Abstract
The tubulin-like FtsZ protein initiates assembly of the bacterial cytokinetic machinery by polymerizing into a ring structure, the Z ring, at the prospective site of division. To block Z-ring formation over the nucleoid and help coordinate cell division with chromosome segregation, Escherichia coli employs the nucleoid-associated division inhibitor, SlmA. Here, we investigate the mechanism by which SlmA regulates FtsZ assembly. We show that SlmA disassembles FtsZ polymers in vitro. In addition, using chromatin immunoprecipitation (ChIP), we identified 24 SlmA-binding sequences (SBSs) on the chromosome. Remarkably, SlmA binding to SBSs dramatically enhanced its ability to interfere with FtsZ polymerization, and ChIP studies indicate that SlmA regulates FtsZ assembly at these sites in vivo. Because of the dynamic and highly organized nature of the chromosome, coupling SlmA activation to specific DNA binding provides a mechanism for the precise spatiotemporal control of its anti-FtsZ activity within the cell.
Keywords: cytokinesis, cytoskeleton, DNA replication, cell cycle
Bacterial cytokinesis is mediated by a ring-shaped, multiprotein machine called the septal ring or divisome (1). At the heart of this machine is the highly conserved, tubulin-like protein, FtsZ. It initiates divisome assembly by polymerizing into a dynamic ring structure, the Z ring, just underneath the cytoplasmic membrane at the prospective site of fission (2). Several FtsZ binding proteins (FtsA, ZipA, and ZapA) have been identified and appear to play important roles in Z-ring formation (1). They are thought to decorate and stabilize the structure as it forms. Once its assembly is complete, the Z ring is thought to serve as a scaffold for the recruitment of a large set of essential and auxiliary division proteins to the division site, which together form the transenvelope septal ring machine capable of synthesizing the new daughter cell poles (1).
Division site placement is primarily determined through the spatial regulation of FtsZ polymerization. Whereas many of the core septal ring proteins are broadly conserved throughout the bacterial domain, the regulatory strategies used to control Z-ring assembly are quite diverse (1, 3). In the model systems of Escherichia coli and Bacillus subtilis, Z-ring positioning is controlled by two partially redundant, negative regulatory systems: Min and nucleoid occlusion (NO) (1). The E. coli Min system is composed of MinC, MinD, and MinE (4). The system has been extensively characterized cytologically and biochemically (5). Membrane binding by the MinCD division inhibitor is dynamically antagonized by MinE such that waves of the membrane-associated complex rapidly oscillate from one cell pole to the other (5). Consequently, the time-averaged concentration of MinCD is highest at the cell poles and lowest at midcell where Z-ring formation becomes favored (5).
By blocking Z-ring assembly in regions of the cell occupied by the nucleoid, NO systems help coordinate cell division with chromosome segregation and prevent cells from dividing over the nucleoid in the event of problems with DNA replication/segregation (6–8). In contrast to the Min system, the factors that promote NO in E. coli and B. subtilis are completely unrelated by sequence, yet they appear to have similar modes of action. SlmA is a putative DNA binding protein of the TetR family that mediates NO in E. coli, whereas Noc is a ParB-family DNA-binding protein that mediates NO in B. subtilis (6–8). Loss of SlmA or Noc function results in a synthetic lethal phenotype with a Min defect (6, 8). In both cases, Min− NO− cells fail to divide and form long filamentous cells with many aberrant FtsZ assemblies observed over their nucleoids. In addition, NO− mutants are unable to block division over nonreplicating nucleoids that remain at midcell following depletion of the essential initiation factor, DnaA (6, 8). Overproduction of either SlmA or Noc in their respective organisms blocks or slows cell division, suggesting that both proteins function as division inhibitors (6–8). The target of Noc regulation is not currently known (6, 7). SlmA, on the other hand, has been shown to interact with FtsZ in vitro and is therefore thought to mediate NO by directly regulating FtsZ assembly (8). However, the mechanism by which SlmA accomplishes this has remained unclear. Here, we investigate the division regulatory activity of SlmA and its effect on FtsZ assembly. We show that SlmA is an antagonist of FtsZ polymerization that is activated by sequence-specific DNA binding.
Results
SlmA Is an FtsZ Antagonist.
FtsZ is a GTPase that assembles into dynamic polymers in the presence of GTP (9). It was previously shown that purified SlmA with N-terminal 6× His and T7 tags promoted the assembly of FtsZ protofilaments into large, ribbon-like structures composed of both FtsZ and SlmA (8). This was unexpected because all of the genetic data regarding SlmA function suggested that it acted as an FtsZ inhibitor in vivo, not a factor that might stabilize FtsZ assemblies. We therefore reinvestigated the in vitro activity of SlmA using an untagged version of the protein and different reaction conditions [lower pH (6.7 vs. 7.2) and higher KCl (200 mM vs. 50 mM)].
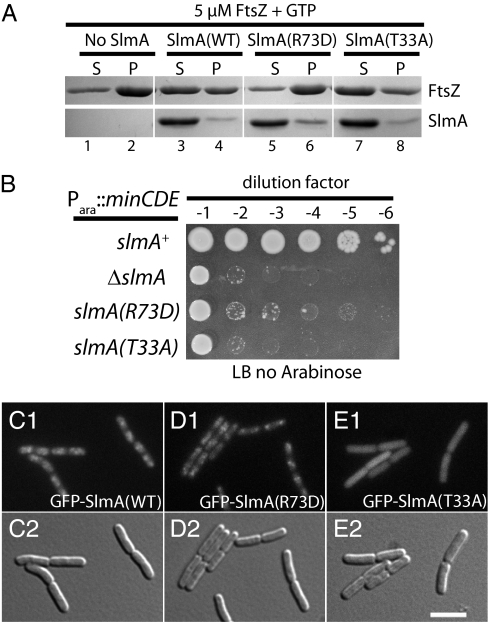
In contrast to previous results, we found that SlmA blocked FtsZ assembly in a KCl-dependent manner (Fig. 1A and SI Appendix, Fig. S1). Maximal disruption of FtsZ polymerization was observed at K+ concentrations near those found in the cytoplasm (∼200 mM) (10). To assess the in vivo relevance of this activity, we isolated two NO-defective slmA missense alleles (Fig. 1B) and tested the activity of the resulting protein variants in vitro (Fig. 1 A and B and SI Appendix, Fig. S2). SlmA(R73D) retained its ability to associate with the nucleoid in vivo (Fig. 1 C and D), but did not cause a severe division block when it was overproduced (SI Appendix, Fig. S2G). It therefore appeared to be defective for an interaction with FtsZ (see below). SlmA(T33A) has a substitution in a conserved residue of the helix-turn-helix (HTH) motif. It failed to associate with the nucleoid (Fig. 1E), but blocked division when it was overproduced (SI Appendix, Fig. S2H), suggesting that it interacts with FtsZ when produced at high concentrations. As expected on the basis of their in vivo phenotypes, the SlmA variants had opposite effects on FtsZ in vitro. SlmA(T33A) retained the ability to block FtsZ polymerization, whereas SlmA(R73D) was defective in this activity (Fig. 1A). Importantly, at low KCl (30 mM), both untagged SlmA(WT) and SlmA(R73D) caused the formation of ribbon-like FtsZ assemblies similar to those observed previously (8) (SI Appendix, Fig. S3). Because such ribbon-like FtsZ assemblies can be formed by both NO-functional and NO-defective variants of SlmA, their formation is unlikely to reflect the key physiological activity of SlmA.
Fig. 1.
SlmA is an FtsZ polymerization antagonist. (A) FtsZ polymerization assays. Purified FtsZ (5 μM) was incubated in polymerization buffer (50 mM PIPES, pH 6.7, 10 mM MgCl2, 200 mM KCl) at room temperature for 20 min with or without SlmA(WT) or SlmA variants (5 μM) as indicated. GTP (5 mM) was then added and FtsZ polymers were sedimented by ultracentrifugation. Proteins found in the resulting pellet (P) and supernatant (S) fractions were separated on an SDS-polyacrylamide gel and visualized by Coomassie brilliant blue staining. (B) NO defect of slmA missense alleles. TB57 [Para::minCDE] cells and its derivatives with the indicated slmA allele were grown overnight in M9-arabinose medium. The resulting cultures were serially diluted in LB (1% NaCl), 5 μL of each dilution was spotted on LB (1% NaCl) agar lacking arabinose, and the plate was incubated at 30 °C overnight. (C–E) Localization of SlmA variants. Cells of HC246 [ΔslmA] with the integrated expression constructs (attHKTB99) [Plac::gfp-slmA] (C), (attHKHC482) [Plac::gfp-slmA(R73D)] (D), or (attHKHC505) [Plac::gfp-slmA(T33A)] (E) were grown to an OD600 of 0.5 in LB with 250 μM IPTG and visualized using GFP (C1, D1, and E1) and DIC (C2, D2, and E2) optics.
Further support for FtsZ assembly being the target of SlmA action in vivo came from the observation that FtsZ overproduction suppressed the lethal division block induced by the overproduction of GFP–SlmA(T33A) (SI Appendix, Fig. S4). Use of GFP–SlmA(T33A) was required for this experiment because the phenotype induced by the overproduction of GFP–SlmA(WT) appears to be confounded by (nonspecific) effects on the nucleoid as well as its ability to interfere with Z-ring formation (8) (SI Appendix, Fig. S4). Overall, our biochemical and genetic results indicate that SlmA is an FtsZ polymerization antagonist and that this activity is essential for its ability to interfere with Z-ring assembly and mediate NO in vivo.
SlmA Is a Sequence-Specific DNA-Binding Protein.
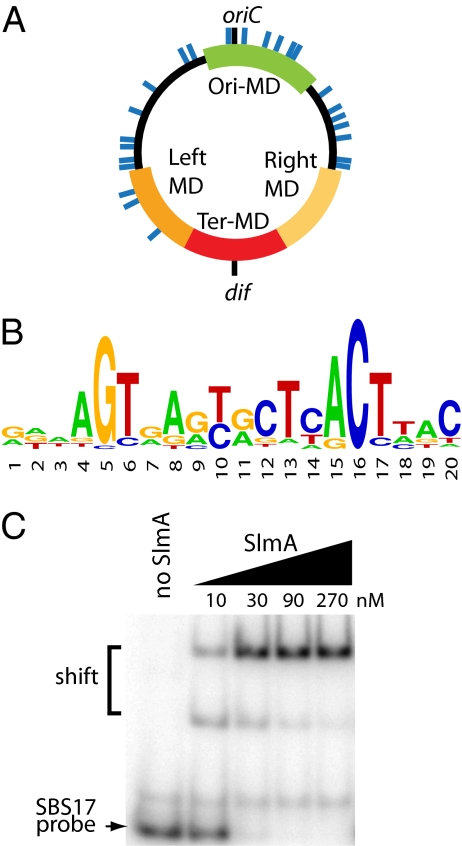
We expected variants defective for nucleoid association like SlmA(T33A) to be highly toxic because the division inhibitor would be free to diffuse throughout the cell and interfere with Z-ring assembly at all locations. However, SlmA(T33A) only inhibited division when overproduced (SI Appendix, Fig. S2H), suggesting that DNA-binding might stimulate the activity of SlmA. Consistent with this idea, high SlmA:FtsZ ratios were required to interfere with FtsZ polymerization in vitro (SI Appendix, Fig. S1A), suggesting that the reactions described above might be missing a critical component. DNA is an obvious candidate. Because we suspected that a specific DNA-binding site may be required, we used ChIP-on-chip to identify SlmA-binding sites on the chromosome. With this approach, 24 chromosomal sites were identified that were significantly enriched following native SlmA immunoprecipitation with affinity-purified anti-SlmA polyclonal antibodies (Fig. 2 and SI Appendix, Fig. S5 and Table S1). Enrichment of these regions was not observed when the analysis was performed using ΔslmA cells. The ChIP-on-chip results were confirmed for several sites using standard ChIP and quantitative PCR (qPCR) analysis (SI Appendix, Fig. S6). Similar to the recently described Noc-binding regions in B. subtilis (7), SlmA-binding sites were not observed in the terminus region (Fig. 2A). Bioinformatic analysis using MEME (11) revealed the presence of potential SlmA-binding sequences (SBS1-24) within the 24 chromosomal regions enriched by ChIP with anti-SlmA antibodies (SI Appendix, Table S1). The majority of these binding sequences were present within genes as opposed to intergenic regions (SI Appendix, Table S1). A sequence logo of the position weight matrix (PWM) representing the consensus SBS is shown in Fig. 2B, and an analysis of the quality of the PWM is presented in SI Appendix, Table S2.
Fig. 2.
Identification of chromosomal SlmA-binding sites. (A) Circular diagram of the E. coli chromosome with approximate locations of SlmA-binding sites shown as blue lines. Green, red, dark- and light-orange colored regions correspond to the Ori, Ter, Left, and Right macrodomains (MDs), respectively (26). (B) Sequence logo of consensus SlmA-binding sequence generated using weblogo. (C) The indicated concentrations of SlmA were incubated with unlabeled poly-dIdC (100 μg/mL) and 0.2 nM [32P]-labeled SBS17-containing DNA (100 bp). The SBS17 site was centered within the probe fragment and flanked by adjacent chromosomal sequence. Protein–DNA complexes were separated from free probe by gel electrophoresis and detected using a phosphorimager. We do not currently know why an intermediate shifted complex is observed.
We used electrophoretic mobility shift assays (EMSA) to test SlmA binding to SBSs directly. For this, we generated a 100-bp probe containing SBS17 (GTTAGTGACCATTTACTTAC) flanked on either side by 40 bp of chromosomal sequence from the SBS17 locus. We also generated a similar 100-bp probe encoding the consensus SBS flanked by vector sequence. As expected, SlmA bound both probes with high affinity (Fig. 2C and SI Appendix, Fig. S7A). Importantly, an unlabeled SBS17 fragment with a mutant SBS (GTTCTGGACCATTTCAGTAC) failed to compete with the WT SBS17 fragment even when it was present at more than a thousand-fold molar excess (SI Appendix, Fig. S7B). This validates the SBS identified using MEME and indicates that SlmA is a sequence-specific DNA-binding protein. In agreement with their in vivo nucleoid association phenotypes, SlmA(R73D) bound the SBS17 probe with wild-type affinity, whereas SlmA(T33A) was defective for binding (SI Appendix, Fig. S7C).
SlmA Is Activated by SBS in Vitro.
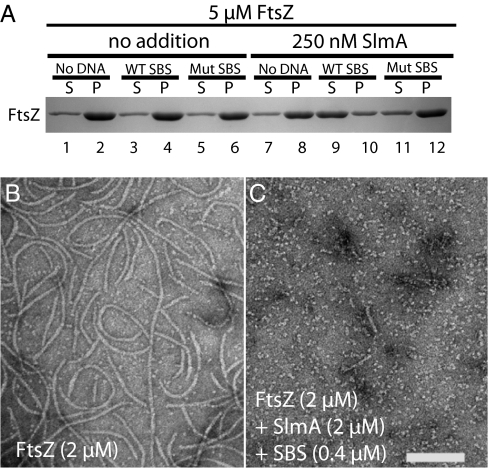
The identification of specific DNA-binding sites for SlmA allowed us to test the hypothesis that DNA binding activates the anti-FtsZ activity of SlmA. For these experiments, we used a synthetic 30-bp DNA fragment encoding SBS17 flanked on either side by 5 bp of chromosomal sequence. As described above, in the absence of added DNA, robust inhibition of FtsZ polymerization required equimolar amounts of SlmA (Fig. 1A and SI Appendix, Fig. S1A). Strikingly, however, the addition of synthetic SBS17 DNA (50 nM) promoted a near complete block of FtsZ (5 μM) assembly by as little as 250 nM SlmA (Fig. 3A). The inhibition observed was equal to or, in some cases, even better than that mediated by 5 μM SlmA alone. SBS17 DNA had no effect on FtsZ assembly in the absence of SlmA. Importantly, mutant SBS17 DNA failed to activate SlmA (Fig. 3A). In addition, when added at low levels (250 nM), the SlmA variants, SlmA(R73D) and SlmA(T33A), were also ineffective in preventing FtsZ assembly in the presence of SBS17 DNA (SI Appendix, Fig. S8A). Thus, as expected, SlmA activation by SBS DNA requires both its anti-FtsZ and DNA-binding activities.
Fig. 3.
SBS binding activates SlmA. (A) Sedimentation assays were performed as in Fig. 1 A–B, except lower levels (250 nM) of SlmA were used and the reactions also contained 30 bp of WT or mutant SBS17 dsDNA fragments (50 nM) as indicated. (B and C) FtsZ polymers were formed in reactions containing FtsZ (2 μM) and GTP (2 mM) without (B) or with (C) the addition of SlmA (2 μM) and 30 bp of SBS17 dsDNA (400 nM). Reactions were spotted onto carbon-coated grids 5 min after GTP addition, negatively stained with uranyl formate, and visualized by electron microscopy. (Scale bar, 100 nm.)
In addition to the sedimentation assay, we also monitored the effect of SlmA–SBS complexes on FtsZ polymerization using electron microscopy (EM). In the absence of added SlmA, FtsZ (2 μM) assembled into protofilaments with a broad length distribution (Fig. 3B and SI Appendix, Fig. S8 B and E). Polymer length appeared to be largely unaffected by SlmA alone or by SlmA in the presence of mutant SBS DNA regardless of the concentrations used (250 nM–2 μM of SlmA and 50–400 nM of DNA) (SI Appendix, Fig. S8 C, D, F, G, I, and K). Polymerization was significantly affected, however, by the addition of SlmA with WT SBS DNA. At low concentrations (250 nM of SlmA/50 nM of DNA), SlmA–SBS complexes caused a decrease in the average polymer length by a factor of 2 (SI Appendix, Fig. S8 C and F). When the concentrations of SlmA and SBS DNA were increased to 2 μM and 400 nM, respectively, protofilaments were all but undetectable (Fig. 3C). Thus, although the sedimentation and EM assays show a different concentration dependence for the activity of SlmA–SBS complexes, they both indicate that SlmA is an antagonist of FtsZ assembly that is activated by specific DNA binding.
FtsZ Is Regulated at SBSs in Vivo.
The in vitro results described above suggest that SlmA antagonizes FtsZ polymerization from chromosomal SBSs in vivo. If true, cells possessing extra copies of SBSs spread throughout the cell should display a division defect because of SlmA-mediated inhibition of Z-ring formation at inappropriate sites. To test this, SBS12 and SBS17 were inserted in tandem into a high-copy number (pUC derived) plasmid. As expected, the resulting plasmid (pHC534) blocked cell division by inhibiting Z-ring formation in a SlmA-dependent fashion (SI Appendix, Fig. S9). The cell division defect appeared to arise from a mislocalization of activated SlmA associated with the SBS sites on the plasmid. In the presence of pHC534, GFP–SlmA was distributed throughout the cell such that it could interfere with FtsZ assembly in the nucleoid-free regions as well as over the nucleoid where it is normally exclusively located (SI Appendix, Fig. S9 C and D). Consistent with this idea, the SlmA-dependent division defect of pHC534 (pUC-2xSBS) can be partially suppressed by overproducing FtsZ (SI Appendix, Fig. S9 E–H).
We reasoned that if FtsZ polymerization is being disrupted by SlmA at native chromosomal SBSs in vivo, we should be able to detect SBS–SlmA–FtsZ complexes by assaying for SBS enrichment following ChIP with anti-FtsZ antiserum. Even if the SlmA–FtsZ complexes formed are transient, as one might expect for the association of a polymerization antagonist with its substrate polymer, we suspected that the crosslinking step in the ChIP procedure would “trap” enough interactions at the SBSs to detect them. Indeed, relative to the control lacZ locus, all of the SBSs tested were significantly enriched (5–10 fold) following anti-FtsZ ChIP (SI Appendix, Fig. S9I). Enrichment of the SBS loci was not observed in samples prepared from ΔslmA, slmA(R73D), or slmA(T33A) mutants (SI Appendix, Fig. S9I). We therefore conclude that FtsZ associates with chromosomal SBSs in a SlmA-dependent manner and that SlmA is likely antagonizing FtsZ assembly at these sites in vivo.
The absence of SBSs in the terminus region of the chromosome (Fig. 2A) suggests that SlmA–SBS complexes are likely to be segregated to the prospective daughter cell halves before replication is complete. As suggested previously for Noc (7), this would create a SlmA-free zone at midcell to allow for the assembly of a Z ring as DNA replication is nearing completion. To test the importance of this SlmA-free zone for normal division timing, we integrated an array of six SBS sites on both sides of the dif site in the terminus region. Interestingly, when cells were grown at low temperature (20 °C) this led to a significant increase in average cell length of SlmA+ cells relative to cells lacking these sites or cells harboring a mutant SBS17 site at identical locations (SI Appendix, Fig. S9J). Importantly, the average length of SlmA− cells was unaffected by SBS arrays in the terminus region, indicating that the division delay caused by these sites was SlmA dependent (SI Appendix, Fig. S9J). We did not observe a significant change in cell length when cells with the SBS arrays in the terminus region were grown at 30 °C. The reason for the temperature-dependent effect of the SBS arrays is not known, but may be related to whether or not cells are engaged in multifork DNA replication.
SlmA Promotes the Disassembly of Preformed FtsZ Polymers.
FtsZ polymers are head-to-tail assemblies of monomers. The GTPase active site is formed at the interface between two FtsZ subunits of a polymer (12, 13). Thus, like tubulin, the polymeric species of FtsZ is the active GTPase. Polymerization is rapid upon the addition of GTP to a solution of FtsZ. Once a significant amount of the GTP is hydrolyzed and converted to GDP, the polymers disassemble (9). FtsZ polymers are therefore dynamic structures, and the nucleotide-bound state of FtsZ appears to control polymerization dynamics (14). GTPase measurements are useful in determining whether an FtsZ antagonist interferes with polymerization by blocking polymer assembly or by promoting the disassembly of preassembled polymers. Factors like the SOS-induced division inhibitor SulA that bind FtsZ monomers and prevent their assembly into polymers inhibit the GTPase activity of FtsZ because they prevent the formation of active sites in the polymer (15, 16). In contrast, antagonists that act on preformed polymers will either leave GTPase activity unaffected or will, in some cases, stimulate GTPase activity as they promote polymer disassembly (3, 17).
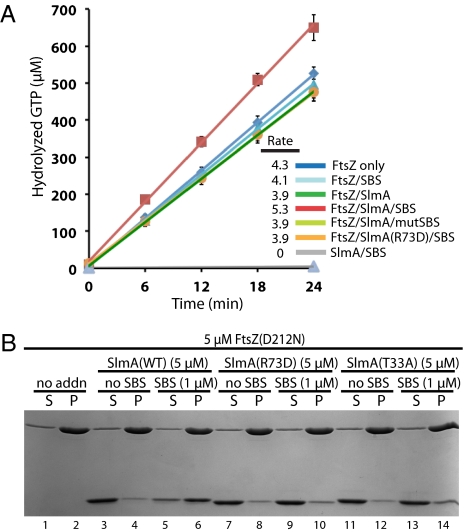
To investigate the mechanism by which SlmA interferes with FtsZ polymerization, we monitored its effect on the GTPase activity of FtsZ with and without the addition of SBS-containing DNA. SlmA alone or in the presence of mutant SBS DNA did not appear to significantly affect FtsZ GTPase activity (Fig. 4A). In the presence of WT SBS DNA, however, SlmA enhanced the GTPase activity of FtsZ, increasing it by 36% relative to the addition of SlmA alone, SlmA with mutant SBS DNA, or SlmA(R73D) with WT SBS DNA (Fig. 4A). No GTPase activity was detected for SlmA or DNA in the absence of FtsZ, and DNA alone had no effect on the GTPase activity of FtsZ. Thus, SlmA is likely to function by disrupting preformed polymers. In support of this possibility, SlmA antagonized FtsZ polymerization whether it was added to assembly reactions before or after the addition of GTP to induce polymerization. Moreover, we failed to detect a stable interaction between SlmA and monomeric FtsZ in pull-down assays containing immobilized SBS DNA, SlmA, and FtsZ(GDP) (SI Appendix, Fig. S10).
Fig. 4.
FtsZ GTPase activity is required for SlmA–SBS complexes to disrupt polymerization. (A) FtsZ GTPase activity measured at 18 °C by hydrolysis of α-[32P]-GTP. Reactions contained 5 mM GTP, 5 μM FtsZ, 10 μM SlmA, or SlmA(R73D) and 30 bp SBS17 fragments (2 μM) as indicated. Results are the average of triplicate measurements and error bars show the SD. Reaction rates (molecules GTP hydrolyzed/min/FtsZ molecule) are listed. Due to overlap, not all lines are readily visible in the graph. (B) Sedimentation assays using FtsZ(D212N) were performed as in Fig. 1A. SlmA(WT) or its variants were included in the reactions along with 30 bp of SBS17 dsDNA as indicated.
SBSs Stimulate SlmA–SlmA and SlmA–FtsZ Interactions.
To determine whether FtsZ GTPase activity is required for SlmA to antagonize FtsZ polymerization, we purified a GTPase defective FtsZ variant, FtsZ(D212N) (18). This variant was completely resistant to high concentrations of SlmA and SlmA–SBS complexes (Fig. 4B), indicating that GTP hydrolysis by FtsZ is important for SlmA to disrupt polymers (Discussion).
The ability to assemble SlmA-resistant FtsZ(D212N) polymers allowed us to investigate the interaction of SlmA with FtsZ polymers by cosedimentation. Interestingly, even when SlmA was added at 5 μM, a level competent to disrupt polymer assembly by FtsZ(WT), we only observed cosedimentation of SlmA with FtsZ(D212N) polymers in the presence of SBS DNA (Fig. 4B). The control proteins, SlmA(R73D) and SlmA(T33A), did not cosediment with FtsZ(D212N) polymers with or without the addition of SBS DNA (Fig. 4B). These results indicate that DNA-binding activity is required for SBS DNA to stimulate the SlmA–FtsZ interaction and that SlmA(R73D) is defective in FtsZ regulation because it is unable to associate with FtsZ polymers. To test if this is also true in vivo, we used a bacterial two-hybrid assay based on the reconstitution of adenylate cyclase activity from the fragments T18 and T25 (19). Consistent with the in vitro results, T18 fusions to FtsZ or FtsZ(D212N) only showed a strong interaction signal when paired with T25–SlmA(WT) (SI Appendix, Fig. S11A). T25–SlmA(R73D) and T25–SlmA(T33A) fusions both appeared to be largely defective for the interaction (SI Appendix, Fig. S11A).
Gel filtration chromatography indicates that SlmA only forms a dimer in solution at high concentrations, suggesting that it only weakly self-associates in the absence of SBS DNA (SI Appendix, Fig. S12). This correlates well with the anti-FtsZ activity of SlmA. Without SBS DNA, it is only active at high concentrations (Fig. 1A). Thus, DNA binding may stimulate SlmA self-association and this, in turn, may activate SlmA for interaction with FtsZ. Accordingly, bacterial two-hybrid results indicate that the DNA-binding defective variant SlmA(T33A) not only has a reduced capacity to interact with FtsZ but that it is also defective for self-association (SI Appendix, Fig. S11B). SlmA(WT) and SlmA(R73D), on the other hand, both showed strong self-interaction signals (SI Appendix, Fig. S11B).
Discussion
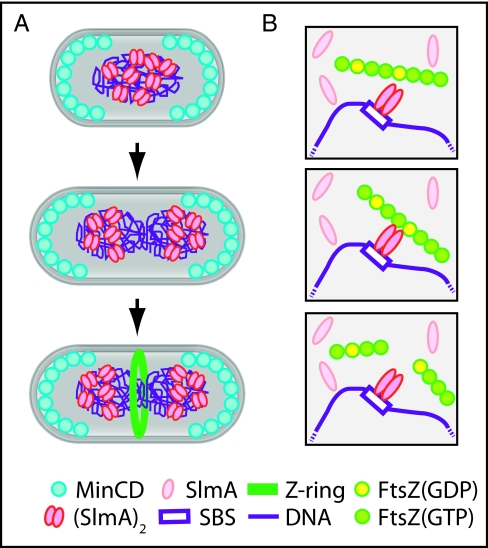
Here, we demonstrated that the NO protein SlmA is an FtsZ antagonist and a sequence-specific DNA binding protein. Importantly, we also showed that the ability of SlmA to interfere with FtsZ polymerization is greatly stimulated upon DNA binding. Our results therefore suggest a molecular mechanism for the NO phenomenon in which SlmA molecules bound to the identified chromosomal SBSs directly antagonize FtsZ polymerization to block Z-ring formation (Fig. 5). Because high concentrations of SlmA are required to interfere with FtsZ polymerization in the absence of SBS DNA in vitro, free SlmA is unlikely to affect FtsZ polymerization in vivo. Accordingly, the DNA-binding defective SlmA variant, SlmA(T33A), did not detectably interact with FtsZ in a bacterial two-hybrid assay. We thus infer that the SBS binding requirement for SlmA activity allows precise spatial control over the division inhibitor such that it will only effectively block FtsZ assembly in the cellular regions or “territories” occupied by chromosomal SBSs (see below).
Fig. 5.
Model for FtsZ regulation by SlmA. (A) In newborn cells, MinCD and SlmA prevent Z-ring assembly at the cell poles and over the nucleoid, respectively. For simplicity, MinCD oscillation is not shown. As the chromosome is replicated, SlmA bound to chromosomal SBSs segregates with the origin-proximal regions of the daughter chromosomes. This leaves the midcell zone free of inhibitors so that the Z ring can form as replication nears completion. (B) SlmA free in the cytoplasm is monomeric and inactive. Binding to SBSs stimulates the ability of SlmA to interact with FtsZ polymers. Upon associating with polymers, it promotes their breakdown. We propose it does so by disrupting FtsZ–FtsZ interactions at interfaces where GTP has been hydrolyzed to GDP. In our model, DNA binding activates SlmA in part by promoting dimerization. It is also possible that higher order oligomers are formed and that they further enhance SlmA activity.
Coordinating Cell Division and Chromosome Segregation.
Similar to the recently described Noc binding sequences (NBSs) (7), the SBSs we identified are absent from the terminus region. Because sequences near the origin are the first to be replicated and segregated (20), this distribution predicts that SlmA bound to chromosomal SBSs is likely to be segregated to the prospective daughter cell halves before the completion of replication. Indeed, it was previously shown that fluorescence from a GFP–SlmA fusion segregates before bulk nucleoid segregation is complete (8). As proposed for Noc (7), the timing of SlmA–SBS segregation determined by the distribution of SBSs on the chromosome is likely important for coordinating chromosome replication and segregation with Z-ring assembly and cytokinesis. SlmA–SBS complexes are expected to be cleared from midcell to allow the initiation of Z-ring assembly while the latter third of the chromosome is still being replicated. This agrees well with a previous cell cycle study indicating that the Z ring forms at about the time chromosome replication is nearing completion (21). Incorporating SBSs in the terminus region can apparently delay this, as evidenced by the elongated cell phenotype that this modification induces. Forming the Z ring before the completion of DNA replication is likely advantageous because it would allow time for septal ring maturation so that cell constriction can initiate soon after replication is complete. Additionally, nascent Z rings are known to promote a zonal mode of cell growth at midcell before the initiation of cell constriction (22, 23). Although it is now well established that this is not the main driving force for chromosome segregation as once proposed by Jacob and coworkers (24), zonal growth of the cell envelope between daughter chromosomes during the latter stages of replication may contribute to the completion of the segregation process.
While this report was under review, Schumacher and colleagues published a similar investigation into the mechanism of division regulation by SlmA (25). They identified 52 chromosomal SBSs with a chromosomal distribution and consensus sequence similar to the 24 sites we identified. The difference in the number of sites identified is probably due to the fact that they ectopically expressed a tagged version of SlmA for the ChIP analysis, whereas we detected sites bound by untagged SlmA produced from its native locus.
SBSs and Chromosomal Macrodomain Organization.
Recombination studies and fluorescent marker tracking has defined four insulated and presumably structured chromosomal macrodomains with limited mobility (Ori, Left, Right, and Ter) and two less constrained (nonstructured) regions of greater mobility (NSR and NSL) (26, 27). The origin proximal half of the chromosome consists of the Ori macrodomain (81′–1′) flanked on either side by NSR and NSL (1′–13′ and 62′–81′, respectively). The Ter macrodomain (26′–47′) is flanked by the Right and Left macrodomains (13′–26′ and 47′–62′, respectively). Each of these domains was found to occupy a defined cellular territory as the chromosome is replicated with the NS domains occupying larger territories due to their greater mobility (27). Interestingly, 15/24 SBSs are found in the NS domains, over twice the number located in the similarly sized Ori macrodomain (6/24) (SI Appendix, Table S1). Thus, whereas the dependence of SlmA activity on SBS binding is likely a mechanism for restricting SlmA action to the binding sites, the bias of the SBSs for the more mobile NS domains may allow a limited number of SlmA-binding sites to promote NO throughout a relatively large volume of the nucleoid.
Diversity of DNA-Associated Division Regulators.
SlmA, Noc, and MipZ are all DNA-associated division regulators (3, 6, 8). SlmA and Noc are sequence-specific DNA binding proteins, whereas MipZ forms a DNA-associated complex with ParB that is specifically bound to a cluster of parS sites near the Caulobacter cresentus replication origin. All of these factors block cell division while bound to the origin proximal regions of the chromosome to help couple cytokinesis with chromosome segregation. Given that they share no sequence similarity and belong to three entirely different protein families, the functional parallels between these factors is remarkable. Their diversity suggests that many solutions to the problem of coupling chromosome segregation with cell division and the prevention of chromosomal bisection by the division apparatus exist within the bacterial domain. All that appears necessary is the (covalent or noncovalent) connection of a division regulatory activity with a sequence-specific DNA binding function and the proper distribution of the binding sites.
Mechanism of FtsZ Regulation by SlmA.
Based largely on a structural model of a SlmA–FtsZ complex and EM data, Tonthat et al. (25) recently proposed a mechanism for SlmA action in which it induces the assembly of antiparallel FtsZ protofilament bundles that are unable to productively participate in Z-ring assembly. Our combined genetic and biochemical results argue for a simpler model in which dimers of SlmA antagonize FtsZ polymerization by disrupting FtsZ–FtsZ interactions (Fig. 6). Because SlmA dimers only form in solution at high concentrations (SI Appendix, Fig. S12) and because a similarly high concentration of SlmA is needed to interfere with FtsZ assembly in the absence of SBS-containing DNA, we propose that a dimer (or higher order oligomer) of SlmA is likely to be the active FtsZ antagonist. It therefore follows that SBS-containing DNA is likely activating SlmA, at least in part, by stimulating dimerization (oligomerization). How dimerization/oligomerization might activate SlmA is not clear, but it may do so by creating an FtsZ-interaction surface at the dimer interface. Interestingly, however, even at concentrations where SlmA alone is likely to be a dimer, it only interacted with FtsZ(D212N) polymers in the presence of SBS-containing DNA. This suggests that, in addition to promoting SlmA dimerization, DNA binding may also induce a conformational change in SlmA that stimulates its interaction with FtsZ polymers for their eventual breakdown. The inability of SlmA(R73D) to antagonize FtsZ polymerization or interact with FtsZ(D212N) polymers in vitro, even though it retained the ability to bind SBS DNA, suggests that Arg73 of SlmA is a key residue at the SlmA–FtsZ interface. Because SlmA or SlmA–SBS complexes did not interfere with FtsZ GTPase activity, it is unlikely that SlmA functions by completely blocking the formation of FtsZ protofilaments. Rather, this result suggests that SlmA functions by shortening preformed polymers. Because this activity requires FtsZ GTPase activity, we propose that, similar to MinC (17), SlmA may sever polymers at positions where GTP has been hydrolyzed to GDP. Additionally, the increased GTPase activity observed in the presence of SlmA and SBS DNA may indicate that SlmA–SBS complexes also stimulate the formation of FtsZ(GDP) within polymers to promote their breakdown. Further genetic and structural studies are required to test this possibility and provide us with higher resolution pictures of the SlmA–FtsZ interaction, how DNA-binding influences this interaction, and how SlmA–DNA complexes disrupt FtsZ–FtsZ interactions within polymers.
Materials and Methods
Media, Bacterial Strains, Plasmids, and Microscopy.
As indicated, cells were grown in LB or minimal M9 medium supplemented with 0.2% casamino acids and 0.2% sugar. Bacterial strains and plasmids used in this study are listed in SI Appendix, Tables S3 and S4, respectively (see SI Appendix, SI Text for a detailed description of their construction).
Fluorescence microscopy was performed essentially as described previously (28). See SI Appendix, SI Text for detailed electron microscopy protocols.
Protein Purification, FtsZ GTPase, and FtsZ Sedimentation Assays.
See SI Appendix, SI Text for protein purification protocols. Both the FtsZ and SlmA proteins used in this analysis were untagged. FtsZ GTPase activity was determined by monitoring the hydrolysis of α-[32P]-GTP and FtsZ sedimentation assays were performed essentially as described previously (16) (see SI Appendix, SI Text for detailed protocols).
ChIP Analysis, SBS Identification, qPCR, and EMSA.
The ChIP procedure, ChIP-on-chip analysis, and qPCR analysis was performed as described previously (29), except that high-density oligonucleotide arrays corresponding to the Escherichia coli MG1655 genome were used (Roche NimbleGen) (see SI Appendix, SI Text for detailed protocols). Detailed protocols for EMSA are presented in SI Appendix, SI Text.
Supplementary Material
Acknowledgments
The authors thank members of our laboratories for critical reading of the manuscript and experimental advice. We also thank Amal Rahmeh and Sean Whelan for help with GTPase assays and Bryan McGuffie for help with SI Appendix, Fig. S6. This work was initiated in the laboratory of Piet de Boer and supported in part by National Institutes of Health (NIH) Grant GM57059, as well as by the Massachusetts Life Science Center (T.G.B), the Burroughs Wellcome Fund (T.G.B), and the NIH (AI083365 to T.G.B., and AI069007 and AI057754 to S.L.D.). T.G.B. holds a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018674108/-/DCSupplemental.
References
- 1.Adams DW, Errington J. Bacterial cell division: Assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol. 2009;7:642–653. doi: 10.1038/nrmicro2198. [DOI] [PubMed] [Google Scholar]
- 2.Bi EF, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 3.Thanbichler M, Shapiro L. MipZ, a spatial regulator coordinating chromosome segregation with cell division in Caulobacter. Cell. 2006;126:147–162. doi: 10.1016/j.cell.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 4.de Boer PA, Crossley RE, Rothfield LI. A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum in E. coli. Cell. 1989;56:641–649. doi: 10.1016/0092-8674(89)90586-2. [DOI] [PubMed] [Google Scholar]
- 5.Lutkenhaus J. Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring. Annu Rev Biochem. 2007;76:539–562. doi: 10.1146/annurev.biochem.75.103004.142652. [DOI] [PubMed] [Google Scholar]
- 6.Wu LJ, Errington J. Coordination of cell division and chromosome segregation by a nucleoid occlusion protein in Bacillus subtilis. Cell. 2004;117:915–925. doi: 10.1016/j.cell.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Wu LJ, et al. Noc protein binds to specific DNA sequences to coordinate cell division with chromosome segregation. EMBO J. 2009;28:1940–1952. doi: 10.1038/emboj.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernhardt TG, de Boer PAJ. SlmA, a nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over chromosomes in E. coli. Mol Cell. 2005;18:555–564. doi: 10.1016/j.molcel.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukherjee A, Lutkenhaus J. Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J. 1998;17:462–469. doi: 10.1093/emboj/17.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shabala L, et al. Ion transport and osmotic adjustment in Escherichia coli in response to ionic and non-ionic osmotica. Environ Microbiol. 2009;11:137–148. doi: 10.1111/j.1462-2920.2008.01748.x. [DOI] [PubMed] [Google Scholar]
- 11.Bailey TL, Williams N, Misleh C, Li WW. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006;34:W369–W373. doi: 10.1093/nar/gkl198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Löwe J, Amos LA. Crystal structure of the bacterial cell-division protein FtsZ. Nature. 1998;391:203–206. doi: 10.1038/34472. [DOI] [PubMed] [Google Scholar]
- 13.Löwe J, Amos LA. Tubulin-like protofilaments in Ca2+-induced FtsZ sheets. EMBO J. 1999;18:2364–2371. doi: 10.1093/emboj/18.9.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mingorance J, Rivas G, Vélez M, Gómez-Puertas P, Vicente M. Strong FtsZ is with the force: Mechanisms to constrict bacteria. Trends Microbiol. 2010;18:348–356. doi: 10.1016/j.tim.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Cordell SC, Robinson EJH, Lowe J. Crystal structure of the SOS cell division inhibitor SulA and in complex with FtsZ. Proc Natl Acad Sci USA. 2003;100:7889–7894. doi: 10.1073/pnas.1330742100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee A, Cao C, Lutkenhaus J. Inhibition of FtsZ polymerization by SulA, an inhibitor of septation in Escherichia coli. Proc Natl Acad Sci USA. 1998;95:2885–2890. doi: 10.1073/pnas.95.6.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen B, Lutkenhaus J. Examination of the interaction between FtsZ and MinCN in E. coli suggests how MinC disrupts Z rings. Mol Microbiol. 2010;75:1285–1298. doi: 10.1111/j.1365-2958.2010.07055.x. [DOI] [PubMed] [Google Scholar]
- 18.Scheffers DJ, de Wit JG, den Blaauwen T, Driessen AJ. Substitution of a conserved aspartate allows cation-induced polymerization of FtsZ. FEBS Lett. 2001;494:34–37. doi: 10.1016/s0014-5793(01)02310-9. [DOI] [PubMed] [Google Scholar]
- 19.Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci USA. 1998;95:5752–5756. doi: 10.1073/pnas.95.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reyes-Lamothe R, Wang X, Sherratt D. Escherichia coli and its chromosome. Trends Microbiol. 2008;16:238–245. doi: 10.1016/j.tim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Den Blaauwen T, Buddelmeijer N, Aarsman ME, Hameete CM, Nanninga N. Timing of FtsZ assembly in Escherichia coli. J Bacteriol. 1999;181:5167–5175. doi: 10.1128/jb.181.17.5167-5175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Pedro MA, Quintela JC, Höltje JV, Schwarz H. Murein segregation in Escherichia coli. J Bacteriol. 1997;179:2823–2834. doi: 10.1128/jb.179.9.2823-2834.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aaron M, et al. The tubulin homologue FtsZ contributes to cell elongation by guiding cell wall precursor synthesis in Caulobacter crescentus. Mol Microbiol. 2007;64:938–952. doi: 10.1111/j.1365-2958.2007.05720.x. [DOI] [PubMed] [Google Scholar]
- 24.Jacob F, Brenner S, Cuzin F. On the regulation of DNA replication in bacteria. Cold Spring Harb Symp Quant Biol. 1963;28:329–348. [Google Scholar]
- 25.Tonthat NK, et al. Molecular mechanism by which the nucleoid occlusion factor, SlmA, keeps cytokinesis in check. EMBO J. 2011;30:154–164. doi: 10.1038/emboj.2010.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valens M, Penaud S, Rossignol M, Cornet F, Boccard F. Macrodomain organization of the Escherichia coli chromosome. EMBO J. 2004;23:4330–4341. doi: 10.1038/sj.emboj.7600434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espeli O, Mercier R, Boccard F. DNA dynamics vary according to macrodomain topography in the E. coli chromosome. Mol Microbiol. 2008;68:1418–1427. doi: 10.1111/j.1365-2958.2008.06239.x. [DOI] [PubMed] [Google Scholar]
- 28.Uehara T, Dinh T, Bernhardt TG. LytM-domain factors are required for daughter cell separation and rapid ampicillin-induced lysis in Escherichia coli. J Bacteriol. 2009;191:5094–5107. doi: 10.1128/JB.00505-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castang S, McManus HR, Turner KH, Dove SL. H-NS family members function coordinately in an opportunistic pathogen. Proc Natl Acad Sci USA. 2008;105:18947–18952. doi: 10.1073/pnas.0808215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.