Abstract
Mitochondria are essential and highly dynamic organelles, constantly undergoing fusion and fission. We analyzed mitochondrial dynamics during infection with the human bacterial pathogen Listeria monocytogenes and show that this infection profoundly alters mitochondrial dynamics by causing transient mitochondrial network fragmentation. Mitochondrial fragmentation is specific to pathogenic Listeria monocytogenes, and it is not observed with the nonpathogenic Listeria innocua species or several other intracellular pathogens. Strikingly, the efficiency of Listeria infection is affected in cells where either mitochondrial fusion or fission has been altered by siRNA treatment, highlighting the relevance of mitochondrial dynamics for Listeria infection. We identified the secreted pore-forming toxin listeriolysin O as the bacterial factor mainly responsible for mitochondrial network disruption and mitochondrial function modulation. Together, our results suggest that the transient shutdown of mitochondrial function and dynamics represents a strategy used by Listeria at the onset of infection to interfere with cellular physiology.
Keywords: bacterial infection, calcium influx, bioenergetics
Mitochondria are essential organelles providing most cellular ATP as well as biosynthetic intermediates. They have emerged as important integrators of several signaling cascades (1). Fusion and fission of mitochondria occurs constantly, regulating their size and subcellular distribution and reflecting their functional state (1, 2). Disturbance of mitochondrial dynamics leads to pathological conditions exacerbated in tissues with high metabolic demand such as neuronal or muscle tissues (3). Although it often is unclear whether defects in mitochondrial dynamics are the cause or effect of the disease, mitochondria have now emerged as drug targets for several pathologies (4, 5).
At the single-cell level, long-term impairment of either fusion or fission can cause respiratory defects (6, 7). Key players in the fusion process include the outer mitochondrial membrane GTPases mitofusin 1 and 2 (Mfn1/2) (8, 9), and the inner membrane GTPase optic atrophy 1 (Opa1) (10, 11), but the molecular details of the fusion mechanism remain obscure (12, 13). Mitochondrial fission critically depends on the GTPase dynamin-related protein 1 (Drp1) (14), which oligomerizes on the outer mitochondrial membrane to constrict mitochondria at division sites (15). Mitochondrial fission also occurs during apoptosis and may be required for apoptosis progression under specific circumstances (16), although it does not induce apoptosis per se (17–19).
Several pathogens including both viruses and bacteria directly or indirectly target mitochondria to interfere with the host apoptotic machinery (20–24). Depending on the pathogen and host-cell type, this interference can inhibit cell death to preserve the pathogen's replication niche or induce cell death to promote infection spreading (25). Mitochondria also function as signaling platforms in the innate immune response, and this function has been linked recently to mitochondrial dynamics during viral infection (26, 27).
To investigate the interrelation between host-cell mitochondrial dynamics and bacterial infection, we focused on Listeria monocytogenes, a facultative intracellular bacterium causing the food-borne disease listeriosis. While listeriosis is a public health issue, L. monocytogenes has been instrumental in elucidating fundamental cell biological questions, e.g., actin polymerization principles (reviewed in refs. 28 and 29).
After cell invasion, L. monocytogenes uses the pore-forming toxin listeriolysin O (LLO) to escape from the phagosome. Although LLO function had been characterized first in Listeria escape from the phagosome under acidic conditions (30), several studies now indicate that this crucial virulence factor also displays activity at neutral pH and acts on cells before bacterial entry (31, 32). Indeed, low levels of LLO secreted before bacterial entry are sufficient to activate prosurvival signaling cascades such as the NFκB and MAPK pathways (33, 34), transcriptionally reprogram host cells (35), and trigger global deSUMOylation (36).
Here we report that L. monocytogenes causes dramatic alterations of mitochondrial dynamics via LLO. Strikingly, mitochondrial fragmentation induced by Listeria infection is a transient phenomenon, indicating that mitochondria are not terminally damaged. We propose that modulation of mitochondrial dynamics and function is a strategy used by pathogenic Listeria at the onset of infection to slow down mitochondrial activity and mitochondria-dependent processes.
Results
Infection with Pathogenic Listeria Induces Mitochondrial Fragmentation.
We first investigated the effects of L. monocytogenes infection on mitochondria of epithelial cells by using confocal laser scanning microscopy. Fig. 1A shows L. monocytogenes infection of HeLa cells [1 h, multiplicity of infection (MOI) of 50] inducing strong and rapid mitochondrial network fragmentation. Importantly, this network fragmentation is specific to pathogenic L. monocytogenes, because it is not observed with the closely related nonpathogenic species Listeria innocua, even when cells are infected with L. innocua overexpressing the L. monocytogenes invasin Internalin B (InlB) to enter cells [L. innocua(InlB), Fig. 1A]. This finding indicates that mitochondrial fragmentation is not a consequence of stress imposed by the engulfment of bacteria. Furthermore, L. monocytogenes-induced mitochondrial fragmentation is not restricted to HeLa cells, because it also occurs in human placental (Jeg3) and green monkey kidney (Vero) cells (Fig. S1A).
Fig. 1.
Mitochondrial fragmentation upon infection with Listeria monocytogenes. (A) HeLa cells infected with L. monocytogenes (1 h; MOI of 50) display strongly fragmented mitochondria, whereas cells infected with L. innocua expressing InlB to enter cells do not. Mitochondria were stained with MitoTracker Deep Red (red). L. innocua or L. monocytogenes were stained with polyclonal antibodies R6 and R11 (green). Arrows indicate infected cells, and insets show 2× enlargements. (B) Infection (1 h, MOI of 50) with E.coli(Inv) as a model for Yersinia infection, Shigella flexneri (M09T), Salmonella enterica serovar typhimurium, and enteropathogenic E. coli (EPEC) do not cause mitochondrial fragmentation. Cell nuclei and bacteria were stained with DAPI (blue). Salmonella additionally expresses GFP (green). Mitochondria were stained as in A. Arrows indicate infected cells, and insets show 2× enlargements.
To assess whether other invasive pathogens also cause mitochondrial fragmentation, we infected cells with Salmonella enterica serovar typhimurium, Escherichia coli(Inv) as a model for Yersinia pseudotubercolosis (37), enteropathogenic E. coli (EPEC), and Shigella flexneri. Importantly, infection with either the extracellular pathogen EPEC or with invasive pathogens that remain confined to a phagocytic vacuole [i.e., E. coli(Inv) and Salmonella enterica serovar typhimurium] did not appear to affect mitochondria (Fig. 1B), even at an MOI of 100 and up to 3 h of infection, supporting the notion that L. monocytogenes-induced mitochondrial fragmentation is not a general stress response to bacterial infection. Moreover, mitochondrial fragmentation is not caused by the presence of bacteria in the cytosol, because infection with Shigella flexneri, which, like Listeria, escapes from the vacuole and polymerizes actin to move intra- and intercellularly, did not cause mitochondrial fragmentation (Fig. 1B).
Correlative light/transmission electron microscopy (TEM) showed that fragmented mitochondria in Listeria-infected cells had a disorganized ultrastructure with remodeled cristae compared with mitochondria in noninfected cells (Fig. S1B).
Impairment of Mitochondrial Dynamics Affects Infection by Listeria monocytogenes.
Having established that L. monocytogenes infection induces mitochondrial fragmentation, we asked whether inhibiting fusion or fission by siRNA would affect early Listeria infection stages. Cells depleted of the fusion proteins Mfn1 and Mfn2 (resulting in fissioned mitochondria) or of the fission protein Drp1 (resulting in hyperfused mitochondria) were infected with L. monocytogenes. Infection was strongly impaired in cells with fissioned mitochondria, and the strongest inhibition was seen with codepletion of both mitofusins (Fig. 2 A–C and Fig. S2 A and B). Infection was more efficient in cells with hyperfused mitochondria (Fig. 2D and Fig. S2C). This result suggests that, for efficient infection, Listeria requires the ability to induce mitochondrial fission, because cells with mitochondria that already are fissioned at the onset of infection provide an unfavorable environment for infection. Silencing of proteins regulating mitochondrial dynamics may also affect later stages of infection or have indirect effects on mitochondrial function and infection.
Fig. 2.
Early Listeria infection is affected by impaired mitochondrial dynamics. (A) HeLa cells treated with two different siRNAs (#A and #B) targeting the mitochondrial fusion protein Mfn1 were infected with L. monocytogenes (MOI of 50) in a gentamicin survival assay. The relative number of intracellular bacteria was determined by cfu count at 3 h postinfection. L. monocytogenes infection is significantly impaired in Mfn1-knockdown cells (P < 0.001, one-tailed Student's t test). Each experiment was performed in triplicate, and a representative experiment with SDs is shown. At least three independent experiments were performed for each condition. (B) Infection of Mfn2-knockdown cells was performed as in A. Mfn2 knockdown also impairs Listeria infection, although a lesser extent. (C) Down-regulation of both Mfn1 and Mfn2 has a cumulative effect in impairing Listeria infection, suggesting that these proteins play nonredundant roles in infection. (D) L. monocytogenes infection is promoted in Drp1-knockdown cells. Experiment and statistical analysis were performed as in A–C. **P < 0.005, ***P < 0.001 by one-tailed Student's t test.
Listeriolysin O Is Sufficient to Cause Mitochondrial Fragmentation.
Because mitochondria appeared to fragment at early stages of infection, we tested whether a secreted effector of L. monocytogenes could cause mitochondrial fragmentation. We first used a noninvasive mutant of L. monocytogenes lacking InlB and found that this mutant was still able to induce mitochondrial fragmentation (Fig. 3A). We then asked whether the best-characterized secreted effector of L. monocytogenes, the pore-forming toxin LLO, could induce mitochondrial fragmentation. Strikingly, mitochondrial fragmentation was abolished when an LLO-deletion mutant (L. monocytogenes Δhly) was used (Fig. 3A), indicating that LLO is required for fragmentation of host-cell mitochondria. Bacteria whose hemolysin (hly) gene carries a point mutation disrupting pore formation (38) did not affect mitochondrial morphology (Fig. 3B), revealing that fragmentation depends on the pore-forming ability of LLO.
Fig. 3.
LLO induces mitochondrial fragmentation without affecting total levels of key mitochondrial dynamics proteins. (A) HeLa cells infected (1 h, MOI of 50) with a noninvasive L. monocytogenes mutant (ΔinlB) display fragmented mitochondria, indicating bacterial entry is not required. In contrast, no fragmentation occurs with infection by an LLO-deficient mutant (Δhly). Bacteria were stained with anti L. monocytogenes (R11, green), mitochondria were stained with anti-cytochrome c (red), and DNA was stained with DAPI (blue). Insets show 2× enlargements of mitochondria. Arrows point to infected cells. (B) L. monocytogenes carrying a point mutation in the hly gene inactivating its pore-forming ability (W492A) do not cause mitochondrial fragmentation. Mitochondria (red) and bacteria (green) were stained as in A. (C) HeLa cell treatment with recombinant LLO (10 min, 6 nM) was sufficient to induce mitochondrial fragmentation. Mitochondria (red) were stained as in A. (D) HeLa cells were treated with different concentrations of LLO for 10 min or with 6 nM LLO for different periods of time. Morphometric analysis indicated that LLO-induced mitochondrial fragmentation is concentration and time dependent at the cell-population level. The percentage of fragmentation was determined by counting at least 100 cells per data point; data from at least two independent experiments were pooled, and P values were calculated using one-tailed Student's t test (***P < 0.005, *P < 0.25). (E) Cells treated for 10 min with the indicated amounts of LLO were analyzed by Western blot for Drp1, Mfn1, or Mfn2, showing that LLO treatment does not affect their total levels.
LLO appears necessary and sufficient to induce mitochondrial fragmentation, because addition of the purified toxin at nanomolar concentrations [3–6 nM, considered noncytotoxic and not causing lactate dehydrogenase (LDH) release (35)] recapitulated the mitochondrial phenotype observed upon infection (Fig. 3C). Mitochondrial fragmentation was found to occur in a fast, all-or-nothing manner (i.e., within less than 10 min) upon addition of recombinant LLO (Movie S1). Increasing incubation time or LLO concentration resulted in an increased number of cells displaying fragmented mitochondria (Fig. 3D).
Mitochondrial fragmentation could result from enhanced fission or decreased fusion (39). We thus analyzed whether LLO treatment would affect total levels of key mitochondrial dynamics mediators, i.e., Mfn1, Mfn2, and Drp1. Total levels of these proteins did not decrease in cells treated with 3–6 nM recombinant LLO (Fig. 3E). Because LLO forms ion-permeable pores in membranes (30), we hypothesized that LLO might modulate mitochondrial dynamics by inducing ion flux. We tested whether blocking K+ efflux or Ca2+ influx would prevent LLO-induced mitochondrial fragmentation, given that changes in these ions are known to affect mitochondrial morphology (40, 41). Blocking K+ efflux from LLO-treated cells by incubation in high extracellular K+ concentrations (135 mM) had no effect (Fig. 4). In contrast, interfering with LLO-induced Ca2+ influx by performing LLO treatment in Ca2+-free medium strongly prevented mitochondrial fragmentation (Fig. 4). This result suggested that Ca2+ influx (rather than K+ efflux) through LLO pores is the signal inducing mitochondrial fragmentation.
Fig. 4.
LLO-induced mitochondrial fragmentation is mediated by calcium influx, not by potassium efflux. Cells were treated for 10 min with 6 nM LLO in the presence or absence of extracellular calcium. Mitochondria (stained with anti-cytochrome c) showed strongly inhibited fragmentation in the absence of calcium, whereas blocking potassium efflux by incubation in 135 mM KCl did not prevent LLO-induced mitochondrial fragmentation.
LLO Treatment Causes Mitochondrial Membrane Potential Loss and a Drop in Respiration and Cellular ATP.
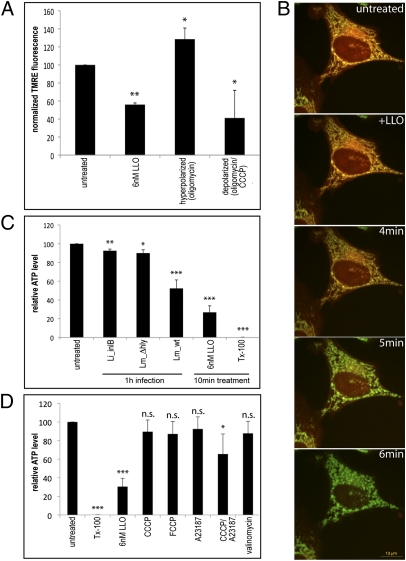
To investigate the functionality of mitochondria in LLO-treated cells, we measured the mitochondrial membrane potential ΔΨ by assessing release of the ΔΨ-dependent mitochondrial dye tetramethylrhodamine ethyl ester (TMRE) and found that LLO treatment caused a significant drop in ΔΨ compared with untreated cells (Fig. 5A). At the single-cell level, time-lapse microscopy of mitochondria loaded with the TMRE derivative tetramethylrhodamine methyl ester (TMRM) indicated that ΔΨ loss was concomitant with or immediately preceded mitochondrial fragmentation (Fig. 5B).
Fig. 5.
LLO causes a decrease in the mitochondrial membrane potential ΔΨ concomitant with fragmentation and loss of intracellular ATP. (A) The mitochondrial membrane potential ΔΨ of HeLa cells was measured in a plate-based TMRE-release assay. Cells were treated with the indicated drugs and loaded with TMRE in a ΔΨ-dependent manner. Mitochondrial TMRE then was released by overnight incubation at 4 °C and measured at 585 nm, reflecting drug-induced changes in ΔΨ. Mitochondria were uncoupled with 10 μM CCCP/0.5 μg/mL oligomycin (control for ΔΨ decrease), whereas oligomycin alone induced higher ΔΨ by blocking the F1FO ATPase (hyperpolarization control). LLO treatment caused a decrease in ΔΨ. Normalized average values from two independent experiments are shown, and P values were calculated (**P < 0.01, *P < 0.25, one-tailed Student's t test). (B) Time-lapse analysis of HeLa cells stained with MitoTracker 488 (green) and the potential-sensitive dye TMRM (red) shows that ΔΨ decreases shortly before mitochondrial fragmentation induced by addition of 6 nM LLO becomes apparent. Fragmented mitochondria lose TMRM and retain only the potential-insensitive MitoTracker 488. (C) Intracellular ATP levels were measured 1 h after infection with L. innocua expressing InlB, L. monocytogenes Δhly, or wild-type L. monocytogenes. Wild-type L. monocytogenes causes the strongest drop in intracellular ATP levels; the effect is more pronounced when cells are incubated with recombinant LLO (10 min, 6 nM). Cell permeabilization with detergent (0.1% Triton X-100, 10 min) causes complete intracellular ATP release. Experiments were performed three times in duplicate, and mean values (normalized to untreated cells) are shown. ***P < 0.0005, **P < 0.005, *P < 0.05, one-tailed Student's t test. (D) HeLa cells were treated for 10 min with 6 nM LLO or 0.1% Triton X-100, for 30 min with the mitochondrial uncouplers CCCP (100 μM), carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) (1 μM), and valinomycin (100 nM), or with the Ca2+ ionophore A23187 (1 μM). Intracellular ATP levels do not decrease significantly upon Ca2+ influx or uncoupling but do decrease significantly with the combination of both treatments. Statistical analysis was performed as in C. n.s., nonsignificant values.
LLO treatment caused a drop in respiratory activity as measured with an oxygen consumption chamber (Fig. S3). Respiration resumed when the mitochondrial respiratory substrate succinate was added, indicating that no major damage occurred to mitochondria, because the respiratory chain (complex II–complex IV) appeared functional. Furthermore, the resumption of respiration demonstrates that LLO does not form pores in the mitochondrial inner membrane, a finding that is supported further by immunofluorescence analysis, showing plasma membrane rather than mitochondrial localization of LLO (Fig. S4). In contrast, LLO appears to permeabilize the otherwise succinate-impermeable plasma membrane, contributing to mitochondrial depolarization by allowing leakage of bioenergetic substrates out of the cell.
Infection also affected cellular ATP levels in an LLO-dependent manner: Infection with wild-type L. monocytogenes (1 h, MOI of 50) induced a 50% decrease in intracellular ATP levels, but this decrease was not observed with L. innocua(InlB) or the L. monocytogenes Δhly mutant (Fig. 5C). Recombinant LLO (6 nM, 10 min) was sufficient to cause an even more pronounced decrease in intracellular ATP levels (Fig. 5C). A similar decrease was obtained by a combination of chemically induced mitochondrial uncoupling with carbonyl cyanide m-chlorophenylhydrazone (CCCP) and Ca2+ influx (A23187), although uncoupling or Ca2+ influx separately did not lead to a significant difference (Fig. 5D). These data strongly suggest that L. monocytogenes infection not only affects mitochondrial dynamics but also interferes with cellular bioenergetics and mitochondrial function.
LLO-Induced Mitochondrial Fragmentation Does Not Correlate with Classical Apoptosis.
Mitochondrial fragmentation has been described in apoptotic cells, prompting us to analyze apoptosis markers such as cytochrome c release. Cytochrome c was not released from fragmented mitochondria upon infection or LLO treatment (Fig. 3A and Fig. S5A). In contrast, cytochrome c release was observed in the positive control, i.e., staurosporine-treated cells (Fig. S5A). Interestingly, the observed mitochondrial fragmentation did not depend on mitochondrial transition pore (mTP) opening, because it was not blocked by the mTP inhibitor cyclosporin A. To test further for apoptosis in cells displaying infection-induced mitochondrial fragmentation, we analyzed activated B-cell lymphoma 2 (Bcl2)-associated X (Bax) protein by immunostaining. The proapoptotic Bcl2 protein family member Bax is activated by several apoptotic signals and translocates to mitochondria, where it forms pores and participates in fission (42, 43). We could not detect mitochondrial recruitment of activated Bax upon infection or LLO treatment (Fig. S5B).
Together, these experiments suggest that neither L. monocytogenes infection nor treatment with sublytic LLO concentrations causes classical apoptosis in HeLa cells.
Infection-Induced Mitochondrial Fragmentation Is Transient.
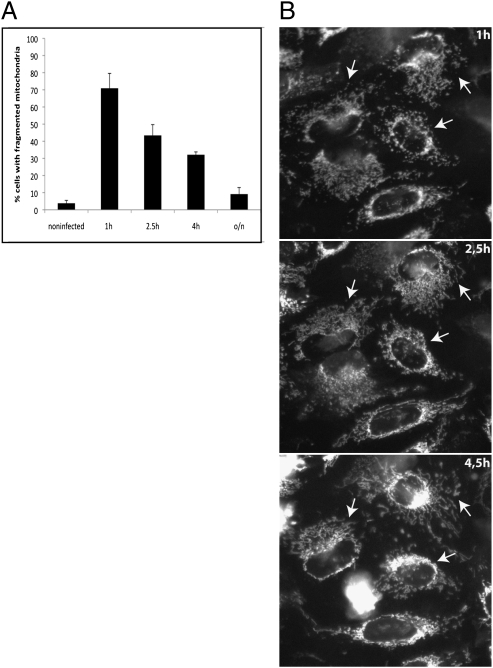
An important question was whether mitochondria that fragment because of LLO produced at initial stages of Listeria infection recover their original shape and reform an interconnected network. To this end, we followed recovery by infecting cells for 1 h and then observing mitochondrial morphology at different time points after bacteria removal. The proportion of cells with fragmented mitochondria decreased steadily (Fig. 6A). Time-lapse imaging indicated that in most cases tubular mitochondrial morphology is recovered within the first few hours (Fig. 6B). In line with these results, we found that intracellular ATP levels recovered by 4 h postinfection (Fig. S6), suggesting that infected cells are not terminally damaged.
Fig. 6.
Mitochondria fragmented upon L. monocytogenes infection can recover tubular morphology. (A) HeLa cells infected with L. monocytogenes (1 h, MOI of 50) were fixed directly or were washed and further incubated for 1.5 h, 3 h, or overnight (i.e., 2.5 h, 4 h, and overnight after inoculation) in the presence of 80 μg/mL gentamicin to kill extracellular bacteria rapidly and to slow intracellular bacterial replication. The number of cells displaying fragmented mitochondria decreases steadily with time, indicating mitochondrial network recovery. The percentage of cells with fragmented mitochondria was determined by counting >100 cells per time point in two independent experiments. (B) HeLa cells were loaded with 100 nM MitoTracker 488, treated as in A, and imaged at the indicated time points. Arrows indicate cells with fragmented mitochondria that recover tubular morphology starting at 2.5 h postinfection.
Discussion
We show here that infection with pathogenic L. monocytogenes causes transient mitochondrial network fragmentation and identify the secreted bacterial toxin LLO as the main factor affecting mitochondrial morphology and function at early time points of infection. LLO induces Ca2+-dependent mitochondrial fragmentation, accompanied by a decrease in the mitochondrial membrane potential ΔΨ and in respiration. As ΔΨ and respiration concomitantly decrease upon LLO treatment, ATP regeneration proceeds inefficiently, contributing to a decrease in intracellular ATP levels. This decrease suggests a transient metabolic “slow-down” of host cells, favoring early stages of infection by interfering with the capacity of the host cell to respond to this event. Such strategy could be common to several bacteria secreting pore-forming toxins, because we found that different recombinant pore-forming toxins had effects comparable to LLO (Fig. S7).
LLO pore formation has been studied at the biophysical and physiological level and induces Ca2+ influx (32, 44). Interestingly, several pathogens manipulate host-cell physiology by inducing Ca2+fluxes (45). Importantly, this Ca2+ influx enhances Listeria entry into cells (31), suggesting that early LLO action is a crucial step in epithelial cell infection. Ca2+ influx probably represents a first bioenergetic insult to the cell, inducing mitochondrial fragmentation and depolarization as well as blocking mitochondrial movement (Movie S1), although such damage is reversible. While neither uncoupling nor Ca2+ ionophore treatment significantly reduce intracellular ATP levels, such an ATP decrease is reproduced partially by the synergistic action of an uncoupler and a Ca2+ ionophore but these drugs do not cause the dramatic mitochondrial fragmentation observed upon LLO treatment (Fig. S8). In the case of Listeria infection, the bioenergetic crisis is probably aggravated by leakage of small molecules, including respiratory substrates or glycolysis intermediates through LLO pores.
LLO-induced ΔΨ decrease also may reflect a host-cell response to prevent mitochondrial Ca2+ accumulation to cytotoxic levels, because mitochondrial Ca2+ uptake is ΔΨ dependent (46). Indeed, several markers of apoptosis were absent in cells with LLO- or infection-induced mitochondrial fragmentation. Our data are consistent with the notion that epithelial cells recover from the attack of pore-forming toxins (including LLO) at sublytic concentrations, regain membrane integrity, and resume the cell cycle (32, 47, 48). The molecular mechanisms underlying LLO recovery are currently unclear: LLO does not colocalize with endocytic markers at early time points (Fig. S4). Infected cells appear to restore their mitochondrial network both morphologically (Fig. 6) and functionally, because intracellular ATP levels recover 4 h postinfection (Fig. S6). Consistent with the view that cytosolic LLO is inactivated rapidly (30), mitochondrial fragmentation is induced only by extracellular LLO, i.e., early during Listeria infection; at late time points of infection intracellular bacteria do not affect mitochondrial morphology, even though they produce LLO to escape from the vacuole (Fig. S9). Together, these data indicate that Listeria affects host-cell mitochondria only transiently. Permanent impairment of mitochondrial function and dynamics would harm host cells and therefore would be counterselected for, because it would eliminate the bacterial replication niche.
Our data suggest that active induction of mitochondrial fragmentation early during infection is critical for infection. Indeed, infection is impaired in cells with previously fissioned mitochondria and is enhanced in cells with hyperfused mitochondria. Treatments that impair mitochondrial respiration cause fragmentation (49), and, conversely, mitochondrial fragmentation has been shown to limit the spread of incoming Ca2+ across the mitochondrial network (50). Accordingly, fragmentation appears to be an appropriate response to avoid propagation of the consequences of Ca2+ influx to the entire mitochondrial network. A prefissioned state would limit the extent of damage caused by LLO action and allow the mitochondrial network to restore cellular bioenergetics more efficiently. Consequently, invading Listeria would have less time to take advantage from the transient bioenergetic “slow-down” it induces. The opposite effect would occur in Drp1-knockdown cells with a hyperfused mitochondrial network.
In conclusion, we propose a scenario in which the normal bioenergetic state of the cell represents a barrier to Listeria invasion. Consequently, an LLO-induced transient metabolic reprogramming of the cell would promote efficient infection. In agreement with this notion, mitochondrial dysfunction has been linked to increased susceptibility to bacterial infection (51, 52). Our work shows that mitochondrial dynamics plays a role in infection with the human pathogen L. monocytogenes. Whether in this case specific signaling cascades are activated downstream of the induced mitochondrial fragmentation and dysfunction is currently unknown, but mitochondrial dynamics and infection do appear to influence each other mutually, because Listeria infection specifically leads to transient disruption of mitochondrial morphology and function, and prior disruption of mitochondrial dynamics by siRNA affects Listeria infection efficiency. Interestingly, the cytomegalovirus protein vMia induces mitochondrial fragmentation and thereby prevents innate immune signaling downstream of mitochondrial antiviral signaling protein (MAVS) (26). Listeria may act similarly, explaining MAVS-independent activation of the innate immune response (53). Given that bioenergetics affect mitochondrial shape, and vice-versa (2, 49), it is likely that mitochondrial localization of innate immunity components serves to coordinate innate immune responses with cellular energy levels via sensing of mitochondrial morphology and function. How innate immunity components and the fission/fusion machinery sense changes in the bioenergetic status of mitochondria is still unknown. The strong and rapid action of LLO described in our work provides an additional model system to address this question.
Materials and Methods
Reagents, cell lines, and bacterial strains used in this study, detailed experimental protocols, and nonstandard abbreviations are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We acknowledge the expert help of C. Schmitt and G. Pehau-Arnaudet of the Plate-Forme de Microscopie Ultrastructurale and the continuous support of the Plate-Forme d'Imagerie Dynamique staff at the Institut Pasteur. We thank E. Veiga and L. Dortet for pioneering experiments, K. Rogers and M. Lecuit for stimulating discussions and laboratory members for critical reading of the manuscript. This work received support from the European Research Council (Advanced Grant 233348), the Fondation Le Roch Les Mousquetaires, and the Fondation Jeantet. P.C. is a Howard Hughes Medical Institute International Fellow. F.S. was funded by a Roux Fellowship (Institut Pasteur) and currently is a European Molecular Biology Organization Long-Term Fellow.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100126108/-/DCSupplemental.
References
- 1.Soubannier V, McBride HM. Positioning mitochondrial plasticity within cellular signaling cascades. Biochim Biophys Acta. 2009;1793:154–170. doi: 10.1016/j.bbamcr.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Sauvanet C, Duvezin-Caubet S, di Rago JP, Rojo M. Energetic requirements and bioenergetic modulation of mitochondrial morphology and dynamics. Semin Cell Dev Biol. 2009;21:558–565. doi: 10.1016/j.semcdb.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 4.Aliev G, et al. Brain mitochondria as a primary target in the development of treatment strategies for Alzheimer disease. Int J Biochem Cell Biol. 2009;41:1989–2004. doi: 10.1016/j.biocel.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet. 2005;14(Spec No. 2):R283–289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- 7.Parone PA, et al. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS One. 2008;3:e3257. doi: 10.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rojo M, Legros F, Chateau D, Lombès A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci. 2002;115:1663–1674. doi: 10.1242/jcs.115.8.1663. [DOI] [PubMed] [Google Scholar]
- 9.Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114:867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- 10.Alexander C, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 11.Delettre C, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eura Y, Ishihara N, Yokota S, Mihara K. Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem. 2003;134:333–344. doi: 10.1093/jb/mvg150. [DOI] [PubMed] [Google Scholar]
- 14.Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol. 1998;143:351–358. doi: 10.1083/jcb.143.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank S, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 17.Estaquier J, Arnoult D. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ. 2007;14:1086–1094. doi: 10.1038/sj.cdd.4402107. [DOI] [PubMed] [Google Scholar]
- 18.Cassidy-Stone A, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parone PA, et al. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol Cell Biol. 2006;26:7397–7408. doi: 10.1128/MCB.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashktorab H, et al. Bax translocation and mitochondrial fragmentation induced by Helicobacter pylori. Gut. 2004;53:805–813. doi: 10.1136/gut.2003.024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faherty CS, Maurelli AT. Staying alive: Bacterial inhibition of apoptosis during infection. Trends Microbiol. 2008;16:173–180. doi: 10.1016/j.tim.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kepp O, Rajalingam K, Kimmig S, Rudel T. Bak and Bax are non-redundant during infection- and DNA damage-induced apoptosis. EMBO J. 2007;26:825–834. doi: 10.1038/sj.emboj.7601533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanke SR. Micro-managing the executioner: Pathogen targeting of mitochondria. Trends Microbiol. 2005;13:64–71. doi: 10.1016/j.tim.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Boya P, Roques B, Kroemer G. New EMBO members’ review: Viral and bacterial proteins regulating apoptosis at the mitochondrial level. EMBO J. 2001;20:4325–4331. doi: 10.1093/emboj/20.16.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnoult D, Carneiro L, Tattoli I, Girardin SE. The role of mitochondria in cellular defense against microbial infection. Semin Immunol. 2009;21:223–232. doi: 10.1016/j.smim.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Castanier C, Garcin D, Vazquez A, Arnoult D. Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep. 2010;11:133–138. doi: 10.1038/embor.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasukawa K, et al. Mitofusin 2 inhibits mitochondrial antiviral signaling. Sci Signal. 2009;2(ra47):1–11. doi: 10.1126/scisignal.2000287. [DOI] [PubMed] [Google Scholar]
- 28.Cossart P, Toledo-Arana A. Listeria monocytogenes, a unique model in infection biology: An overview. Microbes Infect. 2008;10:1041–1050. doi: 10.1016/j.micinf.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 29.Hamon MA, Cossart P. Histone modifications and chromatin remodeling during bacterial infections. Cell Host Microbe. 2008;4:100–109. doi: 10.1016/j.chom.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Schnupf P, Portnoy DA. Listeriolysin O: A phagosome-specific lysin. Microbes Infect. 2007;9:1176–1187. doi: 10.1016/j.micinf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Dramsi S, Cossart P. Listeriolysin O-mediated calcium influx potentiates entry of Listeria monocytogenes into the human Hep-2 epithelial cell line. Infect Immun. 2003;71:3614–3618. doi: 10.1128/IAI.71.6.3614-3618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gekara NO, et al. The multiple mechanisms of Ca2+ signalling by listeriolysin O, the cholesterol-dependent cytolysin of Listeria monocytogenes. Cell Microbiol. 2007;9:2008–2021. doi: 10.1111/j.1462-5822.2007.00932.x. [DOI] [PubMed] [Google Scholar]
- 33.Tang P, Rosenshine I, Cossart P, Finlay BB. Listeriolysin O activates mitogen-activated protein kinase in eucaryotic cells. Infect Immun. 1996;64:2359–2361. doi: 10.1128/iai.64.6.2359-2361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kayal S, et al. Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: Activation of NF-kappa B and upregulation of adhesion molecules and chemokines. Mol Microbiol. 1999;31:1709–1722. doi: 10.1046/j.1365-2958.1999.01305.x. [DOI] [PubMed] [Google Scholar]
- 35.Hamon MA, et al. Histone modifications induced by a family of bacterial toxins. Proc Natl Acad Sci USA. 2007;104:13467–13472. doi: 10.1073/pnas.0702729104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribet D, et al. Listeria monocytogenes impairs SUMOylation for efficient infection. Nature. 2010;464:1192–1195. doi: 10.1038/nature08963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isberg RR, Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985;317:262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- 38.Michel E, Reich KA, Favier R, Berche P, Cossart P. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol Microbiol. 1990;4:2167–2178. doi: 10.1111/j.1365-2958.1990.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 39.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 40.Frieden M, et al. Ca(2+) homeostasis during mitochondrial fragmentation and perinuclear clustering induced by hFis1. J Biol Chem. 2004;279:22704–22714. doi: 10.1074/jbc.M312366200. [DOI] [PubMed] [Google Scholar]
- 41.Han XJ, et al. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol. 2008;182:573–585. doi: 10.1083/jcb.200802164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karbowski M, et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Autret A, Martin SJ. Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol Cell. 2009;36:355–363. doi: 10.1016/j.molcel.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Meixenberger K, et al. Listeria monocytogenes-infected human peripheral blood mononuclear cells produce IL-1beta, depending on listeriolysin O and NLRP3. J Immunol. 2010;184:922–930. doi: 10.4049/jimmunol.0901346. [DOI] [PubMed] [Google Scholar]
- 45.TranVan Nhieu G, Clair C, Grompone G, Sansonetti P. Calcium signalling during cell interactions with bacterial pathogens. Biol Cell. 2004;96:93–101. doi: 10.1016/j.biolcel.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. Calcium in cell injury and death. Annu Rev Pathol. 2006;1:405–434. doi: 10.1146/annurev.pathol.1.110304.100218. [DOI] [PubMed] [Google Scholar]
- 47.Bischofberger M, Gonzalez MR, van der Goot FG. Membrane injury by pore-forming proteins. Curr Opin Cell Biol. 2009;21:589–595. doi: 10.1016/j.ceb.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Babiychuk EB, Monastyrskaya K, Potez S, Draeger A. Intracellular Ca(2+) operates a switch between repair and lysis of streptolysin O-perforated cells. Cell Death Differ. 2009;16:1126–1134. doi: 10.1038/cdd.2009.30. [DOI] [PubMed] [Google Scholar]
- 49.De Vos KJ, Allan VJ, Grierson AJ, Sheetz MP. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr Biol. 2005;15:678–683. doi: 10.1016/j.cub.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 50.Szabadkai G, et al. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 51.Ayres JS, Freitag N, Schneider DS. Identification of Drosophila mutants altering defense of and endurance to Listeria monocytogenes infection. Genetics. 2008;178:1807–1815. doi: 10.1534/genetics.107.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Francione L, et al. Legionella pneumophila multiplication is enhanced by chronic AMPK signalling in mitochondrially diseased Dictyostelium cells. Dis Model Mech. 2009;2:479–489. doi: 10.1242/dmm.003319. [DOI] [PubMed] [Google Scholar]
- 53.Soulat D, Bauch A, Stockinger S, Superti-Furga G, Decker T. Cytoplasmic Listeria monocytogenes stimulates IFN-beta synthesis without requiring the adapter protein MAVS. FEBS Lett. 2006;580:2341–2346. doi: 10.1016/j.febslet.2006.03.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.