Abstract
The brains of ataxia telangiectasia (AT) patients display an aberrant loss of Purkinje cells (PCs) that is postulated to contribute to the observed deficits in motor coordination as well as in learning and cognitive function. AT patients have mutations in the ataxia telangiectasia mutated (ATM) gene [Savitsky et al. (1995) Science 268:1749–1753]. However, in Atm-deficient mice, the neurological defects are limited, and the PCs are not deformed or lost as observed in AT patients [Barlow et al. (1996) Cell 86:159–171]. Here we report that PC-specific deletion of the mouse males absent on the first (mMof) gene (Cre−), which encodes a protein that specifically acetylates histone H4 at lysine 16 (H4K16ac) and influences ATM function, is critical for PC longevity. Mice deficient for PC-specific Mof display impaired motor coordination, ataxia, a backward-walking phenotype, and a reduced life span. Treatment of MofF/F/Pcp2-Cre+ mice with histone deacetylase inhibitors modestly prolongs PC survival and delays death. Therefore, Mof expression and H4K16 acetylation are essential for PC survival and function, and their absence leads to PC loss and cerebellar dysfunction similar to that observed in AT patients.
Keywords: ataxia telangiectasia phenotype, chromatin modification, DNA damage response
The most striking and obvious neurological manifestation seen in ataxia telangiectasia (AT) patients is a progressive cerebellar ataxia (1). Postmortem examination of brains from AT patients demonstrates significant loss and deformities in Purkinje cells (PCs) (2). Although a cause-and-effect relationship has not been proven, PC loss has a strong correlative association with AT. Mice deficient in ataxia telangiectasia mutated (Atm) display no gross ataxia or neuronal degeneration, but motor function is impaired without detectable PC deformity or loss (3, 4). Oxidative stress in PCs of Atm−/− mice is increased (5), probably resulting in increased DNA damage, the sensing and repair of which is ATM dependent (6–8). The mammalian males absent on the first (MOF) is an H4 histone acetylase that interacts with and influences downstream ATM functions in response to DNA damage (9). Through histone H4 acetylated at lysine 16 (H4K16ac), MOF has a critical role at multiple points in the cellular DNA damage response and repair of DNA double-strand breaks (10). Acetylation of histone H4 at K16 by MOF also is an epigenetic signature of cellular proliferation during both embryogenesis and oncogenesis (11), but whether MOF has a role in postmitotic cells is not known. In this study, we determined that MOF is required for postmitotic PC survival and that loss of MOF and H4K16 acetylation in PCs results in mice displaying neurological abnormalities similar to those encountered in AT patients.
Results and Discussion
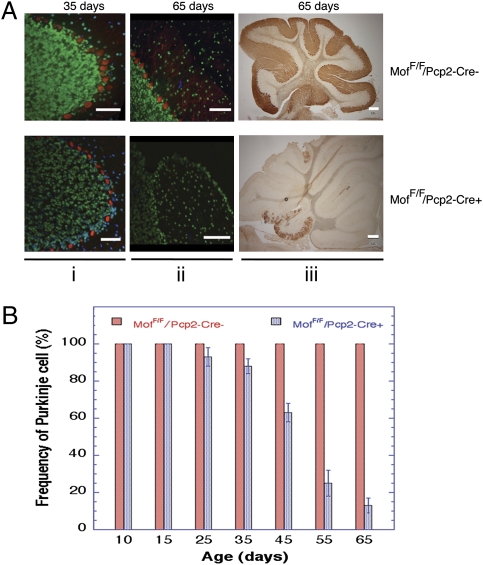
Immunostaining of mouse cerebellum tissue sections confirmed endogenous MOF expression in PCs and the presence of acetylated H4K16 (Fig. S1). To deplete MOF specifically from PCs, mice containing a conditional floxed mMof gene (MofF/F) introduced by homologous recombination were crossed with mice expressing Cre recombinase from the PC-specific Pcp2 gene promoter (12) to obtain MofF/F/Pcp2-Cre− and MofF/F/Pcp2-Cre+ mice. The tissue-specific Pcp2 promoter becomes maximally active 2–3 wk after birth, thus circumventing the embryonic-lethal effect of homozygous mMof deletion (11). In cerebellum collected from MofF/F/Pcp2-Cre− and MofF/F/Pcp2-Cre+ mice at various times (10–65 d) after birth, MOF expression was easily detected in tissue from MofF/F/Pcp2-Cre− or MofF/F/Pcp2-Cre+ mouse PCs at postnatal day 15, but expression was lost by postnatal day 25 in tissue from MofF/F/Pcp2-Cre+ mice. As expected, MOF expression in PC-adjacent cell types was unaltered during the 65-d period examined (Fig. S2). Similarly, H4K16ac was easily detected in PCs from MofF/F/Pcp2-Cre+ mice at postnatal day 15, but expression was gradually lost by postnatal day 35 (Fig. S2). Calbindin (a PC-specific cell marker) histochemical staining of cerebellum from age-matched MofF/F/Pcp2-Cre− and MofF/F/Pcp2-Cre+ mice indicated that detectable PC loss in MofF/F/Pcp2-Cre+ mice was first evident by postnatal day 25, increased to 40% of PCs by postnatal day 45, and finally reached 80% loss by postnatal day 65 (Fig. 1 and Figs. S3 and S4). A significant decrease (P < 0.001; Student's t test) in the number of PCs in MofF/F/Pcp2-Cre+ mice was observed from about postnatal day 35 onwards, but there was no corresponding decrease in PC numbers in MofF/F/Pcp2-Cre− mouse brains (Fig. 1). Along with the loss of PC observed in brains MofF/F/Pcp2-Cre+ mice, granular cells were visibly reduced (Fig. 1Ai and ii). Numerous PCs in tissue from MofF/F/Pcp2-Cre+ mice contained pyknotic nuclei, perhaps reflecting an altered chromatin state caused by decreasing H4K16ac (Fig. S2), a chromatin modification essential for protein–protein interactions (13). After the initial appearance of pyknotic-like PCs in tissue from MofF/F/Pcp2-Cre+ mice, abnormal, coarse PC dendrites were detected with calbindin staining (Fig. 2 and Fig. S4).
Fig. 1.
PCs in cerebellum of MofF/F/Pcp2-Cre− and MofF/F/Pcp2-Cre+ mice. (A) Calbindin staining of cerebellum. (i and ii) Immunofluorescence staining for calbindin (red) and H4k16ac (green) of ventricle IV from cerebellum of MofF/F/Pcp2-Cre− and MofF/F/Pcp2-Cre+ mice at postnatal days 35 (i) and 65 (ii). Loss of PCs is quite prominent in 65-d-old MofF/F/Pcp2-Cre+ mice. (iii) PC identification by calbindin detection by HRP staining. Cerebellums of 65-d-old MofF/F/Pcp2-Cre+ mice show widespread loss of PCs and PC dendritic calbindin activity with some residual PC in the 10th lobe. (B) PC density at various ages, demonstrating progressive PC loss in MofF/F/Pcp2-Cre+ mice.
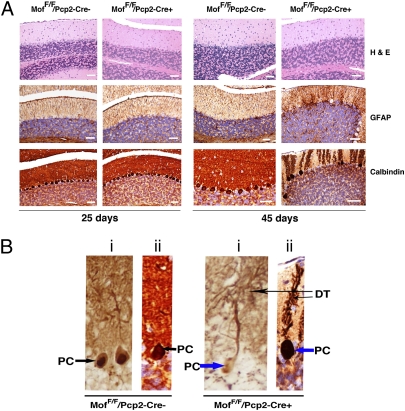
Fig. 2.
Histologically stained sagittal sections of cerebellum from MofF/F/Pcp2-Cre+ and MofF/F/Pcp2-Cre− mice. (A) (Top) H&E staining reveals minimal pyknotic PCs in 25-d-old MofF/F/Pcp2-Cre+ mice, but by postnatal day 45 the frequency of degenerating PCs with pyknotic nuclei and eosinophilic cytoplasm increases substantially. (Middle) GFAP staining reveals that levels are similar in cerebellum of 25-d-old MofF/F/Pcp2-Cre+ and MofF/F/Pcp2-Cre+ mice; however, GFAP levels are increased only in cerebellum of 45-d-old MofF/F/Pcp2-Cre+ mice, most conspicuously in areas of PC loss. (Bottom) Calbindin staining shows similar frequency of PC cells in cerebellum of 25-d-old MofF/F/Pcp2-Cre+ and MofF/F/Pcp2-Cre+ mice; however, PC degeneration along with dendrite loss is quite visible in cerebellum of 45-d-old MofF/F/Pcp2-Cre+ mice. (B) Comparison of calbindin-stained PC from MofF/F/Pcp2-Cre− mice (Left) and MofF/F/Pcp2-Cre+ mice (Right) at higher magnification. DT, dendrite. (i) Twenty-five days. (ii) Forty-five days.
The effect of PC-specific MOF loss on cerebellar cortical cytoarchitecture was analyzed further by H&E staining of cerebellum sections. No differences in the granular, PC or molecular layers were observed in tissue from 15-d-old MofF/F/Pcp2-Cre+ or MofF/F/Pcp2-Cre− mice. However, by postnatal day 25, occasional pyknotic PC nuclei became apparent in tissue from MofF/F/Pcp2-Cre+ mice (Fig. 2 and Fig. S5). Most strikingly, by postnatal day 45 there was a substantial increase in the numbers of pyknotic PCs and degenerating PCs, accompanied by areas of frank PC loss and modestly increased numbers of Bergmann glia at the granule cell–molecular layer interface in tissue from MofF/F/Pcp2-Cre+ mice (Fig. 2 and Figs. S6 and S7).
Cerebellum GFAP is necessary for the integrity of central nervous system white-matter architecture and long-term maintenance of myelination (14). GFAP levels in immunostained sections of cerebellum from 15-d-old MofF/F/Pcp2-Cre+ and MofF/F/Pcp2-Cre− mice were similar. However, at postnatal day 25 the intensity of GFAP staining was higher in tissue from MofF/F/Pcp2-Cre+ mice than in tissue from MofF/F/Pcp2-Cre− mice (Fig. 2 and Fig. S8), and this intensity increased further by day 45 (Fig. 2 and Fig. S9). The increased GFAP expression observed is compatible with the Bergmann gliosis noted in areas of PC loss.
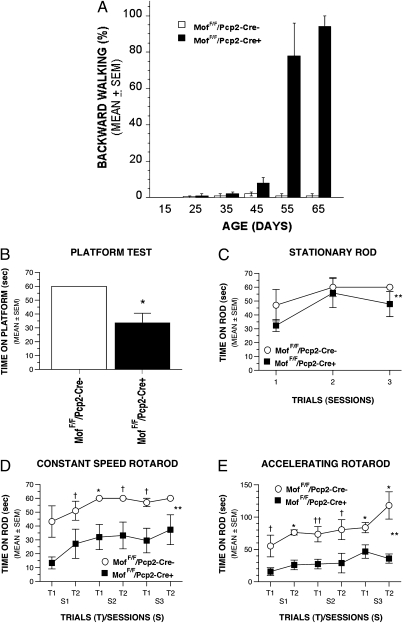
PCs send inhibitory projections to the deep cerebellar and vestibular nuclei, which are the only source of output from the cerebellar cortex (15). The most obvious clinical manifestation seen in AT patients is progressive cerebellar ataxia (1) attributable to the profound loss of PCs seen in postmortem brains (2). Therefore, we investigated whether MOF depletion in mouse PCs results in abnormal motor coordination. We observed that, beginning around postnatal day 35, MofF/F/Pcp2-Cre+ mice are much less active than MofF/F/Pcp2-Cre− mice (Movie S1) and display very slow forward walking (Movie S2). More interestingly, by postnatal day 45 more than 65% of MofF/F/Pcp2-Cre+ mice display the curious phenotype of backward walking (Fig. 3A and Movie S2), and there is a direct correlation between the progression of PC loss and initiation of backward walking. By postnatal day 55, MofF/F/Pcp2-Cre+ mice exhibit only backward walking in open field until they die (Fig. 4A and Movies S3 and S4). A prominent visible ataxia of rapid head shaking was observed only in MofF/F/Pcp2-Cre+ mice after postnatal day 45 (Movie S5), a phenotype that has not been observed in Atm-null mice (3, 4).
Fig. 3.
Behavioral studies of MofF/F/Pcp2-Cre− and MofF/F/Pcp2-Cre+ mice. (A) MofF/F/Pcp2-Cre+ mice showed prominent backward walking as shown in SI movies. Significant backward walking of MofF/F/Pcp2-Cre+ mice was observed after postnatal day 45. (B) Results from the elevated platform test showed that MofF/F/Pcp2-Cre+ mice were able to remain on a small, circular, elevated platform for a significantly shorter time than MofF/F/Pcp2-Cre− mice (*P = 0.007), suggesting impaired balance in MofF/F/Pcp2-Cre+ mice. (C–E) Results from the Rotarod test suggest that the MofF/F/Pcp2-Cre+ mice had mild balance deficits and severe impairments in fine-motor coordination. (C) On average across the test sessions, MofF/F/Pcp2-Cre+ mice spent significantly less time than MofF/F/Pcp2-Cre− mice on the stationary rod of the Rotarod test (**P = 0.042). (D) On the constant-speed Rotarod, MofF/F/Pcp2-Cre+ mice spent a significantly shorter amount of time than MofF/F/Pcp2-Cre− mice on the rotating rod (**P = 0.005) across the test sessions. Differences were significantly different between groups for session 2 of trial 1 (*P = 0.008), and large differences also were observed for session 1 of trial 2, session 2 of trial 2, and session 3 of trial 1 (†P < 0.049). (E) MofF/F/Pcp2-Cre+ mice spent a significantly shorter period than MofF/F/Pcp2-Cre− mice on the accelerating rotating rod (**P = 0.0005) across the test sessions. Pairwise comparisons showed significant differences between groups in session 1 of trial 2, and in session 3 of both trials (*P < 0.003); large differences also were also observed in session 2 of trial 1 (††P = 0.009) and in the other remaining trials (†P < 0.035).
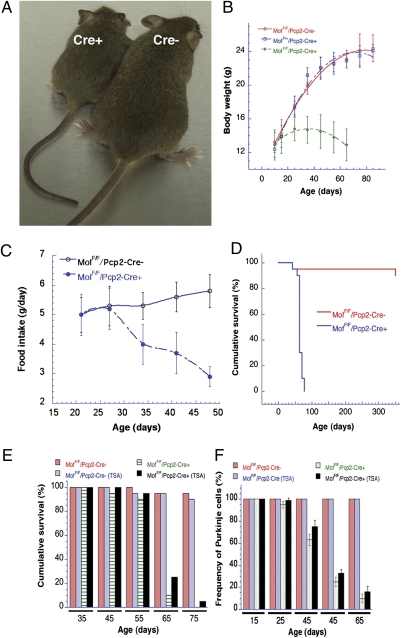
Fig. 4.
Effect of MOF and HDACi treatment on body weight, survival, and PC numbers. (A) MofF/F/Pcp2-Cre+ (Left) and MofF/F/Pcp2-Cre− (Right) mice at postnatal day 45. (B) Body weight of MofF/F/Pcp2-Cre+ mice decreased after postnatal day 30. (C) Comparison of food intake by MofF/F/Pcp2-Cre+ and MofF/F/Pcp2-Cre− mice. (D) Survival of MofF/F/Pcp2-Cre+ mice was significantly decreased relative to MofF/+/Pcp2-Cre− mice. Mice with PC-specific knockout of the mMof gene (MofF/F/Pcp2-Cre+) die at 61 ± 12 d after birth, whereas MofF/F/Pcp2-Cre− mice remain healthy. (E) TSA has a positive effect on the cumulative survival of MofF/F/Pcp2-Cre+ mice but not MofF/F/Pcp2-Cre− mice. (F) TSA affects the frequency of PCs in MofF/F/Pcp2-Cre+ mice but not in MofF/F/Pcp2-Cre− mice.
Results from sensorimotor testing indicated that MofF/F/Pcp2-Cre+ mice were impaired on the elevated platform test relative to MofF/F/Pcp2-Cre− controls (Fig. 3B), as evidenced by the MofF/F/Pcp2-Cre+ mice spending significantly less time on the platform [F(1,12) = 10.48; P = 0.007]. However, the groups did not differ in other measures within the battery (e.g., ledge, pole, or 90° inclined or inverted screen), suggesting that the impaired performance on the platform test represented a relatively specific deficit, possibly involving disturbed balance. These tests were performed at the age of 6–9 wk, when >40% of PCs were lost in MofF/F/Pcp2-Cre+ mice.
Rotarod testing indicated that the MofF/F/Pcp2-Cre+ mice also were impaired on tasks that required fine-motor coordination, with deficits becoming more apparent with increasing task difficulty. For example, the MofF/F/Pcp2-Cre+ mice exhibited mild performance deficits in their ability to remain on the stationary rod (Fig. 3C) [main effect of Genotype (PC-specific mMof knockout in mice vs. control): F(1,12) = 5.21; P = 0.042], whereas their performance on the constant speed and accelerating Rotarod components was impaired (Fig. 3 D and E). Specifically, an analysis of the constant speed Rotarod data revealed that, on average across test sessions, the MofF/F/Pcp2-Cre+ mice remained on the rotating rod for a significantly shorter time than the MofF/F/Pcp2-Cre− mice, although performance generally improved across test sessions [significant effects of Genotype, F(1,12) = 11.98, P = 0.005, and Test Session, F(2,24) = 4.73, P = 0.022]. Even greater performance impairments were observed in the ability of MofF/F/Pcp2-Cre+ mice to stay on the accelerating Rotarod [significant effects of Genotype, F(1,12) = 22.63, P = 0.0005, and Test Session, F(2,24) = 11.60, P = 0.0003]. In summary, the Rotarod data suggest that MofF/F/Pcp2-Cre+ mice are impaired on tasks requiring fine-motor coordination (constant speed Rotarod and accelerating Rotarod tests) (Fig. 3 D and E). These behavioral analyses indicate that MofF/F/Pcp2-Cre+ mice have a behavioral phenotype of severe impairment in fine-motor coordination and possibly mild balance disturbances. These results are consistent with previous findings using the same tests to characterize functional deficits in other strains of mutant mice that have synaptic defects in cerebellar PCs (16), mice that have nonreceptor Abl/Arg tyrosine kinase-related cerebellum defects (17), and Atm-deficient mice (3).
The physiological effect of PC loss induced by MOF depletion was readily visible (Fig. 4). Although the total body weight of MofF/F/Pcp2-Cre+ and MofF/F/Pcp2-Cre− mice was similar at postnatal day 15, the growth of MofF/F/Pcp2-Cre+ mice was significantly reduced subsequently, with maximal total body weight reached 25–45 d after birth that was <50% of the weight of age-matched MofF/F/Pcp2-Cre− mice. After age 45 d, the body weight of MofF/F/Pcp2-Cre+ mice began to decline (Fig. 4 A and B). The loss of body weight in MofF/F/Pcp2-Cre+ mice correlated with a decrease in food intake compared with MofF/F/Pcp2-Cre− mice (Fig. 4C). Overall survival was dramatically decreased in MofF/F/Pcp2-Cre+ mice, with the majority dying within 55–65 d after birth and none surviving longer than 75 d (Fig. 4D).
The H4K16ac modification poses a structural constraint on the formation of higher-order chromatin (13), perhaps inducing an open chromatin configuration that is more readily accessible to transcription as well as DNA repair. Acetylation of histone H4 at K16 also is critical for protein–protein interactions (18), and the only mammalian protein known to perform this function is MOF (9, 10, 19, 20), Recently, we reported that cells treated with trichostatin A (TSA) had increased levels of H4K16ac but unaltered MOF protein levels (10). We therefore investigated whether inhibition of H4K16ac de-acetylation with two different histone deacetylase inhibitors (HDACi), TSA and EX-527 (21), would improve survival by delaying PC loss in MofF/F/Pcp2-Cre+ mice. Short-term drug treatment of MofF/F/Pcp2-Cre+ mice initiated 30–35 d after birth delayed mouse death (Fig. 4E). A further delay in initiating treatment to postnatal day 55 failed to extend lifespan (Fig. 4 and Fig. S10). If drug treatment began at postnatal day 35, a slight, but still significant, increase in PC survival was observed in MofF/F/Pcp2-Cre+ mice, and such mice also had a 10–12% increase in lifespan.
There was no difference in the number of PCs in MofF/F/Pcp2-Cre+ or MofF/F/Pcp2-Cre− mice after treatment with TSA during postnatal days 10–15. This lack of effect probably reflects the period before cellular MOF levels began decreasing because of Cre recombinase expression. However, when mice were treated with TSA beginning on postnatal day 20, PC loss was significantly delayed in MofF/F/Pcp2-Cre+ mice as compared with MofF/F/Pcp2-Cre− mice (Fig. 4F). The delay in loss of PCs in MofF/F/Pcp2-Cre+ mice was most prominent when mice were treated during postnatal days 25–45. However, TSA had a minimal effect on MofF/F/Pcp2-Cre+ mice when treatment was initiated at postnatal day 45. We tested another HDACi, EX-527, which also is known to increase H4K16ac levels (21, 22). MofF/F/Pcp2-Cre+ mice administered EX-527 also displayed a delay in PC loss (Fig. S10). HDACi treatment in MofF/F/Pcp2-Cre+ mice not only delayed the loss of PCs in MofF/F/Pcp2-Cre+ mice but also increased lifespan by about 12% (Fig. 4 and Fig. S10). Because in vitro treatment with TSA as well as EX-527 is known to increase H4K16ac levels (10, 21, 22), the present studies support the argument that H4K16ac has a critical role in maintaining PC functions.
We report here that PC-specific deletion of the Mof gene in mice produces neurological abnormalities attributable to cerebellar dysfunction that are similar to those seen in AT patients, including impaired fine-motor coordination, balance deficits, ataxia, and a most unusual backward-walking phenotype. Examination of the corresponding mouse cerebellum indicates PC depletion, which is also observed postmortem in AT patient brains. Taken together, these results suggest that the PC-specific Mof-deletion mouse mimics the physiological developments seen in AT patients. MOF also interacts with and influences downstream ATM functions in response to DNA damage (9, 10, 23), suggesting defective communication along the MOF/ATM pathway in PCs. Analysis of this pathway under conditions of oxidative stress, as occurs in the absence of ATM, may offer explanations as to the development of other AT-related symptoms. In addition, these mice provide a valuable means for understanding the role of chromatin-modifying factors, such as MOF, in the function and survival of nonproliferating postmitotic cells, especially neuronal cells. Further advances will require the development of specific deacetylation inhibitors, such as ones targeting the MOF modification product histone H4K16ac, which could facilitate future treatment possibilities.
Materials and Methods
Generation of PC-Specific mMof-Deficient Mice.
Targeting vectors for the mMof locus were constructed to generate an in vivo deletion of the mMof gene in mice. The details of generating a conditional mMof allele by inserting a single loxP site together with a flippase recombination target (FRT)-flanked hygromycin resistance gene cassette 1.5 kb upstream of the mMof transcription initiation site and a second loxP site within intron 3 were described recently (11). The construct was electroporated into W9.5 ES cells to generate Mofflox/+ cells. Details for generation of Mofflox/+/Rosa26creERT2/+ and Mof Dflox/flox/Rosa26creERT2/+ ES cell clones have been described (11).
To generate Purkinje cell-specific Mof-deficient mice using the Cre/loxP recombination system, Mofflox/flox mice, which contained two loxP sites flanking exon 3 of the Mof gene, were crossed with pcp2-Cre transgenic mice [B6.129-Tg(Pcp2-cre)2Mpin/J] (Jackson Laboratory) expressing Cre recombinase under the control of a L7/Pcp2 promoter (24). All procedures relating to the care and treatment of the animals were performed in accordance with the National Institutes of Health guidelines at Washington University School of Medicine, St. Louis, Columbia University, New York, and University of Texas Southwestern Medical Center, Dallas.
PCR Analysis of Genomic DNA.
Genomic DNA was prepared from mouse tail and several brain regions, including the olfactory bulb, cortex, and cerebellum, using the HotSHOT method (25). PCR was performed using a RoboCycler (Stratagene) with Taq DNA polymerase (QiaTaq; Qiagen). The procedures for primer selection and conditions used for PCR genotyping were as described previously (11).
Histology and Immunohistochemistry.
We analyzed the status of cerebellum PCs using fresh-frozen sections as well as paraffin-embedded sections. Mice were perfused with 4% paraformaldehyde, and dissected brains were perfusion-fixed for 36 h before paraffin embedding.
Fixation and Sectioning.
After harvest, mouse brains were removed and perfusion-fixed with 4% paraformaldehyde. After fixation, hindbrains (brainstem and cerebellum) were divided into four sections in the sagittal plane. Tissues were dehydrated in xylene, embedded in paraffin, and sectioned on a rotary microtome at 3-mm thicknesses. For routine histology, paraffin sections were rehydrated, stained with H&E, and evaluated by light microscopy.
Immunohistochemistry.
Immunostaining was performed at room temperature on a BenchMarkXT automated immunostainer, using the UltraVIEW system with HRP and diaminobenzidine (DAB) chromogen (Ventana Medical Systems). Optimum primary antibody dilutions were predetermined using known positive-control tissues. Paraffin sections were cut at 3 μm on a rotary microtome, mounted on positively charged glass slides, and air-dried overnight. Sections then were placed onto the BenchMarkXT where the deparaffinization and heat retrieval were performed. Sections then were incubated for 1 h with primary mouse monoclonal antibody to calbindin (1:50 dilution; Novocastra/Leica) or rabbit polyclonal antibody to glial fibrillary acidic protein (1:400 dilution; Biocare) diluted in ChemMate buffer (Ventana Medical Systems) or with buffer alone as a negative reagent control. After washing in buffer, sections were incubated with a freshly prepared mixture of DAB and H2O2 in buffer for 8 min, followed by washing in buffer and then in water. Sections were counterstained with hematoxylin, dehydrated in a graded series of ethanols and xylene, and coverslipped. Slides were reviewed by light microscopy. Positive reactions with DAB were identified as dark brown reaction product.
PC Counting.
The number of PCs was quantified in midsagittal, 100-mm-thick cerebellar sections prepared from age-matched MofF/F/Pcp2-Cre− and Mof F/F/Pcp2-Cre+ mice at different ages (n = 4 for each age group). Optical images of PCs immunostained with anti-calbindin antibody were taken using 2×, 10×, and 20× objective lenses.
Reagents.
TSA purchased from Sigma was dissolved in DMSO and administered in vivo at 1 mg·kg of body weight−1·d−1 for 10 d. EX527 was purchased from Tocris and dissolved in 4% DMSO/10% cyclodextrin in PBS and administered in vivo at 1 mg·kg of body weight−1·d−1 for 10 d.
Quantitation of Food Intake.
Standard procedures (26) for quantitating mouse food intake were used. In brief, mice were acclimatized to individual cages for 1 wk. Food intake was measured every week. The body weight of individual mice was measured before and after each test series. Chow intake was measured by weighing the cage tops containing food pellets. Cage bottoms were covered with polyvinyl sheets when the cage tops were weighed, and any food spilled on sheets was collected and weighed. Food intake was corrected for spillage.
Behavioral Studies.
To provide an initial characterization of the functional deficits in MofF/F/Pcp2-Cre+ mice, they and littermate MofF/F/Pcp2-Cre− controls were evaluated on a battery of sensorimotor measures and the Rotarod test at 6–9 wk of age. These behavioral analyses have been used previously to assess ataxias, including ataxias of cerebellar origin, in several different mutant mouse strains (16, 27, 28), and these methodologies are described briefly in SI Materials and Methods. All behavioral tests were conducted by individuals who were unaware of the genotypic status of the mice (SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank David Woznaik, Sara Converys, Peter McKinnon, Mark Goldberg, and Ann Stowe for help with animal behavior studies, suggestions, and comments. We thank members of the T.K.P. laboratory for helpful discussions and suggestions. We also thank Swati Pandita for editing movies and graphic work. This work was supported by National Institutes of Health/National Cancer Institute Grants R01CA123232, R01CA129537, RO1CA154320, and R13CA130756.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016524108/-/DCSupplemental.
References
- 1.Sedgwick RP, Boder E. Progressive ataxia in childhood with particular reference to ataxia-telangiectasia. Neurology. 1960;10:705–715. doi: 10.1212/wnl.10.7.705. [DOI] [PubMed] [Google Scholar]
- 2.Boder E, Sedgwick RP. Ataxia-telangiectasia; a familial syndrome of progressive cerebellar ataxia, oculocutaneous telangiectasia and frequent pulmonary infection. Pediatrics. 1958;21:526–554. [PubMed] [Google Scholar]
- 3.Barlow C, et al. Atm-deficient mice: A paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 4.Barlow C, et al. ATM is a cytoplasmic protein in mouse brain required to prevent lysosomal accumulation. Proc Natl Acad Sci USA. 2000;97:871–876. doi: 10.1073/pnas.97.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Sloan EA, Boerkoel CF. Chromatin remodeling and human disease. Curr Opin Genet Dev. 2003;13:246–252. doi: 10.1016/s0959-437x(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 6.Lavin MF. Ataxia-telangiectasia: From a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 7.Pandita TK, Richardson C. Chromatin remodeling finds its place in the DNA double-strand break response. Nucleic Acids Res. 2009;37:1363–1377. doi: 10.1093/nar/gkn1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandita TK. A multifaceted role for ATM in genome maintenance. Expert Rev Mol Med. 2003;5:1–21. doi: 10.1017/S1462399403006318. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, et al. Involvement of human MOF in ATM function. Mol Cell Biol. 2005;25:5292–5305. doi: 10.1128/MCB.25.12.5292-5305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma GG, et al. MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and double-strand break repair. Mol Cell Biol. 2010;30:3582–3595. doi: 10.1128/MCB.01476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A, et al. The mammalian ortholog of Drosophila MOF that acetylates histone H4 lysine 16 is essential for embryogenesis and oncogenesis. Mol Cell Biol. 2008;28:397–409. doi: 10.1128/MCB.01045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang XM, et al. Highly restricted expression of Cre recombinase in cerebellar Purkinje cells. Genesis. 2004;40:45–51. doi: 10.1002/gene.20062. [DOI] [PubMed] [Google Scholar]
- 13.Shogren-Knaak M, et al. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311:844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 14.Gerbert J, Burns S, Liedtke LA. Anesthesia blocks of the lower extremity. Comparing the Biojector with needle and syringe. J Am Podiatr Med Assoc. 1996;86:195–204. doi: 10.7547/87507315-86-5-195. [DOI] [PubMed] [Google Scholar]
- 15.Ito M, Yoshida M, Obata K, Kawai N, Udo M. Inhibitory control of intracerebellar nuclei by the Purkinje cell axons. Exp Brain Res. 1970;10:64–80. doi: 10.1007/BF00340519. [DOI] [PubMed] [Google Scholar]
- 16.Grady RM, Wozniak DF, Ohlemiller KK, Sanes JR. Cerebellar synaptic defects and abnormal motor behavior in mice lacking alpha- and beta-dystrobrevin. J Neurosci. 2006;26:2841–2851. doi: 10.1523/JNEUROSCI.4823-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu Z, Cang Y, Goff SP. Abl family tyrosine kinases are essential for basement membrane integrity and cortical lamination in the cerebellum. J Neurosci. 2010;30:14430–14439. doi: 10.1523/JNEUROSCI.2861-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neal KC, Pannuti A, Smith ER, Lucchesi JC. A new human member of the MYST family of histone acetyl transferases with high sequence similarity to Drosophila MOF. Biochim Biophys Acta. 2000;1490:170–174. doi: 10.1016/s0167-4781(99)00211-0. [DOI] [PubMed] [Google Scholar]
- 19.Smith ER, et al. A human protein complex homologous to the Drosophila MSL complex is responsible for the majority of histone H4 acetylation at lysine 16. Mol Cell Biol. 2005;25:9175–9188. doi: 10.1128/MCB.25.21.9175-9188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taipale M, et al. hMOF histone acetyltransferase is required for histone H4 lysine 16 acetylation in mammalian cells. Mol Cell Biol. 2005;25:6798–6810. doi: 10.1128/MCB.25.15.6798-6810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomon JM, et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol. 2006;26:28–38. doi: 10.1128/MCB.26.1.28-38.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajji N, et al. Opposing effects of hMOF and SIRT1 on H4K16 acetylation and the sensitivity to the topoisomerase II inhibitor etoposide. Oncogene. 2010;29:2192–2204. doi: 10.1038/onc.2009.505. [DOI] [PubMed] [Google Scholar]
- 23.Li X, et al. MOF and H4 K16 acetylation play important roles in DNA damage repair by modulating recruitment of DNA damage repair protein Mdc1. Mol Cell Biol. 2010;30:5335–5347. doi: 10.1128/MCB.00350-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000;28:93–98. [PubMed] [Google Scholar]
- 25.Truett GE, et al. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and Tris (HotSHOT) Biotechniques. 2000;29:52, 54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- 26.Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Nutrient preference and diet-induced adiposity in C57BL/6ByJ and 129P3/J mice. Physiol Behav. 2001;72:603–613. doi: 10.1016/s0031-9384(01)00412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, et al. Ataxia and paroxysmal dyskinesia in mice lacking axonally transported FGF14. Neuron. 2002;35:25–38. doi: 10.1016/s0896-6273(02)00744-4. [DOI] [PubMed] [Google Scholar]
- 28.Griffey MA, et al. CNS-directed AAV2-mediated gene therapy ameliorates functional deficits in a murine model of infantile neuronal ceroid lipofuscinosis. Mol Ther. 2006;13:538–547. doi: 10.1016/j.ymthe.2005.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.