Abstract
We have determined the X-ray crystal structures of the pre- and postcatalytic forms of the initiation complex of bacteriophage N4 RNA polymerase that provide the complete set of atomic images depicting the process of transcript initiation by a single-subunit RNA polymerase. As observed during T7 RNA polymerase transcript elongation, substrate loading for the initiation process also drives a conformational change of the O helix, but only the correct base pairing between the +2 substrate and DNA base is able to complete the O-helix conformational transition. Substrate binding also facilitates catalytic metal binding that leads to alignment of the reactive groups of substrates for the nucleotidyl transfer reaction. Although all nucleic acid polymerases use two divalent metals for catalysis, they differ in the requirements and the timing of binding of each metal. In the case of bacteriophage RNA polymerase, we propose that catalytic metal binding is the last step before the nucleotidyl transfer reaction.
Keywords: transcription, DNA polymerases, A family, phosphodiester bond
DNA-dependent RNA polymerases (RNAPs) transcribe DNA genetic information into RNA and play a central role in gene expression. RNAP catalyzes a nucleotidyl transfer reaction, which is initiated by the nucleophilic attack of an O3′ oxyanion at the RNA 3′ terminus to the α-phosphate (αP) of the incoming nucleotide, resulting in phosphodiester bond formation and release of pyrophosphate (PPi). Both single-subunit T7 phage-like RNAPs and the multisubunit cellular RNAPs possess two nucleotide-binding sites for loading the RNA 3′ end (P site) and the incoming NTP (N site) (1, 2). A two metal-ion catalytic mechanism has been proposed, as the enzyme possesses two divalent catalytic and nucleotide-binding metal cations chelated by two or three conserved Asp residues (3). The catalytic metal is a Lewis acid, coordinating the RNA 3′-OH lowering its pKa and facilitating the formation of the attacking oxyanion. The nucleotide-binding metal is coordinated by the triphosphate of the incoming nucleotide and stabilizes a pentacovalent phosphate intermediate during the reaction. Both metal ions are proposed to have octahedral coordination at physiological Mg2+ concentrations (4).
During transcript elongation, RNAP carries out the loading of a single nucleotide substrate at the N site followed by a nucleotidyl transfer reaction with the RNA 3′ end at the P site; this cycle is repeated as elongation proceeds. X-ray crystal structures of the single-subunit T7 phage RNAP (2, 5) have depicted the process of transcript elongation in detail and reveal a conformational change of the Fingers subdomain during substrate loading to the active site as also observed in the A family of DNA polymerases (DNAPs) (6, 7).
Initiation is the only step in the entire transcription process where two nucleotide substrates are loaded at the active site followed by a nucleotidyl transfer reaction. Compared with elongation, the process of initiation has not been well characterized by X-ray crystallography. An X-ray crystal structure of T7 RNAP initiation complex was reported (8), but it was captured by using a substrate analog 3′-deoxyGTP (Fig. S1B). This analog lacks the essential O3′ required for nucleotidyl transfer and catalytic metal coordination resulting in the absence of the catalytic metal ion in the structure and misalignment of the reactive groups of substrate. In the present study, we have used X-ray crystallography and a natural substrate plus a proper substrate analog to capture a set of atomic resolution snapshots, from nucleotide binding to nucleotidyl transfer reaction, (Fig. 1A and Table S1) to elucidate a complete picture of the process of transcript initiation by the central domain of N4 phage virion-encapsulated RNAP (mini-vRNAP).
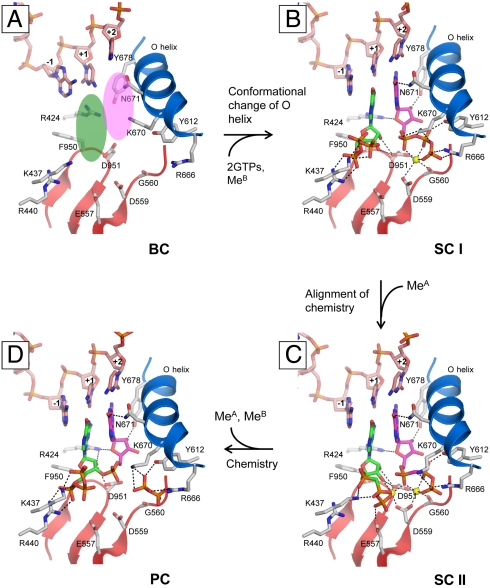
Fig. 1.
The structure of the initiation complex. (A) A schematic representation of the sequential processes during initiation of transcription. The BC comprising RNAP and promoter DNA is depicted as “E,” and catalytic and nucleotide-binding metal ions are shown as MeA and MeB, respectively. (B) Sequences and secondary structures of the two DNA constructs used for crystallization. Regions highlighted by the gray boxes were disordered in the crystal structures. Nucleotide-binding sites (+1 and +2) for transcript initiation are colored in red. (C) Overall structure of the SCII. N4 mini-vRNAP is depicted as a molecular surface model. The N-terminal domain, subdomains, and motifs are labeled. The β-intercalating hairpin, Plug, Thumb, and N-terminal two-thirds of Fingers have been removed from this view for clarity, and only their outlines are shown. The promoter DNA and O helix of the Fingers are depicted by a pink tube and blue ribbon, respectively. (D–F) Electron density maps showing nucleotides, 2-mer RNA, pyrophosphate, and metal ions found in the three initiation complexes. Fo - Fc electron density maps (black net) superimposed on the final models (sticks and spheres) of the SCI (D), SCII (E), and PC (F). These maps were calculated using the native amplitudes and the phase derived from the BC. Template DNA is depicted and labeled. The metal-chelating D559 and D951 are shown as stick models. Divalent metals and waters are depicted by yellow and cyan spheres, respectively.
Results
Design of the X-Ray Crystallographic Experiment to Monitor the Formations of Transcript Initiation Complexes.
Previously, we reported the X-ray crystal structure of the binary complex (BC) of promoter DNA and N4 mini-vRNAP (9), which is a member of the T7-like single-subunit RNAP family (10) that recognizes a specific DNA hairpin sequence with a 5-bp stem, 3-nt loop as its promoter (Fig. 1B) (11–13). In the BC structure, from -1 to +2 template DNA bases point toward the nucleotide entry pore, whereas the +3 template DNA base is flipped in the opposite direction providing an opportunity to analyze the structural transitions of DNA template bases at the +1 and +2 positions and of the enzyme upon nucleotide loading.
The structures reported in this study represent the precatalytic [substrate complex I (SCI); substrate complex II (SCII); mismatch complex (MC)] and postcatalytic [product complex (PC)] stages of transcript initiation (Fig. 1A). Each complex comprises the 120 kDa N4 mini-vRNAP and a 36-nt DNA, which includes the P2 promoter 7 bp stem, stable and well-ordered 3-nt loop hairpin followed by five bases of single-stranded DNA including the start site (+1) (Fig. 1B). Promoter and template DNA regions to +3 ∼ 4 were well resolved in the crystal structures, but were completely disordered downstream. The P2_7a DNA sequence of the transcription start site is CC at positions +1 and +2, to form Watson–Crick base pairs with two molecules of GTP upon nucleotide loading, followed by a nucleotidyl transfer reaction to produce a 2-mer RNA—5′-pppGpG-3′—and a leaving PPi. There are two molecules in the asymmetric unit and, in the cases of the SCI, SCII, and MC, both molecules are quasi-identical. In the case of PC, we observed a clear electron density map corresponding to the 2-mer RNA from only one complex (molecule A); the other complex (molecule B) contained weak and discontinued map for the 2-mer RNA, suggesting that the 2-mer RNA was partially dissociated from molecule B. We used molecule A for the representative structure of transcript initiation.
All complexes were prepared by soaking GTP or its nonhydrolysable analog, guanosine-5′-[(α,β)-methyleno] triphosphate (GMPCPP) (Fig. S1 A and C), and divalent cations, Mg2+ or Mn2+, to the preformed BC crystals. The overall structure of the BC (9) and the initiation complexes determined in this study resemble a canonical “cupped right hand”; the enzyme active site is located at the bottom of this cup (Fig. 1C) and does not interact with any neighboring molecules in the crystals. Indeed, we observed N4 mini-vRNAP-catalyzed RNA synthesis in crystallo that produced the 2-mer RNA product (described below), indicating that the enzyme was active and able to perform any required conformational changes in crystallo.
Each initiation complex structure was determined by rigid body and restrained refinements by using the N4 mini-vRNAP BC (9) as an initial model. After refinements with the BC models against the structure factors from the initiation complex crystals, we observed clear unbiased Fo - Fc electron densities around the active site, which corresponded to nucleotides and metals in the precatalytic complexes and a product 2-mer RNA plus PPi in the postcatalytic complex (Fig. 1 D–F). Compared to the binary complex, the backbone structures of the initiation complexes are almost identical (0.40 ∼ 0.65 Å rmsd) except for distinct deviations in the part of Fingers (residues 657–770, 1.5 ∼ 3.5 Å rmsd) including the O helix (residues 666–678) and DNA bases from -1 to +2 (Figs. 2 and 3, and Movie S1).
Fig. 2.
Structures of active site, DNA, and nucleotides during transcript initiation. The main chains (ribbon models) of motifs A and C (red) and of the O helix (blue), and the main and side chains (stick models) involved in nucleotide and metal binding in the BC (A), SCI (B), SCII (C), and PC (D). NTP binding P and N sites are indicated as green and magenta circles in A. DNA template (from -1 to +2, pink) and nucleotides at +1 (green) and +2 (magenta) positions are shown as stick models. Divalent metals (Mg2+ or Mn2+) are depicted by yellow spheres. Hydrogen bonds and salt bridges are depicted by black dashed lines. Amino acid residues discussed in the text are labeled.
Fig. 3.
Structural transitions of the active site, DNA, and nucleotides associated with transcript initiation. Superposition of the BC and SCI structures (A), SCI and SCII structures (B), and SCII and PC structures (C) showing the conformational changes induced by nucleotide and metal binding and the nucleotidyl transfer reaction. BC, SCI, SCII, and PC are colored in black, yellow, green, and orange, respectively. The O helix is depicted as a ribbon model. DNA template (from -1 to +2, pink), nucleotides, and amino acid side chains involved in nucleotide and metal bindings are shown as stick models and labeled. Divalent metals (Mg2+ or Mn2+) are depicted by spheres, and catalytic and nucleotide metals are indicated as “A” and “B,” respectively. Hydrogen bonds and salt bridges are depicted by yellow (in SCI), green (in SCII), and orange (in PC) dashed lines. Close-up views of reactive groups—O3′(+1) and αP(+2)—of SCI and SCII structures (D) and SCII and PC structures (E). In D, the distance between O3′(+1) and αP(+2) is reduced upon the catalytic metal binding. In E, the [O3′(+1)-catalytic metal-αP(+2)] angle is changed from 84° to 49° by phosphodiester bond formation. (F) Superposition of the BC, SCI, and MC structures showing the partial conformational change of O helix found in the MC. BC, SCI, and MC are colored in black, yellow, and pink, respectively. This view is the same as in A.
Structure of Substrate Complex I: Presence of Two Metals at the Active Site Is Essential for Catalysis.
Precatalytic SCI (Figs. 1 A and D and 2B) was prepared by soaking 5 mM GTP and 10 mM MgCl2 into the BC crystals. SCI contained two molecules of GTP that base pair with DNA bases +1 and +2, and one Mg2+ ion as the nucleotide-binding metal. Mg2+ octahedrally coordinated with ligands that include three atoms of the nonbridging triphosphate oxygens of GTP(+2), two carboxylates of the conserved Asp residues (D559 and D951), and the main-chain carboxyl group of G560 in the metal-binding motifs A and C that are common to the T7-like single-subunit RNAP family.
The binding of the two GTP molecules and the Mg2+ to the BC triggers several conformational changes of DNA, the O helix of the Fingers and side-chain residues of motifs A and C in the Palm (Fig. 3A and Movie S1). Y678 at the O helix C terminus moves 4.3 Å to open the GTP(+2) binding pocket and hydrogen bonds with the 2′-OH of GTP(+2). This movement is linked to a conformational change of the O helix, which swings approximately 8° away from the active site. DNA template bases from -1 to +2 change their positions (-1∶1.4 Å, +1∶3.0 Å, +2∶2.1 Å) to bind GTPs at the P and N sites. The metal-coordinating carboxylates D559 (motif A) and D951 (motif C) rotate their side chains to chelate the nucleotide-binding metal. The triphosphate of GTP(+2) forms extensive interactions with Y612, R666, and K670 in the Fingers and their side chains move their positions upon GTP(+2) binding. In our structural analysis, we identified a conformational change of RNAP that is important for eliciting a proper environment for phosphodiester bond formation (Figs. 2 and 3A, and Movie S1). Although the conformational change of the O helix has been well characterized during substrate loading and catalysis of transcript elongation, our observation clearly proves that the single-subunit RNAP indeed changes its conformation during the initiation process.
To investigate whether correct vs. incorrect base pairing between nucleotide and DNA template is critical for the O-helix conformational change, we determined the structure of an MC prepared by using BC crystals with P2_7c DNA (Fig. 1B) which were soaked with GTP plus MgCl2. In this setup, only a Watson–Crick base pair forms at the +1 position but the +2 position has a G (substrate)-T (DNA template) mismatch. In the MC structure, there is a clear density map corresponding to +1 GTP that forms a Watson-Crick base pair with the +1C DNA base; however, the +2 substrate binding site shows only a subtle density (Fig. S2) likely reflecting the formation of an unstable mismatch between GTP and +2 base of DNA template. The mismatch at +2 position is still able to trigger the conformational change of the O helix and Y678, but their positions deviate from the ones observed in the SCI structure (Fig. 3F), indicating that only the correct base pairing between +2 substrate and DNA base is able to complete the O-helix conformational transition. A partially changed O-helix conformation may function as an intermediate kinetic checkpoint for substrate discrimination and also relate to a unique species, between the open and closed O-helix conformations, found in the mismatch complex of DNAP I by single-molecule FRET analysis (14).
In the SCI, there was no electron density corresponding to the catalytic metal (Fig. 1D), possibly due to the presence of citric acid (0.11 M) in the crystallization solution, which forms a stable metal–ligand complex resulting in a decreased concentration of free Mg2+. The stabilization constant (log 10K, K = [ML]/[M][L], metal, M; ligand, L) between Mg2+ and citric acid is 2.8, which is larger than that between Mg2+ and aspartic acid (2.43) (15). The significantly larger stability constant of Mg2+ and nucleoside triphosphate (4.0) allowed the coordinated nucleotide-binding metal in the SCI to be retained. Other examples of Mg2+ chelation by citric acid in crystal structures, which prevented Mg2+ binding to the catalytic metal site, have been reported [e.g., DNAP λ (16) and CCA adding polymerase (17)]. SCI possesses all of the components required for catalysis except for the catalytic metal; its absence prevents the nucleotidyl transfer reaction even in the presence of reactive GTPs at the active site. The result indicates that substrate loading drives the conformational change of the O helix, although it is not sufficient for nucleotidyl transfer, and that the presence of both metal ions at the active site is essential for catalysis (3).
The GTP(+1) and GTP(+2) Binding Sites.
In the case of transcript initiation, a single nucleotide has to be positioned at the P site prior to the first nucleotidyl transfer reaction, and a single base pair with the template DNA is most likely not sufficient for GTP(+1) binding. The SCI structure revealed extensive interactions between the GTP(+1) triphosphate and two basic residues—K437 and R440—in the Palm core (Fig. 2B). These interactions, which are unique to initiation, because only at this stage is a nucleoside triphosphate loaded at this position, may compensate for the weaker binding of GTP(+1). Accordingly, K437A- and R440A-substituted enzymes had lower affinities for the initiating nucleotide (Km = 200 and 100 μM for K437A and R440A, respectively, vs. 50 μM for the wild-type enzyme) and significantly reduced in vitro transcription activities compared with the wild-type enzyme in the presence of NTP at low concentration (4 μM); higher NTP concentration (500 μM) partially restored the activity of the R440A enzyme but not of the K437A enzyme (Fig. 4A). These results suggest that K437 and R440 play a role in nucleotide binding for transcript initiation and K437 plays a more important role than R440. To ascertain the site of transcript initiation by the mutant enzymes, we cross-linked the hydroxybenzaldehyde ester of GTP to the enzyme; addition of [α - 32P]ATP led to phosphodiester bond formation in a template-directed manner and enzyme autolabeling (10). Catalytic autolabeling of the mutant enzymes at high-NTP concentration confirmed that initiation occurred at position +1 (Fig. 4B).
Fig. 4.
Role of K437, R440, E557, and N671 residues in initiation of transcription by the mini-vRNAP. (A) Effect of K437, R440, and N671 substitutions on mini-vRNAP runoff transcription at increasing NTP concentrations. (B) Effect of Alanine substitutions at K437, R440, and N671 on selection of the site of transcript initiation. Catalytic autolabeling was performed on templates with increasing numbers (n) of As between the promoter hairpin and CTA with increasing concentrations of the hydroxybenzaldehyde derivative of GTP (bGTP). A wild-type vRNAP promoter contains 4As and initiates transcription 11 nt from the center of the hairpin at C. (C) Effect of Mg2+ concentration on runoff transcription by E557A-mutant mini-vRNAP. (D) Effect of E557A substitution on selection of the transcript initiation site. Catalytic autolabeling was performed as described in B at 1-mM bGTP.
The BC structure revealed that residue R318 in the N-terminal domain forms a cation-π interaction with DNA base -2 and salt bridges with the phosphate backbone that induce a DNA kink between bases -2 and -1. During substrate loading, the -1 DNA base changes its position to partially stack with GTP(+1) in the initiation complexes (Fig. 2 A and B). The stacking of purine bases between -1 DNA base and GTP(+1) may facilitate GTP(+1) loading at the active site. This combination, a purine at position -1 on the template strand and at position +1 on the nontemplate strand, is also found in the majority of Escherichia coli σ70-dependent promoters (18), which is consistent with the hypothesis that the -1 template base plays a similar role in initial NTP binding by the bacterial RNAPs.
GTP(+2) is located at the N site (Fig. 2B) and has a unique base-specific hydrogen bond between the keto group of guanosine and the N671 side chain, which is positioned in the middle of O helix. This interaction stabilizes the binding of GTP(+2) because the N671A enzyme had decreased affinity for the second nucleotide (Km = 120 μM for the N671A enzyme vs. 50 μM for the wild-type enzyme) and reduced activity in the presence of low NTP concentration (4 μM), which was restored to approximately 80% at high-NTP concentration (500 μM) with initiation at +1 (Fig. 4A).
The interaction between an amino acid residue at the middle of the O helix and nucleotide +2 might be universal for transcript initiation in the T7-like single-subunit RNAP family. These enzymes contain amino acids with longer side chain with a hydrophilic moiety (Arg, Lys, Asn, or Gln) at this position, which are capable of making base-specific interactions (Fig. S3, Upper). T7 RNAP has a strong sequence preference for GTP at positions +1 and +2 and has Arg at this position, which is capable of making base-specific contact with the 6-keto and/or the 7-imino groups of GTP at position +2. Mitochondrial RNAP, which has a strong sequence preference for ATP or GTP at +2, possesses Gln at this position, which is able to be a hydrogen donor and acceptor at this position. The bifunctional character of Gln may allow this side chain to establish a hydrogen bond with the 7-amine group of ATP and 6-keto group of GTP. This extra interaction between the +2 NTP base and amino acid side chain of the O helix enhances formation of the first phosphodiester bond. However, it may decrease the fidelity of nucleotide selection during transcript elongation by increasing the affinity of the incorrect NTP at active site. Accordingly, the A-family DNAPs, which require a preexisting primer for catalysis, contain a relatively short side-chain residue at this position (Fig. S3, Lower). Furthermore, a substitution of T664 with Arg in Thermus aquaticus DNAP I reduced its specific activity about threefold and increased the mutation frequency about 25-fold (19), suggesting that DNAPs have most likely eliminated the interaction between the amino acid residue at the middle of the O helix and dNTP at the N site in order to enhance DNA replication fidelity.
The ribose ring of GTP(+1) is in the C3′-endo conformation and its O3′(+1) is in line with αP and the leaving bridging oxygen between αP and βP of GTP(+2) (Fig. 3D). However, the distance between O3′(+1) and αP(+2) is 4.1 Å, which is distinctly longer than distances (3.3 ∼ 3.7 Å) reported from other precatalytic forms of polymerase structures, including T7 RNAP in the elongation complex (2) and X-family DNAPs (16, 20). This configuration distance indicates that the geometry of the reactive groups—O3′(+1) and αP(+2)—in the SCI may not be competent for catalysis and suggests that catalytic metal binding at the site will realign these groups for phosphodiester bond formation (4, 21).
Structure of Substrate Complex II: Loading the Catalytic Metal to the Active Site Induces Conformation Changes of the Enzyme Active Site and Nucleotide +1.
To load the catalytic metal but prevent phosphodiester bond formation, we soaked 20 mM MnCl2 and 5 mM of GMPCPP into the preformed BC crystals. The stability constant of the Mn2+-aspartate complex (log 10K = 3.74) is higher than its citric acid counterpart (log 10K = 2.8) (15) allowing Mn2+ binding at both sites. Mn2+ has octahedral coordination with almost identical metal-donor distances as those observed with Mg2+ (22). In addition, both Mg2+ and Mn2+ can activate catalysis in vitro by N4 vRNAP (Fig. S4) and other members of this type of polymerase including T7 RNAP (23) and E. coli DNAP I (24). The structure was determined at 1.8-Å resolution with clear unbiased Fo - Fc electron densities around the active site, corresponding to two molecules of GMPCPP and two Mn2+ ions (Fig. 1E). We termed this precatalytic complex SCII (Figs. 1A and 2C).
Loading the catalytic metal into the active site aligned the reactive groups of substrates and the catalytically essential carboxylates for the nucleotidyl transfer reaction: (i) The O3′(+1) moved in the direction of αP(+2) and the distance between the two groups decreased from 4.1 to 3.1 Å (Fig. 3D); and (ii) the D559 side chain also moved 1.8 Å to chelate both catalytic and nucleotide-binding metals (Fig. 3B), with the metals separated by 3.6 Å. The catalytic metal binding induced an unexpected conformational transition of the triphosphate moiety of nucleotide +1. Compared to the SCI structure, the γ-phosphate (γP) group of GMPCPP(+1) in SCII moved 5.9 Å toward the catalytic metal, thus becoming one of six ligands that coordinate the catalytic metal (Fig. S5A). This drastic motion disrupted the interaction between R440 and the triphosphate, and established a new interaction between the γP group(+1) and E557. To allow this interaction, a nonbridging oxygen associated with γP(+1) is most likely protonated (pKa value for secondary phosphate ionization in unbound nucleotide triphosphate is approximately 7.6) (25). The relevance of this interaction was supported by the behavior of the E557A-mutant enzyme, with decreased runoff transcription activity at 2 mM Mg2+ and some recovery at 10 mM Mg2+ concentration, without a change in the site of initiation (Fig. 4 C and D).
To assess the role of the γP group (+1) in transcript initiation, we determined the kinetic parameters for GTP and GDP incorporation at the RNA 5′ end (Table 1). N4 mini-vRNAP had a fourfold higher affinity (50 μM) and a threefold higher kcat (300 min-1) for GTP than for GDP (200 μM and 100 min-1) at physiological (1 mM) Mg2+ concentration. As a control, we used T7 RNAP, which does not interact with the 5′ phosphate of the initiating nucleotide (8, 26). Accordingly, the kinetic parameters were identical when T7 RNAP initiated with GTP or GDP (200 μM and 20 min-1). Two charged residues—K437 and E557—are involved in positioning of the triphosphate group (+1) in contact with the catalytic metal. The functional roles of K437 and E557 are supported by an analysis of the kinetic parameters of GTP and GDP incorporation by the K437A and E557A enzymes. Both mutant enzymes show similar affinities (200 μM) for GTP and GDP as the initiating nucleotide. Notably, the affinity of the K437A and E557A enzymes for the initiating nucleotide is similar to that of T7 RNAP (Table 1), whose active site is superimposable with that of N4 vRNAP (27); however, T7 RNAP lacks residues equivalent to N4 vRNAP K437 and E557. The 15-fold decrease in kcat upon replacement of E557 by Ala highlights the relevance of E557 in catalysis of the N4 vRNAP.
Table 1.
Summary of N4 mini-vRNAP and T7 RNAP kinetic parameters for GTP and GDP
| Initiation nucleotide |
|||||||
| GTP |
GDP |
Enzyme catalytic efficiency GTP/GDP |
|||||
|
Km, μM |
kcat, min-1 |
kcat/Km, min-1 μM-1 |
Km, μM |
kcat, min-1 |
kcat/Km, min-1 μM-1 |
||
| Wild-type* | 50 | 300 | 6.00 | 200 | 100 | 0.50 | 12.00 |
| K437A* | 200 | 70 | 0.35 | 300 | 70 | 0.23 | 1.52 |
| E557A* | 200 | 20 | 0.10 | 200 | 20 | 0.10 | 1.00 |
| T7 RNAP | 200 | 20 | 0.10 | 200 | 20 | 0.10 | 1.00 |
Conditions as described in SI Experimental Procedures.
*N4 mini-vRNAP.
Structure of the Product Complex: Phosphodiester Bond Formation Releases Both Metals from Their Binding Sites.
The final step of transcript initiation is the nucleotidyl transfer reaction yielding a 2-mer RNA and PPi (Fig. 1A). To understand the structural basis of this chemical reaction, we attempted to carry out the nucleotidyl transfer reaction in crystallo and determine its structure. We found a unique electron density map at the active site in BC crystals with P2_7a DNA soaked with 0.5 mM GTP and 10 mM MgCl2 (Fig. 1F). There was a GTP-like structure at position +1 and a nucleotide at +2 with a single phosphate group. The distance between O3′(+1) and αP(+2) was 1.6 Å, indicating that this density map corresponds to the 2-mer RNA (5′-pppGpG-3′) product. The PPi product, coordinated by residues K666, R660, and Y612, was also observed.
The 5′ and 3′ ends of RNA in the PC were found at the P and N sites, respectively (Fig. 2D), indicating that the PC was in a pretranslocation state (2). The template DNA did not change its position and the enzyme maintained the O-helix closed conformation with the Y678 side chain in the same position as observed in the SCI and SCII (Fig. 3C and Movie S1). In the PC, weak electron densities were present at its metal-binding sites (Fig. 1F); however, the coordination distances of these densities were longer (density at catalytic metal site, 3.1 Å; density at nucleotide-binding metal site, 3.5 Å) than the expected distance for Mg2+ (2.1 ∼ 2.3 Å), and their coordination spheres lacked the octahedral geometry (22). We therefore assigned these densities as isoelectronic water, indicating that both catalytic and nucleotide-binding Mg2+ ions had dissociated after the nucleotidyl transfer reaction. The release of metal ions did not shift the 2-mer RNA to the posttranslocated position or release PPi, but triggered conformational changes of the catalytic carboxylates (D557 and D951); their positions were approximately the same as found in the BC, indicating that these residues form the catalytically relevant conformation only in the presence of metals (Fig. 3C). In addition, metal release moved the triphosphate of nucleotide +1 to a position nearly identical to that observed in SCI. The product PPi remained associated with the O helix through interactions with Y612, R666, and K670, but these residues changed their positions to those found in the BC.
Discussion
Transcript Initiation by Single-Subunit T7 Phage-Like RNAPs.
We have determined the high-resolution X-ray crystal structures of three distinct forms of transcript initiation complexes during the formation of 2-mer RNA, which revealed the formation of two intermediates—SCI and SCII—prior to the nucleotidyl transfer reaction. In SCI, we observed the conformational change of the O helix upon binding of nucleotides +1 and +2 and nucleotide-binding metal (compare BC and SCI, Fig. 3A); nonetheless, the reactive groups—O3′(+1) and αP(+2)—do not possess the catalytically competent configuration. Binding of the catalytic metal results in alignment of the substrates’ reactive groups to allow the reaction to proceed (compare SCI and SCII, Fig. 3 B and D). In the PC, the catalytic metal is released after phosphodiester bond formation, indicating that the catalytic metal coordinating O3′(+1) and nonbridging oxygen of αP(+2) in the 2-mer RNA cannot maintain octahedral coordination geometry (compare SCII and PC, Fig. 3 C and E). In other words, binding of the catalytic metal is sensitive to positions of these ligands that can be easily influenced by correct vs. incorrect base pairing between the nucleotide and DNA template base. Therefore, a small difference in binding energy from correct vs. incorrect Watson–Crick base pairing is able to be converted into a large difference in catalytic efficiency; reactive groups are in an inactive configuration and the 3′ oxyanion cannot be produced in the absence of the catalytic metal, whereas they are properly aligned to generate the 3′ oxyanion for catalysis in the presence of the catalytic metal. Based on these facts, we propose that binding of the catalytic metal at the active site is the last step in the formation of the catalytically competent transcription complex and that catalytic-metal-dependent substrate alignment is the most critical checkpoint for fidelity of nucleotide incorporation by single-subunit RNAPs, and possibly by the A-family of DNAPs.
The published structure of the T7 RNAP transcript initiation complex (8) poses several problems: (i) Distances between the nucleotide-binding metal and its ligands are significantly greater in this structure (average 4.3 Å) than in the elongation complex (average 2.7 Å) (2); (ii) although Y639 has been shown to discriminate NTP against dNTP at the N site for both transcript initiation and elongation (28), Y639 does not contact the 2′-OH of GTP(+2) in the initiation complex structure; (iii) although the interaction between H784 and the 2-amino group of GTP(+1) was shown to play a role in transcription start site selection (29), the H784 side chain contacts GTP(+2) in the structure; and (iv) no motion of RNAP or of the template DNA strand was observed during substrate loading at the active site (8). Therefore, we suspect that the proposed mechanism of transcript initiation based on this T7 RNAP structure requires reevaluation. Furthermore, the X-ray crystal structure of the T7 RNAP initiation complex (8) identified a unique nucleotide-binding site that the authors termed the D site (de novo site), which is distinct from the P site used for transcript elongation. In order to determine whether the N4 vRNAP SCI possesses a D site for GTP(+1) binding, we superposed the N4 SCI with the T7 RNAP initiation (8) and elongation complexes (2) by overlaying their Palm cores including the T/DxxGR motif and motifs A and C (Fig. S6 A and B). Both GTPs at positions +1 and +2 in the N4 SCI overlaid well with the P and N sites of the T7 elongation complex, but not with the GTP binding sites found in the T7 initiation complex, indicating that N4 vRNAP does not use a D site for GTP(+1) binding.
Transcript Initiation by Cellular RNAPs.
All organisms have multisubunit RNAPs that carry out primer-independent transcript initiation. Crystallographic studies of cellular RNAPs have revealed insights into the mechanism of transcript elongation (1, 30). However, due to their larger size and complexity of preparation, X-ray crystal structures capturing transcript initiation with cellular RNAP have been elusive. In order to obtain structural insights into the process of transcript initiation of cellular RNAPs, we compared the coordination geometry of the catalytic metal ion in the N4 mini-vRNAP SCII structure with the γP group (+1) involved in catalytic-metal coordination (Fig. S5A) and the Thermus thermophilus RNAP elongation complex structure (1), as a representative cellular RNAP because it is the highest resolution structure determined to date (Fig. S5B). The cellular RNAP uses three carboxylates in the absolutely conserved 739-DFDGD-743 motif in the largest subunit (region D) (31). The first Asp residue in the DFDGD motif is involved in both catalytic- and nucleotide-metal coordination, whereas the second and third Asp residues coordinate only the catalytic metal. The second Asp residue in cellular RNAP localizes to the same position as the one occupied by the γP group (+1) of the N4 RNAP SCII; however, a direct comparison of the two structures does not take into account the absence of the triphosphate moiety at the P site nucleotide in the T. thermophilus RNAP elongation complex structure. The sixfold decrease in Vmax observed for 2-mer RNA synthesis when ATP is substituted by ADP as the initiating nucleotide in E. coli RNAP transcription from the λ Pr promoter (32) might reflect the role of the γP group (+1) in transcript initiation by cellular RNAPs. Whether the second Asp residue plays a role in interacting with the γP group (+1) in bacterial RNAPs awaits the determination of the structure of their initiation complexes.
In the case of single-subunit RNAP, binding of the catalytic metal requires the presence of template DNA and substrates at the active site, which is in contrast to the multisubunit RNAPs from Bacteria (31), Archaea (33), and Eukaryote (34), which coordinate the catalytic metal at the active site even in the absence of DNA template or substrates. The difference between single-subunit and cellular RNAPs may reflect the fact that two and three carboxylates are involved in coordinating the catalytic metal at the active site in single- and multisubunit enzymes, respectively (Fig. S5). This structural difference may explain the fact that the single-subunit enzyme carries out only RNA synthesis, whereas the multisubunit enzyme is capable of both RNA synthesis and RNA cleavage reactions, which play an important role in transcriptional proofreading and releasing arrested enzyme (35).
Experimental Procedures
Detailed protocols of (i) N4 mini-vRNAP and DNA purifications, (ii) crystallization of binary complexes, (iii) preparing transcript initiation complexes, (iv) X-ray data collections and structure determinations, (v) site-directed mutagenesis of N4 mini-vRNAP, (vi) runoff transcription and catalytic autolabeling, and (vii) transcript initiation assay and kinetics of first phosphodiester bond formation are described in SI Experimental Procedures.
Supplementary Material
Acknowledgments.
We thank the staff at X25 of the National Synchrotron Light Source, F1 of the Macromolecular Diffraction Facility at Cornell High Energy Synchrotron Source (MacCHESS), and H. Yennawar for supporting crystallographic data collection. We thank P.C. Bevilacqua, C.E. Cameron, P.R. Carey, Y. Chen, and R. Yajima for discussion, and S.J. Benkovic and T. Ellenberger for comments. We thank W. Ross and R.L. Gourse for critical reading of the manuscript. Figures were prepared using PyMOL (http://pymol.sourceforge.net/). This work was supported by National Institutes of Health (NIH) Grants AI12575 and GM071897. The Cornell High Energy Synchrotron Source is supported by the National Science Foundation (NSF) and NIH/National Institute of General Medical Sciences via NSF award DMR-0225180, and the MacCHESS resource is supported by NIH/National Center for Research Resources award RR-01646.
Note.
Recently, using fluorescence-based assays and stopped flow kinetics, Bermek et al. proposed a reaction pathway for E. coli DNAP I where the O-helix conformational change and the catalytic metal binding occur at early and late stages of the reaction, respectively (36). These stages coincide with those we have defined based on the in crystallo reaction.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3Q22 for substrate complex I, 3Q23 for substrate complex II, 3Q0A for the mismatch complex, and 3Q24 for the product complex).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016691108/-/DCSupplemental.
References
- 1.Vassylyev DG, et al. Structural basis for substrate loading in bacterial RNA polymerase. Nature. 2007;448:163–168. doi: 10.1038/nature05931. [DOI] [PubMed] [Google Scholar]
- 2.Yin YW, Steitz TA. The structural mechanism of translocation and helicase activity in T7 RNA polymerase. Cell. 2004;116:393–404. doi: 10.1016/s0092-8674(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 3.Steitz TA, Steitz JA. A general two-metal-ion mechanism for catalytic RNA. Proc Natl Acad Sci USA. 1993;90:6498–6502. doi: 10.1073/pnas.90.14.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang W, Lee JY, Nowotny M. Making and breaking nucleic acids: Two-Mg2+-ion catalysis and substrate specificity. Mol Cell. 2006;22:5–13. doi: 10.1016/j.molcel.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Temiakov D, et al. Structural basis for substrate selection by T7 RNA polymerase. Cell. 2004;116:381–391. doi: 10.1016/s0092-8674(04)00059-5. [DOI] [PubMed] [Google Scholar]
- 6.Doublie S, Tabor S, Long AM, Richardson CC, Ellenberger T. Crystal structure of a bacteriophage T7 DNA replication complex at 2.2 Å resolution. Nature. 1998;391:251–258. doi: 10.1038/34593. [DOI] [PubMed] [Google Scholar]
- 7.Johnson SJ, Taylor JS, Beese LS. Processive DNA synthesis observed in a polymerase crystal suggests a mechanism for the prevention of frameshift mutations. Proc Natl Acad Sci USA. 2003;100:3895–3900. doi: 10.1073/pnas.0630532100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy WP, Momand JR, Yin YW. Mechanism for de novo RNA synthesis and initiating nucleotide specificity by T7 RNA polymerase. J Mol Biol. 2007;370:256–268. doi: 10.1016/j.jmb.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 9.Gleghorn ML, Davydova EK, Rothman-Denes LB, Murakami KS. Structural basis for DNA-hairpin promoter recognition by the bacteriophage N4 virion RNA polymerase. Mol Cell. 2008;32:707–717. doi: 10.1016/j.molcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazmierczak KM, Davydova EK, Mustaev AA, Rothman-Denes LB. The phage N4 virion RNA polymerase catalytic domain is related to single-subunit RNA polymerases. EMBO J. 2002;21:5815–5823. doi: 10.1093/emboj/cdf584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davydova EK, Santangelo TJ, Rothman-Denes LB. Bacteriophage N4 virion RNA polymerase interaction with its promoter DNA hairpin. Proc Natl Acad Sci USA. 2007;104:7033–7038. doi: 10.1073/pnas.0610627104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glucksmann MA, Markiewicz P, Malone C, Rothman-Denes LB. Specific sequences and a hairpin structure in the template strand are required for N4 virion RNA polymerase promoter recognition. Cell. 1992;70:491–500. doi: 10.1016/0092-8674(92)90173-a. [DOI] [PubMed] [Google Scholar]
- 13.Haynes LL, Rothman-Denes LB. N4 virion RNA polymerase sites of transcription initiation. Cell. 1985;41:597–605. doi: 10.1016/s0092-8674(85)80032-5. [DOI] [PubMed] [Google Scholar]
- 14.Santoso Y, et al. Conformational transitions in DNA polymerase I revealed by single-molecule FRET. Proc Natl Acad Sci USA. 2009;107:715–720. doi: 10.1073/pnas.0910909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furia TE. Sequestrants in foods. In: Furia TE, editor. CRC Handbook of Food Additives. 2nd Ed. Vol 1. Boca Raton, FL: CRC; 1972. pp. 271–294. [Google Scholar]
- 16.Garcia-Diaz M, Bebenek K, Krahn JM, Pedersen LC, Kunkel TA. Role of the catalytic metal during polymerization by DNA polymerase lambda. DNA Repair. 2007;6:1333–1340. doi: 10.1016/j.dnarep.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomita K, Ishitani R, Fukai S, Nureki O. Complete crystallographic analysis of the dynamics of CCA sequence addition. Nature. 2006;443:956–960. doi: 10.1038/nature05204. [DOI] [PubMed] [Google Scholar]
- 18.Shultzaberger RK, Chen Z, Lewis KA, Schneider TD. Anatomy of Escherichia coli sigma70 promoters. Nucleic Acids Res. 2007;35:771–788. doi: 10.1093/nar/gkl956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki M, Avicola AK, Hood L, Loeb LA. Low fidelity mutants in the O-helix of Thermus aquaticus DNA polymerase I. J Biol Chem. 1997;272:11228–11235. doi: 10.1074/jbc.272.17.11228. [DOI] [PubMed] [Google Scholar]
- 20.Batra VK, et al. Magnesium-induced assembly of a complete DNA polymerase catalytic complex. Structure. 2006;14:757–766. doi: 10.1016/j.str.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W, Woodgate R. What a difference a decade makes: Insights into translesion DNA synthesis. Proc Natl Acad Sci USA. 2007;104:15591–15598. doi: 10.1073/pnas.0704219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harding M. Geometry of metal-ligand interactions in proteins. Acta Crystallogr, Sect D: Biol Crystallogr. 2001;57:401–411. doi: 10.1107/s0907444900019168. [DOI] [PubMed] [Google Scholar]
- 23.Woody AY, Eaton SS, Osumi-Davis PA, Woody RW. Asp537 and Asp812 in bacteriophage T7 RNA polymerase as metal ion-binding sites studied by EPR, flow-dialysis, and transcription. Biochemistry. 1996;35:144–152. doi: 10.1021/bi952037f. [DOI] [PubMed] [Google Scholar]
- 24.Burgers PM, Eckstein F. A study of the mechanism of DNA polymerase I from Escherichia coli with diastereomeric phosphorothioate analogs of deoxyadenosine triphosphate. J Biol Chem. 1979;254:6889–6893. [PubMed] [Google Scholar]
- 25.Saenger W. Principles of Nucleic Acid Structure. New York: Springer; 1983. pp. 108–110. [Google Scholar]
- 26.Martin CT, Coleman JE. T7 RNA polymerase does not interact with the 5′-phosphate of the initiating nucleotide. Biochemistry. 1989;28:2760–2762. doi: 10.1021/bi00433a002. [DOI] [PubMed] [Google Scholar]
- 27.Murakami KS, Davydova EK, Rothman-Denes LB. X-ray crystal structure of the polymerase domain of the bacteriophage N4 virion RNA polymerase. Proc Natl Acad Sci USA. 2008;105:5046–5051. doi: 10.1073/pnas.0712325105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Beaudry A, McSwiggen J, Sousa R. Determinants of ribose specificity in RNA polymerization: Effects of Mn2+ and deoxynucleoside monophosphate incorporation into transcripts. Biochemistry. 1997;36:13718–13728. doi: 10.1021/bi971609o. [DOI] [PubMed] [Google Scholar]
- 29.Brieba LG, Padilla R, Sousa R. Role of T7 RNA polymerase His784 in start site selection and initial transcription. Biochemistry. 2002;41:5144–5149. doi: 10.1021/bi016057v. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Bushnell DA, Westover KD, Kaplan CD, Kornberg RD. Structural basis of transcription: Role of the trigger loop in substrate specificity and catalysis. Cell. 2006;127:941–954. doi: 10.1016/j.cell.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang G, et al. Crystal structure of Thermus aquaticus core RNA polymerase at 3.3 Å resolution. Cell. 1999;98:811–824. doi: 10.1016/s0092-8674(00)81515-9. [DOI] [PubMed] [Google Scholar]
- 32.McClure WR, Cech CL, Johnston DE. A steady state assay for the RNA polymerase initiation reaction. J Biol Chem. 1978;253:8941–8948. [PubMed] [Google Scholar]
- 33.Hirata A, Klein BJ, Murakami KS. The X-ray crystal structure of RNA polymerase from Archaea. Nature. 2008;451:851–854. doi: 10.1038/nature06530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cramer P, et al. Architecture of RNA polymerase II and implications for the transcription mechanism. Science. 2000;288:640–649. doi: 10.1126/science.288.5466.640. [DOI] [PubMed] [Google Scholar]
- 35.Conaway RC, Kong SE, Conaway JW. TFIIS and GreB: Two like-minded transcription elongation factors with sticky fingers. Cell. 2003;114:272–274. doi: 10.1016/s0092-8674(03)00607-x. [DOI] [PubMed] [Google Scholar]
- 36.Bermek O, Grindley ND, Joyce CM. Distinct roles of the active-site Mg2 ligands, Asp882 and Asp705, of DNA polymerase I (Klenow fragment) during the prechemistry conformational transitions. J Biol Chem. 2011;286:3755–3766. doi: 10.1074/jbc.M110.167593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.