Abstract
Serotonin [i.e., 5-hydroxytryptamine (5-HT)]–targeted antidepressants are in wide use for the treatment of mood disorders, although many patients do not show a response or experience unpleasant side effects. Psychostimulants, such as cocaine and 3,4-methylenedioxymethamphetamine (i.e., “ecstasy”), also impact 5-HT signaling. To help dissect the contribution of 5-HT signaling to the actions of these and other agents, we developed transgenic mice in which high-affinity recognition of multiple antidepressants and cocaine is eliminated. Our animals possess a modified copy of the 5-HT transporter (i.e., SERT, slc6a4) that bears a single amino acid substitution, I172M, proximal to the 5-HT binding site. Although the M172 substitution does not impact the recognition of 5-HT, this mutation disrupts high-affinity binding of many competitive antagonists in transfected cells. Here, we demonstrate that, in M172 knock-in mice, basal SERT protein levels, 5-HT transport rates, and 5-HT levels are normal. However, SERT M172 mice display a substantial loss of sensitivity to the selective 5-HT reuptake inhibitors fluoxetine and citalopram, as well as to cocaine. Through a series of biochemical, electrophysiological, and behavioral assays, we demonstrate the unique properties of this model and establish directly that SERT is the sole protein responsible for selective 5-HT reuptake inhibitor-mediated alterations in 5-HT clearance, in 5-HT1A autoreceptor modulation of raphe neuron firing, and in behaviors used to predict the utility of antidepressants.
The biogenic amine serotonin [i.e., 5-hydroxytryptamine (5-HT)] modulates a wide variety of physiological processes in the brain and periphery (1–4). Whereas the actions of 5-HT are mediated by more than a dozen different receptors, 5-HT inactivation is dictated largely by a single protein, the presynaptic 5-HT transporter (i.e., SERT, SLC6A4). Recognition of the mood regulating actions of 5-HT in the CNS and a desire to eliminate the side effects of first-generation antidepressants led to the development of selective 5-HT reuptake inhibitors (SSRIs), typified by fluoxetine (Prozac) (5). Subsequent drug development resulted in more selective SSRIs, including citalopram (Celexa), s-citalopram (Lexapro), paroxetine (Paxil), and sertraline (Zoloft), among others. Although SERT blockade occurs within minutes of administration, antidepressant efficacy arises only after many weeks of treatment (6), leading some to question whether SERT inhibition is responsible for the beneficial actions of SSRIs. Indeed, despite their high affinity for SERT, SSRIs have been found to interact with many other proteins, including ion channels (7, 8), receptors (9), signaling proteins (10–14), and transcription factors (15–17), conceivably accounting for dose-limiting side effects and/or therapeutic actions (18). Finally, many patients treated with SSRIs have a poor treatment course outcome or do not show a response and may abandon treatment, with potentially life-threatening consequences (5). Tools are needed that can clarify the role of SERT in the actions of SSRIs and psychostimulants (and thereby 5-HT) in vivo. SERT-KO mice and rats (19–21) provide models for tests of SSRIs action, as well as the SERT involvement of psychostimulants (19–23). However, SERT-KO animals exhibit profound compensatory alterations in 5-HT levels and 5-HT receptors, and exhibit many behavioral changes (19, 20), limiting conclusions regarding drug specificity. Such issues encouraged us to establish an alternative animal model in which SERT-dependent 5-HT transport is unperturbed, but antagonist recognition is significantly altered.
Based on hydrophobicity assessments and high-resolution crystal structures of the SLC6 family member LeuTAa, SERT is proposed to contain 12 transmembrane domains (TMs), four of which—TMs 1, 3, 6, and 8—provide the key residues for ion and substrate recognition (24–27). By using the pharmacological differences displayed by human and Drosophila melanogaster SERTs (hSERT and dSERT), we identified variation at a single residue in TM3 (I172 in human and mouse, M167 in fly) proximal to the proposed binding site for 5-HT (28). Remarkably, when we substituted M172 for I172 in hSERT and mouse SERT (mSERT) cDNAs, we observed a significant, 100- to 1,000-fold reduction in potency of many SERT antagonists in transfected cells without an impact on basal 5-HT transport (28). Conversely, dSERT I167 displayed increased potencies for these same drugs.
In this report, we describe the successful generation of knock-in mice expressing SERT M172. We demonstrate that serotonergic terminals from the brains of these mice, studied ex vivo and in vivo, retain normal SERT protein expression and 5-HT transport, and exhibit normal SERT-mediated 5-HT clearance. In contrast, and as predicted by heterologous expression studies, we observe markedly reduced sensitivity of SERT to multiple SSRIs and cocaine. Neurochemical, physiological, and behavioral studies validate the utility of the I172M mice for dissecting the role of SERT and altered 5-HT signaling in the in vivo actions of 5-HT reuptake inhibitors.
Results
SERT M172 Mice Display Normal Growth, SERT Expression, and Saturation Uptake Kinetics.
We subjected a genomic fragment spanning exons 2 through 5 of the SERT gene, from a 129S6 mouse BAC clone, to site-directed mutagenesis with the engineered mutation in exon 4, and a neomycin resistance (Neor) cassette, flanked by lox-p sites, within intron 5 (Fig. S1A). Following successful targeting of ES cells, generation of chimeric animals, germline transmission of the SERT M172 variant, and excision of the Neor cassette (Fig. S1B), SERT M172 heterozygous and homozygous mice were generated along with SERT I172 littermates. SERT M172 homozygous offspring displayed normal growth rates of both male and female animals [repeated-measures ANOVA (RMANOVA), P > 0.05; Fig. S1 C and D], consistent with a lack of interference of the M172 mutation on developmental and adult SERT function and 5-HT signaling. Forebrain synaptosomal SERT protein expression, normalized to β-actin levels, also did not differ between SERT I172 and SERT M172 mice (I172, 0.396 ± 0.056 AU; M172, 0.469 ± 0.058 AU; n = 11; P > 0.05, two-tailed, unpaired Student t test; Fig. S2 A and B). Finally, no statistically significant difference in forebrain synaptosomal 5-HT transport activity of SERT M172 compared with SERT I172 littermates was observed (I172, Km of 175.6 ± 55.92 nM; Vmax of 4.474 ± 0.500 pmol/mg protein; M172, Km of 248.5 ± 73.43 nM; Vmax of 5.827 ± 0.667 pmol/mg protein: P > 0.05, unpaired Student t test for Km and Vmax; Fig. S2C).
Synaptosomes of SERT M172 Mice Display Reduced Sensitivity to Transporter Antagonists.
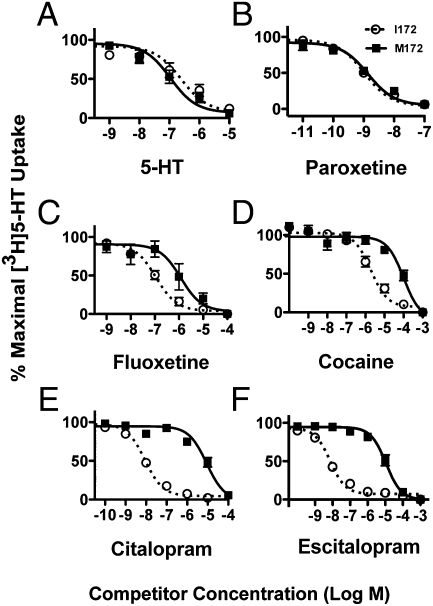
Previously, our heterologous expression studies demonstrated that the M172 substitution produces significant reductions in the potency of multiple SSRIs, cocaine, and cocaine analogues to inhibit 5-HT transport (28), despite the retention of normal potency of substrates (e.g., 5-HT, 3,4-methylenedioxymethamphetamine). Competition studies that used forebrain synaptosomes from M172 mice produced very similar results (Fig. 1 A–F). Thus, forebrain SERT M172 synaptosomes demonstrated no change in the competitive potency of unlabeled 5-HT to compete with [3H]5-HT transport, whereas reductions of 10 to 1,000 fold were observed in the potency of multiple SSRIs and cocaine. Also as seen with transfected cell studies (28), the M172 substitution had no impact on the potency of the SSRI paroxetine to compete with [3H]5-HT transport.
Fig. 1.
Competition uptake analysis of forebrain-derived synaptosomes. (A) Serotonin, (B) paroxetine, (C) fluoxetine, (D) cocaine, (E) citalopram, and (F) escitalopram were assessed for their ability to compete with [3H]5-HT uptake. Fluoxetine, cocaine, citalopram, and escitalopram demonstrated significantly reduced potencies in SERT M172 relative to SERT I172 synaptosomes (n = 6–8 per drug; P < 0.05, one-tailed Student t test).
Diminished SSRI-Mediated Inhibition of Serotonergic Neurons in Brain Slices of SERT M172 Mice.
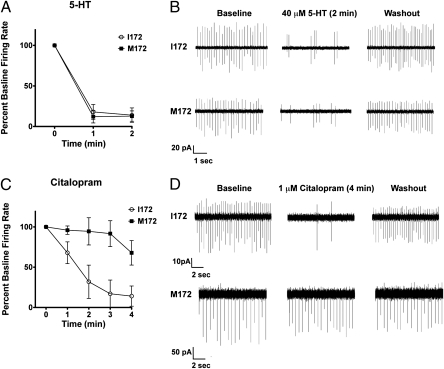
Next, we sought to determine the impact of the SERT M172 substitution on serotonergic synaptic transmission. HPLC analysis of 5-HT, dopamine, and norepinephrine levels in the forebrain and midbrain revealed no significant changes in SERT M172 animals relative to SERT I172 controls (Table S1). We next sought to establish an impact of the SERT M172 substitution on the synaptic actions of 5-HT. Whole-cell patch recordings of neurons in the dorsal raphe provide a measure of SERT-mediated 5-HT clearance, which limits 5-HT1A receptor-dependent suppression of neuronal firing rates (29, 30). As illustrated in Fig. 2 A and B, baseline firing rates of dorsal raphe neurons recorded from slices of SERT I172 and SERT M172 mice were indistinguishable. Furthermore, 5-HT application led to an immediate and equivalent reduction in neuronal firing in both genotypes. Following a 10-min washout protocol, firing rates recovered to baseline levels over an equivalent time course for I172 and M172 animals. Finally, application of 1 μM citalopram to slices from SERT I172 mice displayed the predicted rapid reduction in neuronal firing. However, slices from SERT M172 mice demonstrated little or no inhibition by citalopram (Fig. 2 C and D; P < 0.05, RMANOVA between genotypes).
Fig. 2.
Loss of SSRI sensitivity for inhibition of tonic raphe neuron activity. (A and C) Time-dependent inhibition of dorsal raphe neuron activity by 5-HT or citalopram in SERT I172 and M172 mice. (B and D) Exemplary baseline and drug-modulated firing of dorsal raphe neurons in midbrain slices from SERT I172 and M172 mice. In response to 40 μM 5-HT, raphe neuron firing was inhibited to an equivalent extent in either genotype. In response to 1 μM citalopram, SERT I172 neurons exhibited a rapid reduction in firing rate, whereas SERT M172 neurons were significantly less sensitive to citalopram [two-way RMANOVA, n = 4 (I172) and n = 5 (M172); *P < 0.05 vs. I172].
SERT M172 Mice Display Loss of SSRI Sensitivity in Vivo.
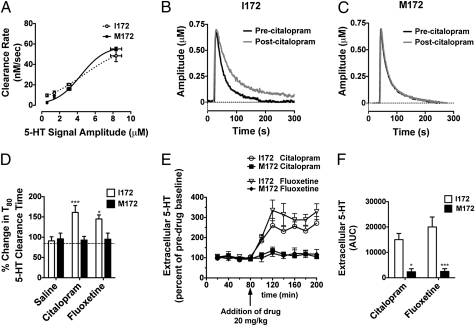
To assess the relative impact of the SERT M172 substitution on 5-HT transport rates and sensitivity to antagonists in vivo, we pursued amperometric recordings of 5-HT clearance in the CA3 region of the hippocampus. In these studies, we determined that maximal baseline 5-HT clearance rates did not differ significantly between genotypes (Vmax, 57.7 ± 21.4 nM and 58.6 ±5.0 nM, respectively; Fig. 3A), providing important in vivo evidence of a lack of functional perturbation of the SERT M172 substitution as predicted from in vitro studies. Preinjection of citalopram or fluoxetine resulted in a significant reduction in 5-HT clearance rates in SERT I172 mice (Fig. 3 B and D and Fig. S3A; P < 0.05, two-way ANOVA with Bonferroni posttest), comparable to those produced by elimination of SSRI-dependent 5-HT clearance in the SERT-KO mouse (31). In contrast, citalopram and fluoxetine injections at the same concentrations were without effect on 5-HT clearance in the SERT M172 mice (Fig. 3 C and D and Fig. S3B).
Fig. 3.
In vivo analysis of SERT-mediated 5-HT clearance and extracellular 5-HT. (A–D) 5-HT clearance measured in the CA3 region of the hippocampus by in vivo amperometry. (A) Baseline clearance rates do not differ between SERT I172 and M172 animals (n = 6 per group; apparent Vmax, 57.7 ± 21.4 nM and 58.6 ± 5.0 nM, respectively). (B) Preapplication of 20 pmol citalopram results in delayed 5-HT clearance in the SERT I172 animals (P < 0.05, Student t test). (C) Preapplication of 20 pmol citalopram has no impact on 5-HT clearance rates in the M172 animals. (D) Percent change in T80 5-HT clearance time (time for signal to decline by 80% of peak signal amplitude) in comparison with baseline T80. I172 SERT mice displayed an increase in the 5-HT T80 clearance time in response to citalopram and fluoxetine, whereas M172 mice did not (*P < 0.05 and ***P < 0.0001 vs. saline solution controls, two-way ANOVA with Bonferroni post-tests; n = 4–8). (E and F) Measurements of extracellular 5-HT by in vivo microdialysis. (E) Time course of the effects of citalopram and fluoxetine administration (20 mg/kg i.p.) on extracellular 5-HT concentrations in the hippocampus of SERT I172 or M172 mice (n = 3–5 for each genotype). SERT I172 mice display increased extracellular levels of 5-HT in response to citalopram, whereas SERT M172 mice do not (P < 0.05, two-way RMANOVA). Values are the mean ± SEM of the percent of predrug baseline. Arrow indicates administration of SSRIs at 80 min. (F) Cumulative elevations of 5-HT across 120-min sample period, expressed as area under the curve, were significantly diminished in the SERT M172 substitution relative to SERT I172 littermate values (*P < 0.05 and ***P < 0.0001 vs. I172, two-way ANOVA with Bonferroni post-tests).
The altered sensitivity of SERT-mediated clearance of exogenously applied 5-HT described earlier suggests that SSRIs should also fail to elevate extracellular levels of endogenously produced 5-HT. To validate this hypothesis, we monitored extracellular levels of 5-HT in the mouse brain of SERT I172 and M172 mice by using in vivo microdialysis. In Fig. 3E, we show that, whereas injection of citalopram or fluoxetine (i.p., 20 mg/kg) induced a rapid and sustained elevation (∼300%) in extracellular 5-HT in SERT I172 mice, these injections were without effect in SERT M172 animals (P < 0.05, RMANOVA). No significant changes were noted in dopamine or norepinephrine levels following these manipulations in SERT I172 or SERT M172 animals (Fig. S3 E and F). The cumulative elevations of 5-HT across our sample period produced by both citalopram and fluoxetine were also significantly diminished by the SERT M172 substitution (P < 0.05, two-way ANOVA with Bonferroni posttest; Fig. 3F). Similar results were observed with escitalopram, the active enantiomer of citalopram (Fig. S3 C and D), following striatal microdialysis and are included here to document a lack of regional specificity of SSRI potency reductions.
SERT M172 Reduces Impact of SSRIs on Behavior.
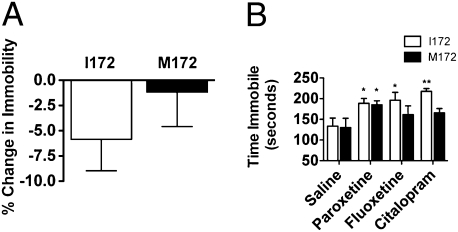
Although the therapeutic actions of antidepressants in humans require weeks of administration, the potential clinical efficacy of these compounds can often be gauged by behavioral alterations in rodents after acute administration (32, 33). Two commonly used tests for assessing a drug's potential antidepressant efficacy are the tail-suspension test (TST) and the forced swim test (FST). The dependent variable in these tests is the struggling behavior of a rodent when subjected to an inescapable stressor (e.g., a brief suspension by the tail or temporary placement in a cylinder of water). In the TST, citalopram exhibited only a modest ability to reduce immobility in SERT I172 mice and thus achieved only a suggestive level of significance in comparison with saline solution treatment (P = 0.076, one-sample t test; Fig. 4A). We achieved a much greater response to citalopram and other SSRIs in the FST (Fig. 4B). It should be noted that the I172 animals on a 129S6/S4 background respond with a significant increase in immobility when treated with citalopram rather than a decrease (Fig. 4B; P < 0.01, Tamhane T2 pairwise comparison). Similar effects were seen in the I172 mice with fluoxetine (P < 0.05) and paroxetine (P < 0.05). Importantly, neither citalopram nor fluoxetine produced a behavioral response in SERT M172 mice, whereas paroxetine produced the same response in both I172M and M172 mice.
Fig. 4.
Behavioral response of SERT M172 mice to acute SSRI administration. (A) Immobility of mice in the TST. Immobility of I172 SERT and M172 SERT mice was assessed in a 6-min TST performed 30 min after i.p. injection of saline solution or 20 mg/kg citalopram (n = 20, I172; and n = 17, M172). (B) Time immobile in the last 4 min of a 6-min FST. I172 SERT mice responded with significant increases in immobility in response to treatment with paroxetine, fluoxetine, or citalopram. In contrast, SERT M172 animals showed a significant increase in immobility only in response to paroxetine, as predicted from in vitro pharmacological studies (n = 8–10; *P < 0.05 and **P < 0.01, Kruskal-Wallis test followed by planned contrasts with Tamhane T2 pairwise comparisons).
Discussion
The data presented here document altered pharmacological properties, both ex vivo and in vivo, of SERT M172 mice as predicted from previous in vitro heterologous expression studies (28), without an impact on SERT protein expression or transport function. An important control for our studies is the SSRI paroxetine, which retains normal potency for SERT blockade. We suspect the inability of the SERT M172 substitution to reduce paroxetine potency reflects a distinct orientation of paroxetine compared with other SSRIs in the 5-HT binding pocket, particularly given that high-affinity SSRI recognition at SERT can be strongly influenced by relatively small changes in transporter structure (34). SERT M172 cell lines should be useful in further exploring the physical basis of interactions of different antidepressants in vitro (26), whereas the SERT M172 mice should help define the requirements for SERT and 5-HT signaling in the in vivo actions of antidepressants and cocaine. Although significant evidence supports a primary role for the 5-HT transporter in the reinforcing properties of psychostimulants (35–37), SERT blockade also appears to contribute to reinforcement (38–40). Indeed, SERT appears to be primarily responsible for the sustained reinforcing properties of cocaine in the 5-HT transporter–KO mouse (22, 23, 39–41). The significant compensatory alterations evident in 5-HT transporter–KO mice encouraged Chen and colleagues (42, 43) to develop a mouse bearing knock-in mutations in 5-HT transporter that, in vitro, reduced cocaine potency. Studies with these mice have yielded convincing evidence that 5-HT transporter is a key determinant of many synaptic and behavioral actions of cocaine. Likewise, the M172 mice should provide evidence as to what roles SERT blockade plays in the initiation of addiction to psychostimulants and/or the negative features associated with drug withdrawal in the context of normal 5-HT transporter expression.
Our assessment of SSRI actions on behavior in the SERT M172 mice focused on acute tests that have proven to be predictive of antidepressant efficacy (32, 33), as opposed to the more chronic actions of antidepressants thought to model the therapeutic course of SSRI action in man. Future studies will use the SERT M172 mice in tests that require chronic antidepressant treatment, such as novelty-induced food neophobia and hippocampal stem cell proliferation (44, 45). The 129S6/S4 background on which our SERT M172 substitution resides derives from our use of popular 129S6-derived ES cells for gene targeting and a cross to 129S4 mice that express Cre 129-based mouse models have been shown by others to respond poorly to SSRIs (46, 47), or as we show in the FST, in an opposite direction, relative to other mouse and rat models (48). In the TST, meager responses to SSRIs were seen with the SERT I172 line. We did obtain evidence for a small, but consistent, reduction in immobility with citalopram, whereas SERT M172 mice displayed no such effects. In the FST, we were able to document a much stronger SSRI effect and here could document a significant loss of response to citalopram and fluoxetine, with no loss of response to paroxetine. The direction of the response to citalopram and fluoxetine in promoting immobility, rather than reducing it, has been noted by others by using mice on a related 129/Svemj background (48). In contrast, it has been reported that mice on the 129Sv substrain display decreased immobility in response to citalopram treatment (49), further documenting the behavioral variations evident across inbred mouse lines (46, 48). The action of SSRIs in the 129 S6/S4 background of our model is not the typical direction monitored for SSRI effects in the FST in many other strains. However, this test is agreed to be of operational use only in predicting antidepressant utility, as opposed to a test for mood alterations, and as such we believe the directionality of responses to drugs is less important than the demonstrated loss of SSRI sensitivity. It is interesting to speculate that the enhanced immobility produced by SSRIs in our model may reflect the anxiogenic nature experienced by human subjects within the first few days of SSRI administration (50, 51), that, if validated, presents a novel paradigm to identify medications that can ameliorate this undesirable effect of many SSRIs. Finally, we are backcrossing the SERT I172 mutation to other mouse strains to provide additional perspectives on the role SERT plays in the behavioral actions of SSRIs.
For more than 20 y, the actions of antidepressant molecules with high affinity for SERT have been interpreted as arising from blockade of the 5-HT transporter. We have not attempted to reproduce all the actions evident in rodents treated with SSRIs, nor tested the full spectrum of molecules whose potency for SERT antagonism is shifted. Nonetheless, the data we obtained with the SERT M172 mice, gained across a spectrum of in vitro and in vivo neurochemical, physiological, and behavioral paradigms, demonstrates how gene products whose activities have never been, or cannot presently be, monitored can be eliminated from consideration (to the limit afforded by rodent models). It is now possible to secure comparable data on SSRI actions in chronic studies, to test whether lifelong changes in behavior following neonatal SSRI action derive from SERT, and to tease out the role of SERT in mixed action drugs such as cocaine.
Methods
Generation of mSERT Ile172Met Knock-in Mice.
All animal studies were performed in accordance with protocols approved by the respective institutions’ (Vanderbilt University or University of Texas Health Science Center at San Antonio) animal use and care committees. To introduce the M172 allele into mice, a targeting construct carrying this substitution was created using exons 2 to 4 of the mouse 129S6 strain Slc6a4 gene and the slc6a4 locus of 129S6 ES cells was targeted in the Vanderbilt University Transgenic Mouse/Embryonic Stem Cell Shared Resource Facility (SI Methods). Heterozygous breeders were used to generate mice homozygous for the SERT M172 substitution and homozygous SERT I172 littermate control animals.
Synaptosomal Uptake.
Synaptosomes were prepared as described previously (52) with 5-HT transport activity assessed at 37 °C as previously described (53). For competition uptake assays, samples containing 25 to 100 μg were incubated in duplicate with 20 nM [3H]5-HT and varying concentrations of nonradioactive inhibitors, obtained either directly from the pharmaceutical supplier or Sigma. For saturation uptake assays, samples were incubated with increasing concentrations of 5-HT containing 2.5% [3H]5-HT as a tracer. Parallel saturation studies were performed in the presence of 1 μM paroxetine and the residual counts for each concentration were subtracted from the experimental curve.
Electrophysiologic Slice Preparation and Recordings.
For electrophysiology experiments, mice were euthanized by decapitation, and brains were removed and blocked in cold sucrose-substituted saline solution. The solutions and procedures used are detailed in a previous publication (54). Briefly, coronal, midbrain slices (200 μm) containing the dorsal raphe nucleus were cut on a Vibroslicer at 4 °C to 10 °C and maintained in 50% sucrose saline/50% normal saline solution for 10 min at room temperature followed by 100% normal saline solution maintained at 34 ± 0.5 °C. Neurons were visualized with an Axioskop microscope (Carl Zeiss) equipped for near-IR digital image correlation. Extracellular patch electrodes with a pipette resistance of 3 to 5 MΩ were filled with filtered, normal Hepes solution (54). Firing rate frequency was measured as the average of a 30-s recording. All neurons were treated first with 5-HT (40 μM) for 2 min, followed by a 5-min application of citalopram (1 μM). At least 10 min between each treatment allowed for complete firing rate recovery, and the order of the SSRIs were counterbalanced. All solutions contained 3 μM phenylephrine hydrochloride and 40 μM L-tryptophan to maintain the spontaneous firing rate in the absence of noradrenergic tone and tryptophan availability (55). Data were analyzed for each drug by using a two-way RMANOVA.
In Vivo Chronoamperometry.
In vivo chronoamperometry with carbon fiber electrodes (30 μm diameter) was carried out according to the methods described previously (56) and is described in greater detail in SI Methods. The electrode–micropipette recording assembly was lowered into the CA3 region of the dorsal hippocampus [anteroposterior (AP), −1.94 from bregma; mediolateral (ML), +2.0 from midline; dorsoventral (DV) −2.0 from dura] of anesthetized mice. To assess 5-HT clearance kinetics, 5-HT was pressure-ejected in increasing volumes to attain signal amplitudes matching in vitro calibration standards of approximately 0.5 to 10 μM. The sequence of 5-HT signal amplitudes was randomized between each mouse. To examine the effect of citalopram and fluoxetine on 5-HT clearance, exogenous 5-HT was intrahippocampally applied by pressure-ejection to attain at least three replicate signals with peak amplitudes within the range of 0.5 to 1.0 μM, conditions at which we find that 5-HT clearance is predominantly SERT-mediated (31, 57). Citalopram (20 pmol) or fluoxetine (54 pmol) was then applied via the same route to the CA3 region of hippocampus. Two min later and then at 5-min intervals thereafter, 5-HT was delivered again until the drug effect dissipated and the signal returned to pretreatment baseline.
In Vivo Microdialysis.
Mice were anesthetized with isoflurane and placed in a stereotaxic frame using mouse-specific ear bars (Kopf Instruments). A guide cannula (CMA7) was placed 1 mm above the dorsal hippocampus (AP, −1.94 from bregma, ± 2.0 ML and −1.0 DV from dura) and secured to the skull with epoxy adhesive (Plastics One). After an overnight recovery from surgery, animals were placed in individual dialysis chambers and dialysate fractions were collected every 20 min and stored at −80 °C until analysis by HPLC/electrochemical detection (SI Methods provides additional details).
Behavioral Studies.
TST.
Mice were tested as previously described (58). Briefly, mice were injected intraperitoneally, 30 min before a 6-min TST, with 0.9% saline solution or citalopram (20 mg/kg, 10 μL/g body weight). Mice were suspended by taping the tail to a vertical aluminum bar connected to a strain gauge. Time immobile in a 6-min TST was recorded by an automated testing apparatus (Med Associates). The following settings were used: threshold 1, 7; gain, 8; time constant, 0.25; and resolution, 200 ms. The time immobile in the last 4 min of the test was analyzed by using the Student t test. A P value lower than 0.05 was considered to be significant.
FST.
Mice were injected with saline solution, fluoxetine, citalopram, or paroxetine 30 min before testing. Mice were subjected to a 6-min FST by placing them in the center of a 15-cm-diameter cylinder filled with water (25–27 °C) to a depth of approximately 15 cm. Mice were considered to be immobile when making only movements required to maintain balance. The time immobile in the last 4 min of the test was recorded. As immobility data for the FST violated the assumption of homogeneity for the drug factor [Levene (3,62) = 3.6; P < 0.05]. We used a nonparametric Kruskal–Wallis test followed by planned contrasts with Tamhane T2 pairwise comparisons to compare genotype at each level of drug as well as saline solution versus each SSRI for each genotype. The omnibus test was significant [χ2(7) = 21.2; P < 0.01], and post-hoc analysis revealed a genetic difference for citalopram (P < 0.05), and drug effects (vs. saline solution) for all three SSRIs given to I172 mice (P < 0.05) and for paroxetine only when given to M172 mice (P < 0.05).
Supplementary Material
Acknowledgments
We thank the R.D.B. laboratory staff for excellent research support, Irwin Lucki for advice on microdialysis and HPLC analyses, Raymond Johnson for the HPLC analysis, and Gregg Stanwood for advice on behavioral studies. This work was supported by National Institutes of Health Awards DA07390 and MH078028 (to R.D.B., L.C.D., and D.G.M.) and a research award from the Forest Research Institute (to R.D.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 3463.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011920108/-/DCSupplemental.
References
- 1.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esteve JM, Launay J-M, Kellermann O, Maroteaux L. Functions of serotonin in hypoxic pulmonary vascular remodeling. Cell Biochem Biophys. 2007;47:33–44. doi: 10.1385/cbb:47:1:33. [DOI] [PubMed] [Google Scholar]
- 3.Hart CM, Block ER. Lung serotonin metabolism. Clin Chest Med. 1989;10:59–70. [PubMed] [Google Scholar]
- 4.Nguyen TT, et al. Placental biogenic amine transporters: in vivo function, regulation and pathobiological significance. Placenta. 1999;20:3–11. doi: 10.1053/plac.1998.0348. [DOI] [PubMed] [Google Scholar]
- 5.Serretti A, Artioli P. The pharmacogenomics of selective serotonin reuptake inhibitors. Pharmacogenomics J. 2004;4:233–244. doi: 10.1038/sj.tpj.6500250. [DOI] [PubMed] [Google Scholar]
- 6.Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- 7.Hamplová-Peichlová J, et al. Citalopram inhibits L-type calcium channel current in rat cardiomyocytes in culture. Physiol Res. 2002;51:317–321. [PubMed] [Google Scholar]
- 8.Tytgat J, Maertens C, Daenens P. Effect of fluoxetine on a neuronal, voltage-dependent potassium channel (Kv1.1) Br J Pharmacol. 1997;122:1417–1424. doi: 10.1038/sj.bjp.0701545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrasco JL, Sandner C. Clinical effects of pharmacological variations in selective serotonin reuptake inhibitors: an overview. Int J Clin Pract. 2005;59:1428–1434. doi: 10.1111/j.1368-5031.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 10.Carneiro AMD, Blakely RD. Serotonin-, protein kinase C-, and Hic-5-associated redistribution of the platelet serotonin transporter. J Biol Chem. 2006;281:24769–24780. doi: 10.1074/jbc.M603877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad HC, et al. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci USA. 2005;102:11545–11550. doi: 10.1073/pnas.0501432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steiner JA, et al. cGMP-dependent protein kinase Ialpha associates with the antidepressant-sensitive serotonin transporter and dictates rapid modulation of serotonin uptake. Mol Brain. 2009;2:26. doi: 10.1186/1756-6606-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu C-B, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- 14.Zhu C-B, Hewlett WA, Francis SH, Corbin JD, Blakely RD. Stimulation of serotonin transport by the cyclic GMP phosphodiesterase-5 inhibitor sildenafil. Eur J Pharmacol. 2004;504:1–6. doi: 10.1016/j.ejphar.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 15.Beck CH. Acute treatment with antidepressant drugs selectively increases the expression of c-fos in the rat brain. J Psychiatry Neurosci. 1995;20:25–32. [PMC free article] [PubMed] [Google Scholar]
- 16.Di Benedetto M, D'Addario C, Candeletti S, Romualdi P. Alterations of CREB and DARPP-32 phosphorylation following cocaine and monoaminergic uptake inhibitors. Brain Res. 2007;1128:33–39. doi: 10.1016/j.brainres.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 17.Frechilla D, Otano A, Del Río J. Effect of chronic antidepressant treatment on transcription factor binding activity in rat hippocampus and frontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:787–802. doi: 10.1016/s0278-5846(98)00040-2. [DOI] [PubMed] [Google Scholar]
- 18.Keiser MJ, et al. Predicting new molecular targets for known drugs. Nature. 2009;462:175–181. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy DL, Lesch K-P. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- 20.Kalueff AV, Olivier JD, Nonkes LJ, Homberg JR. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci Biobehav Rev. 2010;34:373–386. doi: 10.1016/j.neubiorev.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Olivier JD, et al. A study in male and female 5-HT transporter knockout rats: an animal model for anxiety and depression disorders. Neuroscience. 2008;152:573–584. doi: 10.1016/j.neuroscience.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 22.Sora I, et al. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sora I, et al. Cocaine reward models: Conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Field JR, Henry LK, Blakely RD. Transmembrane domain 6 of the human serotonin transporter contributes to an aqueously accessible binding pocket for serotonin and the psychostimulant 3,4-methylene dioxymethamphetamine. J Biol Chem. 2010;285:11270–11280. doi: 10.1074/jbc.M109.093658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry LK, Meiler J, Blakely RD. Bound to be different: neurotransmitter transporters meet their bacterial cousins. Mol Interv. 2007;7:306–309. doi: 10.1124/mi.7.6.4. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann KW, et al. Structural determinants of species-selective substrate recognition in human and Drosophila serotonin transporters revealed through computational docking studies. Proteins. 2009;74:630–642. doi: 10.1002/prot.22178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl—dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 28.Henry LK, et al. Tyr-95 and Ile-172 in transmembrane segments 1 and 3 of human serotonin transporters interact to establish high affinity recognition of antidepressants. J Biol Chem. 2006;281:2012–2023. doi: 10.1074/jbc.M505055200. [DOI] [PubMed] [Google Scholar]
- 29.Gartside S, Umbers V, Hajos M. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: Effects on 5-HT cell firing and extracellular 5-HT. Br J Pharmacol. 1995;115:1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51:215–235. doi: 10.1016/s0165-0327(98)00221-3. [DOI] [PubMed] [Google Scholar]
- 31.Baganz NL, et al. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci USA. 2008;105:18976–18981. doi: 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 33.Lucki I, Singh A, Kreiss DS. Antidepressant-like behavioral effects of serotonin receptor agonists. Neurosci Biobehav Rev. 1994;18:85–95. doi: 10.1016/0149-7634(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 34.Barker EL, et al. High affinity recognition of serotonin transporter antagonists defined by species-scanning mutagenesis. An aromatic residue in transmembrane domain I dictates species-selective recognition of citalopram and mazindol. J Biol Chem. 1998;273:19459–19468. doi: 10.1074/jbc.273.31.19459. [DOI] [PubMed] [Google Scholar]
- 35.Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 36.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 37.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine self-administration appears to be mediated by dopamine uptake inhibition. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:233–239. doi: 10.1016/0278-5846(88)90040-1. [DOI] [PubMed] [Google Scholar]
- 38.Hnasko TS, Sotak BN, Palmiter RD. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci. 2007;27:12484–12488. doi: 10.1523/JNEUROSCI.3133-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rocha BA. Stimulant and reinforcing effects of cocaine in monoamine transporter knockout mice. Eur J Pharmacol. 2003;479:107–115. doi: 10.1016/j.ejphar.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 40.Rocha BA, et al. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- 41.Mateo Y, Budygin EA, John CE, Jones SR. Role of serotonin in cocaine effects in mice with reduced dopamine transporter function. Proc Natl Acad Sci USA. 2004;101:372–377. doi: 10.1073/pnas.0207805101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen R, et al. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci USA. 2006;103:9333–9338. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomsen M, Han DD, Gu HH, Caine SB. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther. 2009;331:204–211. doi: 10.1124/jpet.109.156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 45.Dranovsky A, Hen R. Hippocampal neurogenesis: Regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobson LH, Cryan JF. Feeling strained? Influence of genetic background on depression-related behavior in mice: a review. Behav Genet. 2007;37:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- 47.Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology (Berl) 2005;183:257–264. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- 48.Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- 49.Cervo L, et al. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J Neurosci. 2005;25:8165–8172. doi: 10.1523/JNEUROSCI.1816-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: A fear-potentiated startle study. Neuropsychopharmacology. 2007;32:225–231. doi: 10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- 51.Pollack MH. The pharmacotherapy of panic disorder. J Clin Psychiatry. 2005;66(suppl 4):23–27. [PubMed] [Google Scholar]
- 52.Ansah T-A, Ramamoorthy S, Montañez S, Daws LC, Blakely RD. Calcium-dependent inhibition of synaptosomal serotonin transport by the alpha 2-adrenoceptor agonist 5-bromo-N-[4,5-dihydro-1H-imidazol-2-yl]-6-quinoxalinamine (UK14304) J Pharmacol Exp Ther. 2003;305:956–965. doi: 10.1124/jpet.102.047134. [DOI] [PubMed] [Google Scholar]
- 53.Zhu C-B, et al. Rapid stimulation of presynaptic serotonin transport by A(3) adenosine receptors. J Pharmacol Exp Ther. 2007;322:332–340. doi: 10.1124/jpet.107.121665. [DOI] [PubMed] [Google Scholar]
- 54.Nunemaker CS, DeFazio RA, Moenter SM. A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol Proced Online. 2003;5:53–62. doi: 10.1251/bpo46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evans AK, et al. Evidence for serotonin synthesis-dependent regulation of in vitro neuronal firing rates in the midbrain raphe complex. Eur J Pharmacol. 2008;590:136–149. doi: 10.1016/j.ejphar.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 56.Toney GM, Daws LC. Juxtacellular labeling and chemical phenotyping of extracellularly recorded neurons in vivo. Methods Mol Biol. 2006;337:127–137. doi: 10.1385/1-59745-095-2:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daws LC, et al. Transport mechanisms governing serotonin clearance in vivo revealed by high-speed chronoamperometry. J Neurosci Methods. 2005;143:49–62. doi: 10.1016/j.jneumeth.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 58.Carneiro AM, et al. Functional coding variation in recombinant inbred mouse lines reveals multiple serotonin transporter-associated phenotypes. Proc Natl Acad Sci USA. 2009;106:2047–2052. doi: 10.1073/pnas.0809449106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.