore, the majority of subjects with signs of COPD appeared to have mild to moderate disease, and secondary preventive measures may reduce the future burden of COPD, for both morbidity and mortality.
Acknowledgment
This study was sponsored by Boehringer Ingelheim and Pfizer Aps, Denmark.
Footnotes
Contributors
As principal investigators CSU, AL, RD, JD, GH, PHC, and KKA had full access to all the study data, and take responsibility for their integrity and the accuracy of their analysis. CSU, AL, RD, JD, and GH participated in the study design, and supervised the study. PHC and KKA analyzed the data and provided the statistical expertise. CSU drafted the report, and the report was revised for important intellectual content by AL, RD, JD, GH, and KKA.
Disclosure
As members of the steering committee of the TOP study, CSU, AL, and RD have received consulting fees from Boehringer-Ingelheim and Pfizer Aps, Denmark. JD and GH are employed by Pfizer Aps, Denmark. PHC and KKA declare that they have no conflicts of interests.
References
- 1.Celli BR, McNee W ATS/ERS committee members. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 2.COPD – more than just tobacco smoke (editorial) Lancet. 2009;374:663. doi: 10.1016/S0140-6736(09)61535-X. [DOI] [PubMed] [Google Scholar]
- 3.Blanc PD, Menezes AM, Plane E, et al. Occupational exposures and COPD: an ecological analysis of international data. Eur Respir J. 2009;33:298–304. doi: 10.1183/09031936.00118808. [DOI] [PubMed] [Google Scholar]
- 4.Celli BR. Update on the management of COPD. Chest. 2008;133:1451–1462. doi: 10.1378/chest.07-2061. [DOI] [PubMed] [Google Scholar]
- 5.Soriano JB, Zielinski J, Price D. Screening for and early detection of chronic obstructive pulmonary disease. Lancet. 2009;374:721–732. doi: 10.1016/S0140-6736(09)61290-3. [DOI] [PubMed] [Google Scholar]
- 6.Danish National Board of Health; 2007. KOL: Anbefalinger for tidlig opsporing, opfølgning, behandling, og rehabilitering [COPD: Recommendations for early detection, monitoring, treatment and rehabilitation; in Danish] [Google Scholar]
- 7.Nielsen LM, Brorson S, Gorlen T, et al. KOL i Almen Praksis [COPD in General Practice; in Danish] Danish College of General Practitioners; 2008. [Google Scholar]
- 8.Bestall JC, Paul EA, Garrod R, Granham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Diagnosis, Management and Prevention of COPD. [Accessed February 2010]. Guidelines available from the GOLD website www.goldcopd.com.
- 10.Quanjer PH, Tammeling GJ. Summary of recommendations. Standardized lung function testing. Report Working Party, European Community for Coal and Steel. Bull Eur Physiopathol Respir. 1983;19(suppl 5):7–20. [PubMed] [Google Scholar]
- 11.Ihaka I, Gentleman RR. A language for data analysis and graphics. J Comput Graph Stats. 1996;5:299–314. [Google Scholar]
- 12.EFPIA code on the promotion of prescription-only medicines to, and interactions with, healthcare professionals. European Federation of Pharmaceutical Industries and Associations (Council Directive 2001/ 83/EC). www.efpia.eu. Accessed February 11, 2010
- 13. [Accessed February 11, 2010]. Lægemiddelindustriforeningen www.lifdk.dk.
- 14.Connett JE, Bjornsen-Benson WM, Daniels K. Recruitment of participants in the Lung Health Study II. Assessment of recruiting strategies: Lung Health Study Research Group. Control Clin Trials. 1993;14:38S–51S. doi: 10.1016/0197-2456(93)90023-7. [DOI] [PubMed] [Google Scholar]
- 15.Zielinski J, Bednarek M the Know the Age of Your Lung Study Group. Early detection of COPD in a high-risk population using spirometric screening. Chest. 2001;119:731–736. doi: 10.1378/chest.119.3.731. [DOI] [PubMed] [Google Scholar]
- 16.Maravitlles M, Soriano JB, Garcia-Rio F, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64:863–868. doi: 10.1136/thx.2009.115725. [DOI] [PubMed] [Google Scholar]
- 17.Hansen JG, Pedersen L, Overvad K, Omland Ø, Jense HK, Sørensen HT. The prevalence of chronic obstructive pulmonary disease among Danes aged 45–84 years: population-based study. COPD. 2008;5:347–352. doi: 10.1080/15412550802522635. [DOI] [PubMed] [Google Scholar]
- 18.van Schayck GP, Loozen JMG, Wagena E, Akkermans RP, Wesseling GJ. Detecting patients at a high risk of developing chronic obstructive pulmonary disease in general practice: cross sectional case finding study. BMJ. 2002;321(324):1370–1375. doi: 10.1136/bmj.324.7350.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chronic obstructive pulmonary disease: national clinical guideline for management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. (National Institute for Clinical Excellence (NICE)) 2004;59(Suppl 1):1–232. [PMC free article] [PubMed] [Google Scholar]
- 20.Peces-Barba G, Barbera JA, Agusti A, et al. Diagnosis and management of chronic obstructive pulmonary disease: joint guidelines of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) and the Latin America Thoracic Society (ALAT) Acta Bronchopneumol. 2008;44:271–281. [PubMed] [Google Scholar]