Abstract
A common explanation of molecular recognition by the olfactory system posits that receptors recognize the structure or shape of the odorant molecule. We performed a rigorous test of shape recognition by replacing hydrogen with deuterium in odorants and asking whether Drosophila melanogaster can distinguish these identically shaped isotopes. We report that flies not only differentiate between isotopic odorants, but can be conditioned to selectively avoid the common or the deuterated isotope. Furthermore, flies trained to discriminate against the normal or deuterated isotopes of a compound, selectively avoid the corresponding isotope of a different odorant. Finally, flies trained to avoid a deuterated compound exhibit selective aversion to an unrelated molecule with a vibrational mode in the energy range of the carbon–deuterium stretch. These findings are inconsistent with a shape-only model for smell, and instead support the existence of a molecular vibration-sensing component to olfactory reception.
Olfactory systems perform remarkable feats of molecular recognition, but although much is known about the neurophysiology of olfaction (1–5), how olfactory receptors ”read” molecular structure remains unknown. Parts of odorant molecules (odotopes) have been proposed to engage particular receptors in a “lock-and-key” manner and this molecular shape recognition mechanism is thought sufficient for odor discrimination (2). An alternative hypothesis (6) posits that molecular vibrations of all atoms, or of particular functional groups of odorant molecules, contribute to odor recognition, and odorants with similar vibrational spectra should elicit similar olfactory responses (7). Molecules in which deuterium replaces nonexchangeable hydrogens constitute appropriate probes to test these alternatives because deuteration does not alter atom size or bond length or stiffness (8). Thus, the conformation of a deuterated molecule should be identical to that of a hydrogen-only (i.e., normal) odorant and, according to the molecular recognition theory, the two isotopes should smell identical. However, atoms in a molecule vibrate in normal modes at particular energies that depend on the molecular structure. The doubling of nuclear mass upon deuteration will change all the vibrational modes of an odorant molecule to differing extents. The second hypothesis predicts that deuteration will alter its smell in comparison with the hydrogen-only isotope. A recent attempt to test the latter hypothesis in humans was rather inconclusive, potentially confounded by interference from previous experience, olfactory training or habituation of the subjects, or shortcomings of human olfaction as suggested by the authors (9). A major experimental difficulty is that, in general, isotopes are prepared and purified differently, and therefore perceived differences in smell could be attributed to different impurities. Purity can, in principle, be ensured by smelling single-molecule peaks exiting a gas chromatographic column, but this makes simultaneous two-compound comparisons difficult and animal experiments harder still.
Here we describe a different approach to test whether molecular vibrations contribute to recognition of odor character. We assumed that, if molecular vibrations are indeed detected, the deuterated isotopes of different odorants may share a common odor character, as deuteration shifts particular peaks—e.g., the carbon–deuterium (C-H) stretch—of the compound-specific vibrational spectra to lower frequencies. If so, the deuterium odor character could be distinct and identifiable, irrespective of the structure and chemical properties of the odorant molecules that carry it. Significantly, we used Drosophila as unbiased and objective subjects to address this issue. They possess a relatively well understood olfactory system (10–13), exhibit keen olfactory discrimination (14–16), and can be conditioned to selectively avoid or seek odors with the use of established methodology (17, 18). We ask whether Drosophila can detect deuterium as a distinguishing molecular feature in odorant isotopes and a salient cue for conditioning. The results of these experiments provide support for the notion that flies can smell molecular vibrations.
Results
Spontaneous Differential Responses to Deuterated Odorants.
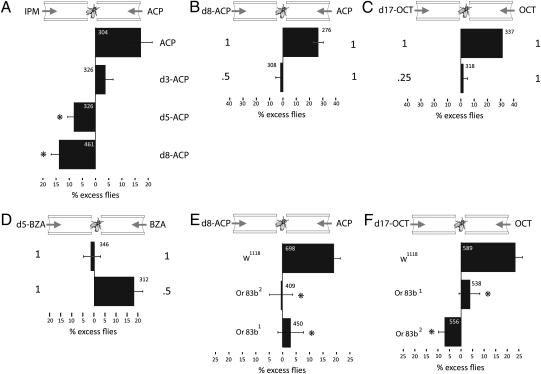
Although deuteration does not appreciably change molecular shape, atom size, or bond length or stiffness, it doubles hydrogen mass, thus affecting the overall vibrational modes of an odorant. Therefore, if recognition of molecular shape alone was the sole determinant for odor character (2, 3), then flies should not respond differentially to deuterated [d, where d(x) denotes replacement of x nonexchangeable hydrogens with deuterium atoms] and nondeuterated/normal (i.e., H-) odorants. To address this hypothesis, we took advantage of the commercial availability of acetophenone (ACP) carrying three, five, or eight deuterium atoms (d3, d5, and d8) in place of the respective hydrogens in the normal molecule (h-ACP). Equal amounts (75 μL) of each odorant were diluted to 1 mL in isopropyl myristate and we quantified (Fig. 1A) the response of groups of flies to each odorant versus unscented air traversing the arms of a standard T-maze (Materials and Methods) (19, 20). When given a choice between normal ACP (i.e., h-ACP) and air, the excess flies present in the odor-delivering maze arm indicated that, at the dilution used, the odorant was attractive. However, replacing only three hydrogen atoms with deuterium eliminated this spontaneous preference as the flies distributed nearly equally in the arms of the maze. The d5-ACP was mildly aversive and the fully substituted d8-ACP was the most aversive of the four isotopes. Moreover, flies consistently avoided d3, d5, and d8 ACP against equal concentration of h-ACP (Fig. S1). Therefore, a spontaneous switch in osmotaxis (14) to ACP was precipitated by substituting hydrogen atoms with deuterium.
Fig. 1.
Differential spontaneous responses to odorants containing deuterium. The mean relative distribution of flies in the arms of the maze (% excess flies) carrying the indicated odorants ± SEM is shown in all graphs. This metric reflects the prevailing distribution within the arms of the maze of groups of 40 to 60 flies tested each time. The total number of flies tested in each group is shown and the number of groups tested is n ≥ 6 for all groups. (A) Spontaneous responses to 75 μL normal or d3-, d5-, or d8-ACP, each diluted with isopropyl myristate (IPM) to 1 mL. Flies spontaneously preferred h-ACP over the solvent. In contrast, incorporation of five or eight deuterium atoms results in significantly different distribution (P < 0.001) from that toward h-ACP (attraction) to aversion. In contrast, the response to d3-ACP was not significantly different (P = 0.012) from that of h-ACP. (B) Flies discriminate against d8-ACP if presented with equal amount (1:1) of h-ACP (75 μL odorant/925 μL IPM). However, a 50% reduction in the amount of deuterated odorant yielded an equal distribution of the flies in the arms (% excess flies not significantly different from zero), defined as a balanced maze. (C) Similarly equal amounts (200 μL) of h-1-octanol (OCT) and d17-1-octanol yielded strong discrimination against the deuterated odorant, which was eliminated upon reducing it by 75% (1:0.25). (D) In contrast, equal amounts of normal and deuterated benzaldehyde (90 μL) did not result in differential discrimination. In accord, decreasing the amount of h-BZA resulted in differential avoidance of d5-BZA. (E) The preferential discrimination against d8-ACP was eliminated in Or83b1 and Or83b2 mutants (P < 0.002 and P < 0.001, respectively, Dunnett test). (F) Similarly, discrimination against d17-octanol was eliminated in Or83b1 and Or83b2 mutants (P < 0.001 for both, Dunnett test).
To verify that flies are generally capable of spontaneous discrimination between airborne deuterated odorants and their normal counterparts, we additionally presented them with equal concentrations of normal and deuterated 1-octanol, normal and d5-benzaldehyde, or a normal–perdeuterated ACP pair. In confirmation of the previous results, h-ACP was preferred versus an equal concentration of the d8 odorant (Fig. 1B). Reducing the concentration of the deuterated compound by 50% resulted in balanced distribution of the flies in the maze arms, indicative of equal response to the two isotopes (i.e., balanced maze). Similarly, d17-1-octanol was preferentially discriminated against versus equal concentration of the normal odorant. In this case, however, the deuterated isotope appeared even more aversive, as 75% reduction in its concentration equalized aversion with h-1-octanol (Fig. 1C). In contrast, flies did not exhibit spontaneous differential avoidance of d5-benzaldehyde, which occurred only upon reducing the concentration of the normal odorant (Fig. 1D). These differences cannot result from reduced evaporation of the slightly heavier deuterated odorants because two of the three elicited increased aversion, known to be proportional to the concentration of airborne odorants in this T-maze system (14, 20, 21).
If the differential response to the deuterated odorants relied solely on olfaction and not any other sensory modality, it should be completely eliminated in anosmic mutants. To that end, we obtained two anosmic Drosophila strains carrying the null alleles Or83b1 and Or83b2 of the gene encoding the common subunit of the dimeric Drosophila olfactory receptor (13, 22, 23). Clearly, the spontaneous avoidance of deuterated d8-ACP and d17-1-octanol were eliminated and the mutant flies distributed equally in the maze arms as expected (Fig. 1 E and F). Therefore, Drosophila use olfaction alone to discriminate between deuterated and normal odorants. Furthermore, the spontaneous discrimination against deuterated odorants indicates that they likely present to the flies recognizable, salient features distinct from their hydrogen counterparts.
Conditioned Discrimination of Isotopes.
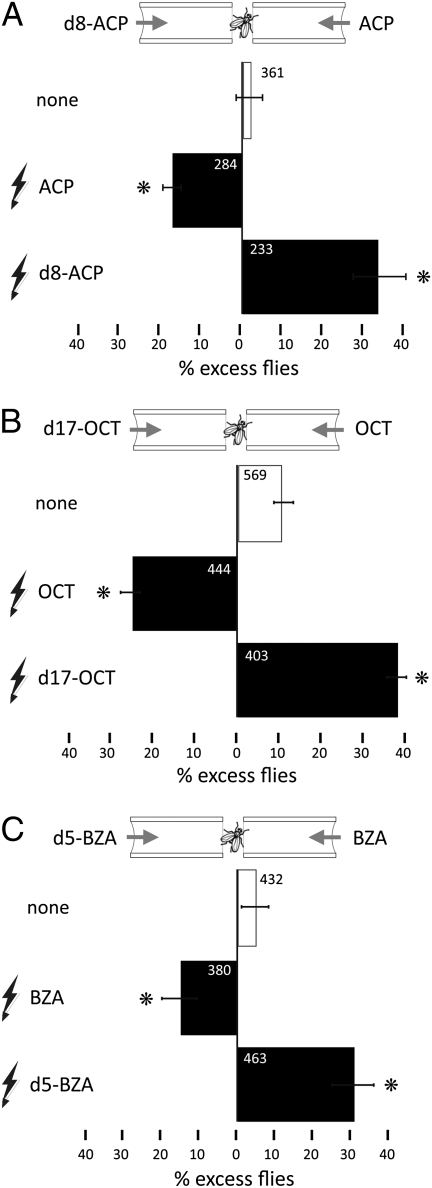
If the deuterated and normal isotopes of a pair exhibit salient odor character differences, then Drosophila should be able to associate either odorant with a punishing stimulus (20). To eliminate bias, the amounts of each odorant in a pair were adjusted as shown in Fig. 1 B–D to yield spontaneous preference as near zero as possible (i.e., balanced maze), so any postconditioning distribution changes would be a consequence of training. Flies were conditioned to associate electric foot-shock punishment with the presence of the deuterated or normal odorant of a pair. Drosophila successfully associated either odorant with punishment, demonstrated by the conditioned selective aversion of the shock-associated odor upon testing (Fig. 2). Flies shocked in the presence of the normal odorant distributed preferentially in the arm carrying its deuterated counterpart and vice versa. The shock-associated learning extended to h-benzaldehyde and d5-benzaldehyde, for which the flies exhibited no spontaneous preference, suggesting, as expected (24), that conditioned discrimination is independent from spontaneous preference.
Fig. 2.
Conditioned avoidance of normal and deuterated chemically identical odorants. The mean relative distribution of flies in the arms of the maze (% excess flies) carrying the indicated odorants ± SEM is shown. The total number of flies tested in each group is shown and mean was derived from at least six repetitions per group. Drosophila was conditioned to selectively avoid the indicated member of an odorant pair by coupling it to electric foot shock (indicated by the lightning symbol). The complementary odor of each pair was used as the unpunished control. Each experiment included a naive group (open bar) to establish balancing of the odors used for testing and was used as control for all statistical comparisons on how conditioning resulted in subsequent selective avoidance of the punished odorants. Asterisks indicate significant such differences that are significant. (A) Flies selectively avoided h-ACP or d8-ACP as expected based on the conditioning scheme and illustrated by their differential distribution in the arms of the maze. The performance of both groups was significantly different from that of naive animals (P < 0.001, Dunnett test). (B) Selective discrimination (P < 0.001 vs. naïve distribution; open bars) against h-octanol and d17-1-octanol in accord with the punished odorant during training. (C) Conditioned discrimination against h-BZA (P = 0.001 vs. naive) and d5-BZA (P < 0.001 vs. naive, Dunnett test).
This was not an exclusive property of the w1118 control strain (14), as experiments were repeated with Canton-S, a different WT strain, with identical results (Fig. S2 A and B). We also reversed the order of stimulus presentation, such that the shock-associated odor was delivered immediately before testing. Flies continued to selectively avoid the shock-associated odor (Fig. 2C), eliminating the possibility that they were simply attracted to the odor presented last in the absence of the shock reinforcer. Thus, deuterated and normal odorants present salient differences to the fly olfactory system, which can be used to predict punishment or its absence by association with either isotope.
Generalization of Conditioned Isotope Discrimination Across Odorant Pairs.
The results so far are consistent with an unambiguous difference between deuterated and normal odorants for all three pairs tested. Is the difference associated with the majority (≥99%) compound or with impurities? Although the odorants used were of the highest purity available (Figs. S3 and S4), they nevertheless may contain small amounts of impurities that, in principle, could account for the spontaneous preferences and the shock-associated learning. To examine this possibility, we asked whether Drosophila could recognize deuterium as a salient feature from one odorant pair to another (i.e., generalize). If different impurities were present, salient features(s) of the training odors allowing their discrimination would be absent if testing with a different odorant pair. Hence, the flies would be unable to generalize (25) and thus distribute equally in the maze arms, as if faced with novel odors. In contrast, if the deuterium odorants had a common salient feature or odor character, then flies could, in principle, generalize between pairs.
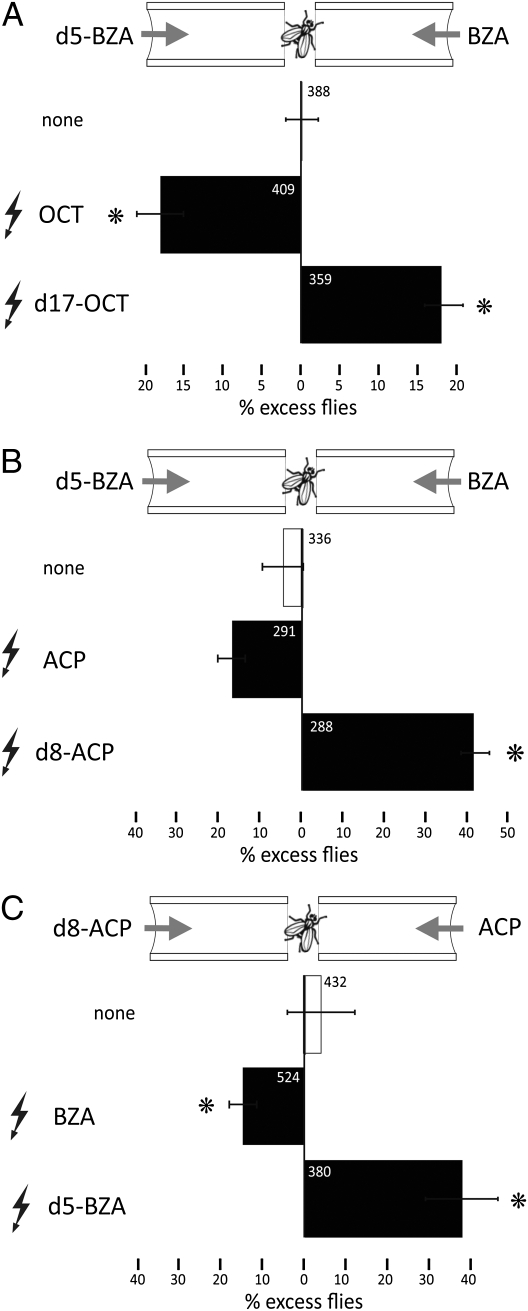
To address this hypothesis, Drosophila were trained to selectively discriminate against deuterated or normal 1-octanol or deuterated versus normal ACP, but tested against the novel pair h-benzaldehyde versus d5-benzaldehyde. We found that flies trained to avoid h-1-octanol avoided h-benzaldehyde selectively when tested with the h-benzaldehyde/d5-benzaldehyde pair. Conversely, conditioning to discriminate against d17-1-octanol resulted in selective avoidance of d5-benzaldehyde (Fig. 3A). Similarly, flies trained to selectively avoid h-ACP discriminated preferentially against benzaldehyde and those trained against d8-ACP avoided the deuterated isotope selectively (Fig. 3B). Because h-ACP is attractive, the task of discriminating against the aversive h-benzaldehyde was expectedly more difficult than the converse choice, as indicated by the modest selective discrimination (Fig. 3B). To further substantiate these findings, we also trained with the h-benzaldehyde/d5-benzaldehyde pair, which do not elicit differential naive discrimination, and tested against normal and deuterated ACP. Again, flies exhibited differential avoidance of d8-ACP if conditioned with d5-benzaldehyde and of h-ACP if trained with h-benzaldehyde (Fig. 3C). Therefore, generalization occurred irrespective of the odorant pairs used for training and testing, suggesting that salient differences independent of their chemical identity and shape allow learned selective aversion to be transferred from the training to the testing pair.
Fig. 3.
Drosophila can be conditioned to selectively avoid deuterium. The mean relative distribution of flies in the arms of the maze (% excess flies) carrying the indicated odorants ± SEM is shown and the total number of flies in each group is denoted. The mean was derived from at least six repetitions per group. Drosophila was conditioned with the indicated pairs of isotopes, but tested against a different pair as indicated. The test odorants were adjusted such that avoidance of naive animals was as near zero as possible (open bar) and was used to compare the distribution of conditioned animals. (A) Animals conditioned with h-1-octanol, but tested after training with h-BZA versus d5-BZA exhibited significantly different distribution than that of naive animals (P < 0.001 vs. naive for both, Dunnett test). Animals trained with d17-1-octanol selectively avoided the deuterated test odorant and vice versa. (B) Animals punished to h-ACP discriminated selectively against h-BZA, albeit not as robustly as previously described (P = 0.004 vs. naive, Dunnett test), but ones trained to avoid d8-ACP avoided the deuterated BZA efficiently (P < 0.001 vs. naive, Dunnett test). (C) Flies conditioned to with h-BZA/d5-BZA exhibited efficient selective avoidance of d8-ACP and h-ACP, respectively (P < 0.001 vs. naive for both, Dunnett test).
Significantly, even if impurities were present, it is not expected that they would be similar in all three odorant pairs and account for differential conditioning, because the syntheses of the six compounds are different. Furthermore, the graded switch from attraction to aversion for a single molecule, ACP, clearly depended on the number of deuterium atoms it carried (Fig. 1A). Hence, it would be remarkable for an aversive impurity to fortuitously follow the number of deuterium atoms in that odorant. Nevertheless, could a single aversive impurity present only in every deuterated compound conceivably account for these results? If this were the case, it is difficult to explain how this impurity is ineffective in d5-benzaldehyde, consistent with the observed lack of spontaneous avoidance (Fig. 1D), but renders d8-ACP and d17-1-octanol aversive. Furthermore, it is possible that benzaldehyde masks the putative impurity, a potential explanation for the equal aversion of its two isotopes. However, if the putative impurity was masked, then, in contrast to our results (Fig. 3 A and B), the benzaldehyde isotopes would not elicit differential discrimination in flies conditioned with 1-octanol/d17-1-octanol or ACP/d8-ACP. Collectively, then, impurities cannot account for the observed across odorant differential conditioned discrimination. In contrast, the generalization results are explained if we hypothesize that the salient cue(s) is a common property that differentiates the isotopes of each pair and distinguishes all deuterated odorants from their normal counterparts. As this cannot be molecular shape, differential evaporation, or impurities, it is likely that the common salient cue is the presence or absence of deuterium.
Odorant Molecular Vibrations Are Salient Cues for Conditioning.
The doubling of hydrogen mass by deuteration will affect every molecular vibration in which movement of the hydrogen atoms occurs. When the vibrational mode involves mostly heavy atoms (i.e., C, N, O), the change in frequency is modest because of the relatively small change in mass of the deuterated versus normal compounds. In contrast, when most of the mode motion is in the hydrogen atoms themselves, as is the case for C-H stretch, wag, and scissor modes, the effect of deuteration on mode frequency will be large. A plausible biophysical mechanism for detection of molecular vibrations has been proposed previously (6), and a detailed study of the underlying physics was recently presented (26). If indeed flies are detecting deuteration by sensing molecular vibrations, it is not unreasonable to suppose that they do so by detecting the modes most affected by deuteration, e.g., the C-H stretch. Each C-H bond has a slightly different stretch frequency clustered around approximately 3,000 cm−1. Deuteration reduces the stretch frequencies by approximately the square root of 2, to 2,100 to 2,200 cm−1. This in turn could confer the salient olfactory difference used to distinguish between isotopes and discriminate them differentially after conditioning.
The fact that the C-D stretch occurs in the 2,200 cm−1 region is useful because only a few triple-bonded functional groups possess vibrations in this region, such as acetylenes, nitriles, isonitriles, and azides. If our hypothesis is correct, flies should be able to generalize the C-D stretch vibration in deuterated compounds to a similar vibration not carried by the C-D stretch, but instead by a triple-bonded functional group similar only in stretch frequency.
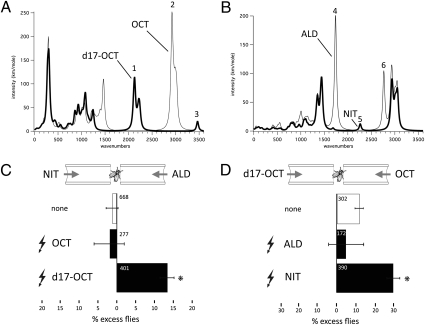
Therefore, the test pair should be molecules of similar odor, only one of which includes a triple bond. We took advantage of the fact that, at least to humans, many nitriles possess odors similar in character to the corresponding aldehydes. A well known such aldehyde–nitrile pair is citronellal (3,7-dimethyloct-6-enal) and citronellyl nitrile (3,7-dimethyloct-6-enenitrile), both citrus compounds with a lemongrass smell, with the nitrile slightly more metallic/oily (27). Consistent with what we perceive as their similar odor character, flies did not show spontaneous preference for or against citronellal or citronellyl nitrile (Fig. 4C). Moreover, in agreement with these empirical observations, the calculated IR spectra of the two compounds in the fingerprint region are very similar (Fig. 4B). The main difference lies in the vibrations of the aldehyde with a C=O stretch around 1,740 cm−1 and the aldehydic C-H stretch around 2,765 cm−1. The nitrile lacks these two vibrations, replaced by a –CN stretch near 2,265 cm−1. The 2,200-cm−1 region is the only one shared by d17-1-octanol and citronellyl nitrile but not by citronellal or octanol. Therefore, in conjunction with the d17-1-octanol/h-1-octanol pair, we now have two pairs of chemically unrelated molecules in which vibrations in the 2,200-cm−1 region are present or absent.
Fig. 4.
Drosophila can be conditioned to selectively avoid a vibrational frequency. (A) Computed vibrational spectra (IR intensities) of h-OCT versus d17-OCT. Salient spectral peaks are indicated on the graphs and show that deuteration of octanol shifts the group of C-H stretch vibrations from around 3,000 cm−1 to 2,150 cm−1. Deuteration also shifts downward all of the peaks on the fingerprint region (1,000–1,500 cm−1). (B) Computed IR intensities of citronellal versus citronellyl nitrile with the salient spectral peaks indicated. The spectra of citronellal and citronellyl nitrile are remarkably similar in the fingerprint region. They differ chiefly in the vibrations involving the terminal functional group: in citronellyl nitrile the aldehyde carbonyl stretch around 1,750 cm−1 is absent, replaced by a nitrile stretch around 2,150 cm−1. The low-lying aldehyde C-H stretch vibration is also absent. The vibration band centered at 2,150 cm−1 is the only one common to d17-1-octanol and citronellyl nitrile but not present in h-octanol or citronellal. The spectra were computed using the Amsterdam Density Functional software at DZP/PBE level of theory. (C) Drosophila selectively avoid the molecular vibrations of deuterium. Flies conditioned to selectively avoid d17-1-octanol exhibited strong preferential avoidance of citronellyl nitrile (P < 0.001 vs. naive), but flies punished to h-octanol did not selectively avoid citronellal (P = 0.691 vs. naive). The only common element potentially recognizable in the test odor pair to aid in selective avoidance is the overlap in the vibrational spectrum of the C-D bonds in d17-1-octanol and the C≡N triple bond in citronellyl nitrile as illustrated in A and B. In contrast, they were not selective toward a novel odor without any recognizable molecular features. (D) In the converse experiment, flies conditioned to selectively avoid citronellyl nitrile exhibited highly significant avoidance of d17-1-octanol as a testing odor (P < 0.001 vs. naive), but flies punished to citronellal did not selectively avoid h-octanol (P = 0.999 vs. naive).
If the salient character recognized in the deuterated odor is indeed the C-D stretch vibrations, flies should be able to generalize the odor character from C-D to C≡N and vice versa. Because the C-D vibration is at the same energy as the nitrile's C≡N stretch, the nitrile should be selectively avoided by animals conditioned to avoid d17-1-octanol. The results of experiments designed to test this idea are shown in Fig. 4 C and D. Flies trained to discriminate against d17-1-octanol selectively avoided the citronellyl nitrile, whereas flies trained to avoid h-1-octanol did not show preferential avoidance (Fig. 4C). The latter outcome was not unexpected, as both citronellyl compounds have similar spectra aside from the functional group region, which do not overlap that of h-1octanol, thus lacking recognizable salient features to guide behavioral responses. Furthermore, flies trained to avoid citronellyl nitrile discriminated selectively against d17-1-octanol, whereas discrimination against citronellal did not yield selective avoidance of h-1-octanol for the reasons outlined earlier. Therefore, in accord with our hypothesis, the presence of nitrile or deuterium resulted in cross-learning in either direction. Moreover, these results further constraint possible explanations invoking a hypothetical impurity, because they can be explained only if d17-1-octanol contains traces of citronellyl nitrile. This seems unlikely, not least because no nitrile solvent was used during its synthesis. As the nitrile is not deuterated, these results also make it very unlikely that the isotope effects we observe are caused by a property of isotopes unrelated to their vibrational modes.
Discussion
Flies, like humans, perceive odor quality and intensity and can be conditioned to discriminate differences in its concentration (28, 29), and at sufficiently different concentrations, the same odorant may appear as distinct qualities (30). Therefore, it can be argued that flies discriminate d8-ACP and d17-1-octanol from their normal isotopes based on putative (small) concentration differences because of the reduced evaporation of the heavier deuterated molecules. However, such differences would have to be greater than 30% (28), which is rather improbable under our experimental conditions, and flies seem to generalize concentration differences smaller than that (29). This explanation is additionally improbable because, except for benzaldehyde, the “heavier” deuterated compounds are more aversive than their normal isotopes. Moreover, this hypothesis is inconsistent with the identification of deuteration as a salient mediator of conditioned responses across chemically distinct odorants and with the selective aversion of molecules with vibrational resonance in the range of the C-D stretch. Therefore, a parsimonious explanation for our results is strongly indicative of a molecular vibration-sensing component in Drosophila olfaction. This is also consistent with independent recent evidence suggesting contributions of the vibrational spectra of odorants in the electrophysiological response of isolated Drosophila olfactory receptors (31).
In the past there have been four main objections to the notion that olfaction detects molecular vibrations. First, that molecular shape is an adequate predictor of smell (2, 30, 32), which currently seems unlikely (33, 34). In fact, Drosophila has only 62 olfactory receptors (35), suggesting that a single receptor must generally respond to multiple odorants, but in addition that a single odorant can activate multiple receptors (10, 15). Clearly, therefore, the structural recognition mechanism alone does not suffice to explain odorant recognition, suggesting that additional properties of these volatile molecules likely contribute to the process. The second objection was to the main assertion of vibrational theory that odorants with similar spectra should produce similar olfactory responses and, conversely, molecules of identical molecular shape but distinct spectra should smell different. Our data are consistent with this hypothesis, at least in flies, and suggest that with gas chromatography-pure odorants, and perhaps with the use of aversive conditioning, similar effects may be revealed for vertebrates—even humans—as shown for perceptual discrimination of enantiomers (36). The third objection has been that no known biological mechanism could behave as the equivalent of a vibrational spectroscope. However, the proposal (6) that olfaction uses inelastic electron tunneling spectroscopy (IETS) has made the idea physically plausible. IETS is a quantum mechanism whereby electrons move from a donor to an acceptor site at constant total energy, although the acceptor is energetically lower than the donor (37). To satisfy conservation of energy, tunneling occurs only if a molecule is present between donor and acceptor, possessing vibrational mode(s) at or near this excess energy, absorbing it, and becoming excited. A modified IETS mechanism appropriate to proteins was recently described (26), suggesting a testable model applicable to olfactory receptors. Finally, it was objected that enantiomers with identical vibrations should always smell the same, whereas some smell different (38). Given that proteins are chiral, a shape-only theory cannot account for the identical odors of most enantiomer pairs. In contrast, this objection is easily answered by IETS because it exhibits the pronounced polarization effects expected when sensing molecules in fixed orientations such as enantiomers (6).
Individual olfactory receptors in Drosophila and other species can mediate excitatory and inhibitory responses to different odorants (10). Our data suggest that the vibrational mode and frequency of particular atoms and active groups of odorant molecules may also provide discriminatory cues that could broaden the recognition repertoire of odorant receptors, but still retain specificity. Currently, we do not know whether the same or different olfactory receptors are recruited to sense the normal and deuterated versions of the odorant molecules, and this question is currently under investigation broadly guided by the following considerations. A single receptor may recognize, by broad odotope features, a given odorant whose particular vibrational resonance may contribute to the odor-specific activity patterns of odorant receptor neurons (39), potentially modifying its particular odor character. This hypothesis predicts that receptors with distinct shape selectivities must also recognize similar vibrational modes and frequencies to explain our data of selective avoidance of citronellyl nitrile after conditioning with d17-1-octanol and vice versa. By analogy to color vision, it is possible that the multiple olfactory receptors may be divided into spectral classes, the members of each class sensing the same vibrational range and differing in their affinity for molecules of different shapes and physical properties. For example, we would expect to find several receptors in the 2,150-cm−1 band, able to sense deuterated molecules but not their undeuterated counterparts.
If the proposed vibrational sensing component is arranged in broad frequency bands of 100 to 200 cm−1 wide, 10 to 20 receptors would suffice to cover the entire vibrational range. However, why then do flies (and mammals) have many more receptors (2, 3)? A possible answer may be that the olfactory system has evolved to reconcile conflicting requirements, namely, to sense a wide range of odorants, thus being nonspecific, but also to possess high affinity to detect them in small concentrations. Therefore, multiple receptors may serve to ensure that one or more will always bind with adequate affinity to any molecule to provide broad olfactory recognition through the combined activities of multiple receptors. Testing these and other predictions is well within reach of available behavioral, imaging, and genetic techniques.
Materials and Methods
Drosophila were cultured as described previously (40) under a 12-h dark, 12-h light cycle. Mixed-sex groups of 2- to 3-d-old flies were lightly anesthetized and segregated in groups of 50 to 70 animals in vials containing food. Twenty-four hours later they were changed to fresh vials and placed in the dark to adapt 2 to 3 h before behavioral experiments commenced. Details of the behavioral procedures can be found in SI Materials and Methods.
All behavioral experiments were performed at 25 °C and 80% to 85% relative humidity. Training and testing were performed in complete darkness, with dim red light used only during manipulations not requiring exposure to odors. The standard T-maze was modified by replacing the odorant holding cups (20) with glass cylinders of 2.25 cm diameter and 14 cm height as described previously (19, 40). An air stream of 600 mL/min passing over the meniscus carried the odors to the maze arms via silicon rubber tubing. The meniscus area was kept constant by maintaining the volume of the odorant and the solvent, isopropyl myristate (Fluka), at a total of 1 mL in all experiments. Deuterated compounds were from CDN Isotopes. The amounts of odorants added to isopropyl myristate to yield equivalent avoidances were 200 μL of 1-octanol (Fluka) and 50 μL of the fully deuterated d17-1-octanol, 90 μL of benzaldehyde (Fluka) and 90 μL of perdeuterated benzaldehyde-d5 150 μL of ACP (Fluka) and 75 μL of the perdeuterated d8-ACP, 100 μL of citronellal and 100 μL of citronellyl nitrile (gift of International Flavors and Fragrances). As for d8-ACP (C6D5COCD3), 75 μL d3-ACP (C6H5OCD3), d5-ACP (C6D5OCH3), and normal odorant were also used for discrimination experiments. Different sets of silicone rubber tubing holding cylinders and maze arms were used for each odor. Complete descriptions of the behavioral procedures are presented in SI Materials and Methods.
Computation of IR Intensity Spectra.
Spectra were computed by using the Amsterdam Density Functional software package (www.scm.com) at DZP/PBE level of theory. Frequencies were computed numerically by differentiation of energy gradients in slightly displaced geometries. The force constants and hence the frequencies were computed by comparison of the gradients (in the harmonic approximation of the energy surface). Under these conditions, intensities are proportional to the change in dipole occurring during atom movements in a given vibrational mode (41). Accuracy of mode calculations is typically fewer than 10 wave numbers for molecules comprising C, O, N, and H atoms.
Supplementary Material
Acknowledgments
We thank Victoria Frolova for the sample of citronellyl nitrile and Dr. Jim Ounsworth of CDN Isotopes for information about synthetic routes and purity of deuterated odorants. This work was supported by Defense Advanced Research Projects Agency Grant N66001-10-1-4062.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012293108/-/DCSupplemental.
References
- 1.Araneda RC, Kini AD, Firestein S. The molecular receptive range of an odorant receptor. Nat Neurosci. 2000;3:1248–1255. doi: 10.1038/81774. [DOI] [PubMed] [Google Scholar]
- 2.Axel R. Scents and sensibility: A molecular logic of olfactory perception (Nobel lecture) Angew Chem Int Ed Engl. 2005;44:6110–6127. doi: 10.1002/anie.200501726. [DOI] [PubMed] [Google Scholar]
- 3.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 4.Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- 5.Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- 6.Turin L. A spectroscopic mechanism for primary olfactory reception. Chem Senses. 1996;21:773–791. doi: 10.1093/chemse/21.6.773. [DOI] [PubMed] [Google Scholar]
- 7.Turin L. A method for the calculation of odor character from molecular structure. J Theor Biol. 2002;216:367–385. doi: 10.1006/jtbi.2001.2504. [DOI] [PubMed] [Google Scholar]
- 8.Wade D. Deuterium isotope effects on noncovalent interactions between molecules. Chem Biol Interact. 1999;117:191–217. doi: 10.1016/s0009-2797(98)00097-0. [DOI] [PubMed] [Google Scholar]
- 9.Keller A, Vosshall LB. A psychophysical test of the vibration theory of olfaction. Nat Neurosci. 2004;7:337–338. doi: 10.1038/nn1215. [DOI] [PubMed] [Google Scholar]
- 10.Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Laissue PP, Vosshall LB. The olfactory sensory map in Drosophila. Adv Exp Med Biol. 2008;628:102–114. doi: 10.1007/978-0-387-78261-4_7. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa T, Vosshall LB. Controversy and consensus: noncanonical signaling mechanisms in the insect olfactory system. Curr Opin Neurobiol. 2009;19:284–292. doi: 10.1016/j.conb.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 14.Acevedo SF, Froudarakis EI, Tsiorva AA, Skoulakis EM. Distinct neuronal circuits mediate experience-dependent, non-associative osmotactic responses in Drosophila. Mol Cell Neurosci. 2007;34:378–389. doi: 10.1016/j.mcn.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 16.Fiala A. Olfaction and olfactory learning in Drosophila: Recent progress. Curr Opin Neurobiol. 2007;17:720–726. doi: 10.1016/j.conb.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Berry J, Krause WC, Davis RL. Olfactory memory traces in Drosophila. In: Sossin WS, Lacaille J-C, Castelucci VF, Bellville S, editors. Progress in Brain Research: The Essence of Memory. Vol. 169. Amsterdam: Elsevier; 2008. pp. 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skoulakis EMC, Grammenoudi S. Dunces and da Vincis: The genetics of learning and memory in Drosophila. Cell Mol Life Sci. 2006;63:975–988. doi: 10.1007/s00018-006-6023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavlopoulos E, Anezaki M, Skoulakis EMC. Neuralized is expressed in the α/β lobes of adult Drosophila mushroom bodies and facilitates olfactory long-term memory formation. Proc Natl Acad Sci USA. 2008;105:14674–14679. doi: 10.1073/pnas.0801605105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 21.Skoulakis EM, Davis RL. Olfactory learning deficits in mutants for leonardo, a Drosophila gene encoding a 14-3-3 protein. Neuron. 1996;17:931–944. doi: 10.1016/s0896-6273(00)80224-x. [DOI] [PubMed] [Google Scholar]
- 22.Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wicher D, et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 24.A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Rescorla RA, Wagner AR, editors; Black AH, Prokasy WK, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- 25.Rescorla RA. Stimulus generalization: Some predictions from a model of Pavlovian conditioning. J Exp Psychol Anim Behav Process. 1976;2:88–96. doi: 10.1037//0097-7403.2.1.88. [DOI] [PubMed] [Google Scholar]
- 26.Brookes JC, Hartoutsiou F, Horsfield AP, Stoneham AM. Could humans recognize odor by phonon assisted tunneling? Phys Rev Lett. 2007;98:038101. doi: 10.1103/PhysRevLett.98.038101. [DOI] [PubMed] [Google Scholar]
- 27.Bedoukian PZ, editor. Perfumery and Flavoring Synthetics. Carol Stream, IL: Allured; 1986. [Google Scholar]
- 28.Borst A. Computation of olfactory signal in Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1983;152:373–383. [Google Scholar]
- 29.Masek P, Heisenberg M. Distinct memories of odor intensity and quality in Drosophila. Proc Natl Acad Sci USA. 2008;105:15985–15990. doi: 10.1073/pnas.0804086105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buck LB. Olfactory receptors and odor coding in mammals. Nutr Rev. 2004;62(suppl):S184–S188. doi: 10.1111/j.1753-4887.2004.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 31.Guo S, Kim J. Dissecting the molecular mechanism of Drosophila odorant receptors through activity modelling and comparative analysis. Proteins. 2010;78:381–399. doi: 10.1002/prot.22556. [DOI] [PubMed] [Google Scholar]
- 32.Rossiter KJ. Structureminus signodor relationships. Chem Rev. 1996;96:3201–3240. doi: 10.1021/cr950068a. [DOI] [PubMed] [Google Scholar]
- 33.Sell CS. On the unpredictability of odor. Angew Chem Int Ed Engl. 2006;45:6254–6261. doi: 10.1002/anie.200600782. [DOI] [PubMed] [Google Scholar]
- 34.Turin L, Yoshii F. Structure-odor relations: A modern perspective. In: Doty R, editor. Handbook of Olfaction and Gustation. New York: Marcel Dekker; 2003. [Google Scholar]
- 35.Robertson HM, Warr CG, Carlson JR. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc Natl Acad Sci USA. 2003;100(suppl 2):14537–14542. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W, Howard JD, Parrish TB, Gottfried JA. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science. 2008;319:1842–1845. doi: 10.1126/science.1152837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambe J, Jaklevic RC. Molecular vibration spectra by inelastic electron tunneling. Phys Rev. 1968;165:821–832. [Google Scholar]
- 38.Bentley R. The nose as a stereochemist. Enantiomers and odor. Chem Rev. 2006;106:4099–4112. doi: 10.1021/cr050049t. [DOI] [PubMed] [Google Scholar]
- 39.Wilson RI. Neural and behavioral mechanisms of olfactory perception. Curr Opin Neurobiol. 2008;18:408–412. doi: 10.1016/j.conb.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moressis A, Friedrich AR, Pavlopoulos E, Davis RL, Skoulakis EMC. A dual role for the adaptor protein DRK in Drosophila olfactory learning and memory. J Neurosci. 2009;29:2611–2625. doi: 10.1523/JNEUROSCI.3670-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan L, Ziegler T. Application of density functional theory to infrared absorption intensity calculations on main group molecules. J Chem Phys. 1992;96:6937–6941. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.