Abstract
TAp63, but not its homolog p53, eliminates oocytes that suffered DNA damage. An equivalent gene for guarding the male germ line is currently not known. Here we identify hitherto unknown human p63 transcripts with unique 5′-ends derived from incorporated exons upstream of the currently mapped TP63 gene. These unique p63 transcripts are highly and specifically expressed in testis. Their most upstream region corresponds to a LTR of the human endogenous retrovirus 9 (ERV9). The insertion of this LTR upstream of the TP63 locus occurred only recently in evolution and is unique to humans and great apes (Hominidae). A corresponding p63 protein is the sole p63 species in healthy human testis, and is strongly expressed in spermatogenic precursors but not in mature spermatozoa. In response to DNA damage, this human male germ-cell–encoded TAp63 protein (designated GTAp63) is activated by caspase cleavage near its carboxyterminal domain and induces apoptosis. Human testicular cancer tissues and cell lines largely lost p63 expression. However, pharmacological inhibition of histone deacetylases completely restores p63 expression in testicular cancer cells (>3,000-fold increase). Our data support a model whereby testis-specific GTAp63 protects the genomic integrity of the male germ line and acts as a tumor suppressor. In Hominidae, this guardian function was greatly enhanced by integration of an endogenous retrovirus upstream of the TP63 locus that occurred 15 million years ago. By providing increased germ-line stability, this event may have contributed to the evolution of hominids and enabled their long reproductive periods.
Keywords: spermatogonia, genomic surveillance, primate evolution, seminoma, suberoylanilide hydroxamic acid
Maintaining genomic integrity in the germ line is a fundamental prerequisite for the evolutionary stability of a species. To achieve this, germ cells with damaged DNA need to be efficiently eliminated. This elimination is particularly important when an individual's fertility is maintained over several decades, as is the case in humans and great apes (Hominidae). In these species, the already long time frame of fertility in females is even more extended in males. However, only very limited knowledge exists on how the genomic integrity of the male germ line is controlled and preserved in humans (1).
p63 is a homolog of the tumor suppressor p53 (2, 3). In somatic cells, p53 is the quintessential mediator of apoptosis in response to DNA damage, thus acting as the guardian of the genome (4). Tumor suppressor p53 binds and transcriptionally activates multiple proapoptotic genes (5). Moreover, p53 directly associates with mitochondria, and by interacting with anti- and proapoptotic members of the Bcl2 family of outer membrane permeability regulators, triggers the release of cytochrome c, thus jumpstarting apoptosis (6, 7). A similar mitochondrial association was observed for the third p53 family member, p73 (8), but so far not for p63.
Targeted deletion of the TP63 gene results in severe developmental and morphogenic defects of multilayered epithelia (e.g., skin) and limb buds (9). In these somatic tissues, the predominantly expressed p63 isoforms are the aminoterminally truncated ΔNp63 proteins, resulting from the use of an alternate internal promoter. On the other hand, in the female germ line high levels of full-length TAp63 isoforms are expressed in the nuclei of primordial and primary oocytes (10). In contrast to oocytes from wild-type and p53-null mice, primordial and primary oocytes of p63 knockout mice are highly resistant toward ionizing irradiation-mediated apoptosis. This finding indicates that TAp63 acts as a guardian of the female germ line by eliminating oocytes with damaged DNA (10). Of note, however, although TAp63 mRNA had also been identified in murine testis (11–13), no evidence could be obtained for a role of the p63 protein in the apoptosis of murine male germ cells upon irradiation (10). It was therefore concluded that germ-line expression of TAp63 is biased toward the female, at least in rodents (10).
Endogenous retroviruses (ERVs) have spread through mammalian genomes throughout evolution, resulting in thousands of copies or fragments of ERVs, encompassing an estimated 7% of the human genome (14, 15). However, despite their abundance, specific functions could only rarely be assigned to ERVs. The LTRs of such viruses can serve as strong promoters/enhancers, boosting the transcription of viral or adjacent cellular genes. In a recent discovery of a pathologic example in humans, the derepression of an LTR was found to induce the driver proto-oncogene CSF1R in Hodgkin's lymphoma (16). The promoter activity of LTRs can display various tissue specificities and is sometimes limited to germ cells. For example, an LTR from the human endogenous retrovirus 9 (ERV9) predominantly transcribes in testis (17).
We show here that unique isoforms of p63 are highly and specifically expressed in human testis. This expression is the result of an upstream insertion of an ERV9 LTR with strong promoter activity that occurred 10 to 15 million years ago during primate evolution at the branching point to long-lived Hominidae. Upon DNA damage, the resulting germ cell-associated, transcriptionally active p63 (GTAp63) suppresses proliferation and induces apoptosis. Conversely, GTAp63 expression is frequently lost in human testicular cancers.
Results
Unique Isoforms of p63 Are Highly Expressed in Human Testis.
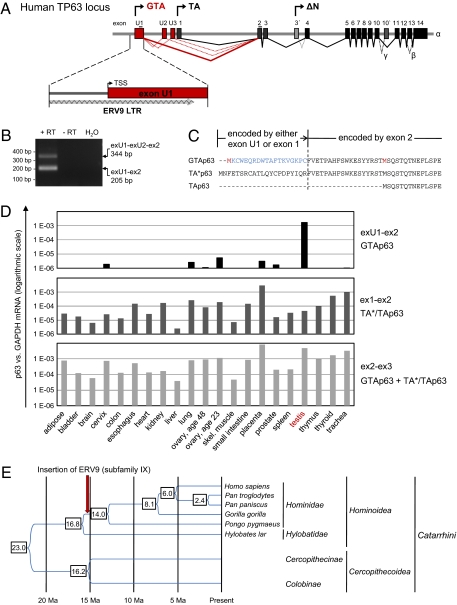
We performed a rapid amplification of cDNA ends (5′-RACE) to amplify endogenous mRNA species upstream of exon 2. Multiple unique p63 isoforms were found (scheme in Fig. 1A; sequence data and RACE primers in Fig. S1A and Table S1). They are characterized by three unique exons located upstream of the previously described exon 1 of the human TP63 gene and are designated exon U1, U2, and U3. PremRNA splicing fuses one or more of these exons directly to the previously described exon 2, thus omitting exon 1. The unique p63 isoforms are readily detectable in human testicular tissues, and the direct fusion of exon U1 with exon 2 constitutes by far the predominant splice product (Fig. 1B). We refer to these mRNAs and their corresponding p63 protein isoforms as GTAp63, for germ-cell–encoded transactivating p63. The main predicted GTAp63 protein sequence (exU1 to ex2, used from here on) differs from the two previously described TAp63 proteins (named TA* and TA, here both termed “conventional TAp63”) by a novel 19-residue long amino terminus (Fig. 1C). Mutation scanning of the two possible candidate upstream ATG codons within the GTAp63 mRNA revealed that the first ATG is the predominantly used initiation codon (Fig. S1B). Of note, GTAp63 transcripts were expressed at fivefold higher levels than conventional TAp63 when tested under identical conditions from the same vector backbone in H1299 human lung carcinoma cells (Fig. S1C), also yielding more abundant protein (Fig. S1B; compare lanes 2 and 5). On the other hand, protein stabilities were comparable in cycloheximide chase experiments (Fig. S1D). The main GTAp63 transcript was highly and specifically expressed in testis; conversely, in most somatic tissues, GTAp63 was completely undetectable, although in a few others it was only expressed at 500- to 1,000-fold lower levels compared with testis (Fig. 1D). In contrast, expression of conventional TAp63 transcripts (containing exons 1 and 2) in testis was over 10-fold lower compared with GTAp63. In addition, conventional TAp63 showed a broad distribution among all tissues (Fig. 1D). Therefore, transcripts containing exons 2 and 3 reflecting the sum of conventional TAp63 and GTAp63 were found in all tissues, although even this combined mRNA level was highest in testis (besides placenta) (Fig. 1D). Some GTAp63 was also seen in ovaries from young but not older females, possibly originating from oocytes (Fig. 1D and Fig. S1E).
Fig. 1.
Testis-specific p63 isoforms are encoded by unique upstream exons, as a result of recent retroviral LTR insertion. (A) Genomic architecture of the human TP63 gene, including all known (black and gray) and previously unexplored (red) exons. The recently identified upstream exons U1, U2, and U3 encode GTAp63. PremRNA splicing fuses these exons directly to the previously described exon 2, thus omitting exon 1. Predominant splicing is indicated by solid lines, less abundant splicing patterns by dotted lines. The three start sites of transcription (GTA, TA, and ΔN) are indicated with arrows above their first exons. Alternative splicing at the downstream exons 10 to 14 generates isoforms designated α (the major variant), β, and γ. (Inset) ERV9 LTR overlaps with the first upstream exon U1 and contains the transcriptional start site (TSS) of GTAp63. (B) Expression of ERV9 LTR-driven p63 transcripts in human testis. Human testicular cDNA was amplified with primers binding to exon U1 and exon 2, as indicated in Fig. S1A and Table S1. The size of the predominantly amplified PCR product corresponds to exon U1 directly spliced onto exon 2. An additional, less-abundant transcript also contains exon U2. (C) Predicted amino acid sequence of human GTAp63 derived from the major transcript present in testis (B). Note that GTAp63 differs from the previously described, long TAp63, termed TA*p63 (2), by a unique 19-residue long amino terminus. TAp63 is a shorter isoform because it uses the second ATG within exon 2 as start codon (2). (D) The unique GTAp63 transcripts are highly expressed in testis, but barely or not at all in somatic tissues. GTAp63 also shows some expression in the ovary of a young female donor but much less so from an older woman (see for example, Fig. S1E). Relative mRNA levels of GTAp63 and conventional TAp63 in an array of normal human tissues were determined by quantitative RT-PCR. Human testis contained 500- to 1,000-fold more GTAp63 transcripts than any of the few somatic tissues with detectable expression (note the logarithmic scale). Most tissues do not express detectable GTAp63. (E) Timing of primate evolution and ERV9 insertion adjacent to TP63. The pattern and sequence of TP63-associated ERV9 LTR in primate genomes (Fig. S1G) indicates that the insertion of the ERV9 LTR upstream of TP63 occurred during the time shortly after Hominidae separated from Hylobatidae, roughly 15 million years ago. This theory is compatible with the model that this insertion contributed to enhanced genomic stability of the male germ line, perhaps enabling the preservation of species with a much prolonged time frame of fertility and increased complexity in gene regulation.
LTR of ERV9 Was Inserted Upstream of TP63 in the Ancestor of Hominidae.
Strikingly, the 5′ portion of exon U1 corresponds to the LTR sequence of ERV9. This LTR is stably integrated in the human genome, adjacent to the TP63 gene on the long arm of chromosome 3, thus partially forming the 5′ portion of exon U1 (Fig. 1A, Inset). This finding strongly suggests that the recently identified p63 isoforms are a result of ERV9 LTR-derived promoter activity.
Of note, the integration of the ERV9 LTR into the vicinity of the TP63 gene is a very recent event in the genomic evolution of humans and great apes. This theory is supported by previous paleogenomic analysis indicating that ERV9 underwent repeated mobilization when great apes (hominids) branched off from other primate lineages (18). The GTAp63-associated LTR is most closely related to subfamily IX of ERV9 sequences, which emerged 18 to 19 million years ago (19) (Fig. S1F and Table S2). PCR analysis of an extended collection of genomic DNA from primates revealed that the LTR insertion upstream of TP63 was found in Hominidae, but not in our closest nonhominid relative, Hylobates lar (gibbon) or other primate species (Fig. S1G), in agreement with available genome sequences (Table S3). Amplification with a primer pair spanning the ERV9 LTR resulted in a long PCR fragment, containing the LTR, when using human genomic DNA as a template; only a short PCR product was obtained using the same primers from the DNA of gibbon and Rhesus macaque (Fig. S1, G and H). Taken together, these findings suggest that the insertion of the ERV9 LTR upstream of TP63 occurred about 15 million years ago, shortly after Hominidae separated from the other primate lineages (Fig. 1E), enabling a more stringent surveillance of male germ line genomic stability, and thus a longer fertile life span in these species.
GTAp63 Protein Is Expressed in Germ-Cell Precursors of Human Testis.
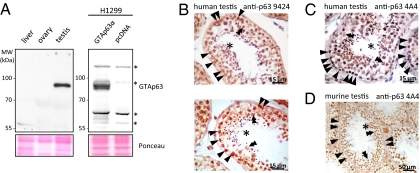
In agreement with the testis-specific expression of GTAp63 mRNA, a single band corresponding to GTAp63α protein was detected in human testicular tissue lysate by immunoblot analysis (Fig. 2A). To further delineate the specific testicular cell types that express p63, we immunostained tissue sections from normal human testis with various antibodies to p63. Of note, the p63 protein was highly expressed in germ-cell precursors of the spermatogenic epithelium lining the seminiferous tubules. Specifically, diploid basal spermatogonia, which tended to exhibit the strongest staining, as well as suprabasal spermatocytes and spermatids, which undergo meiosis, expressed high levels of nuclear p63 (Fig. 2 B and C, and Fig. S2A). In contrast, the final end product (i.e., haploid spermatozoa) failed to stain positive for p63. Hence, a p63 gradient was observed as male germ cells undergo maturation, compatible with a role of p63 in stem and precursor cells of the male germ line. Next, we examined p63 expression in mice (i.e., a species that does not contain LTR-derived GTAp63). Germ-cell precursors of testis from wild-type but not p63 knock-out mice also contained nuclear p63 (Fig. 2D and Fig. S2 B and C). However, a distribution difference of p63 expression was apparent within the murine vs. human spermatogenic epithelium. Whereas in human seminiferous tubules p63 staining tended to be strongest in the basal mitotic stem-cell layer (Fig. 2 B and C), murine testes showed the maximum staining intensity in the meiotic suprabasal layers (Fig. 2D). This differential tissue distribution was in contrast to the moderate differences regarding the overall mRNA levels observed in testes from humans, Rhesus macaques, and mice (Fig. S2D). It is tempting to speculate that the LTR insertion in Hominidae shifted this pattern in favor of spermatogonia, which are the true germ stem cells, thereby ensuring long-term protection of the male germ line.
Fig. 2.
High levels of GTAp63 in human spermatogonia. (A) Human testis expresses high levels of GTAp63 protein as the sole p63 species, as revealed by immunoblot analysis. The band detected by the p63 antibody 4A4 in human testis but not in liver or ovarian tissue corresponds to recombinant GTAp63. Overexpression of GTAp63 in H1299 cells. Nonspecific cross-reactive bands are labeled (asterisk). (B) Expression of p63 in normal human testis. Two representative examples of seminiferous tubules were immunostained with a polyclonal antibody to p63 (# 9424). Although all layers of precursors except the mature luminal spermatozoa (asterisk) stain positively, the highest level of p63 tends to be present in the mitotic spermatogonia at the base (single arrowheads). Suprabasal, meiotic spermatozytes (double arrowheads) tend to express less p63. (C) A similar pattern of p63 expression in normal human testis is seen with monoclonal p63 antibody 4A4. For a lower magnification, see Fig. S2A. (D) Expression of p63 in normal murine testis. Testicular tissue from a 4-mo-old SV129 mouse was immunostained with monoclonal p63 antibody 4A4. Basal spermatogonia reveal only a weak signal (single arrowheads), although the maximum staining intensity is shifted to suprabasal spermatocytes (double arrowheads). Mature luminal spermatozoa (asterisk) are completely negative. For a control staining, see Fig. S2B. The absence of a specific signal upon staining of p63−/− testis with antibody 4A4 is documented in Fig. S2C.
GTAp63 Induces Apoptosis via Proapoptotic Genes upon Genotoxic Stress.
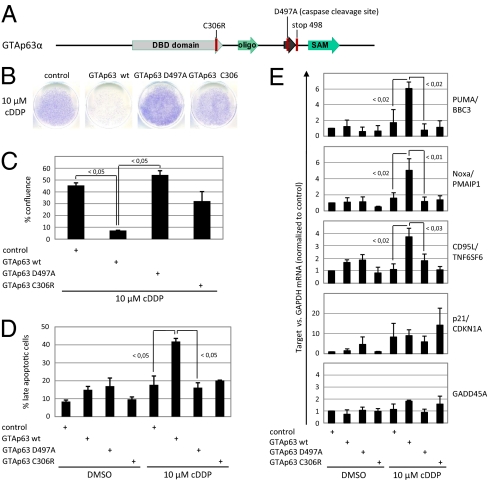
To address the capability of GTAp63 to induce apoptosis, we generated stable H1299 transfectants overexpressing GTAp63α, with or without mutations in the DNA binding domain or at a previously identified caspase cleavage site. Cleavage at this position by caspases 3, 6, 7, and 8 removes the transcription-inhibitory Sterile Alpha Motif (SAM) and post-SAM domains of p63, thereby generating p63 proteins with greater transcriptional activity (20) (see scheme in Fig. 3A). Although GTAp63 did not suppress proliferation or survival of untreated cells, making it possible to generate stable transfectants in the first place, wild-type GTAp63 markedly decreased clonogenic survival upon cisplatin treatment; this was not seen when either DNA binding or caspase cleavage were prevented by point mutations in GTAp63 (Figs. 3 B and C and Fig. S3A). Flow cytometry revealed that wild-type but not mutant GTAp63 substantially increased the acute apoptotic response to genotoxic treatment (Fig. 3D and Fig. S3B). Next, to mimic the effect of genotoxic treatment-induced caspase cleavage in GTAp63, we introduced a stop codon immediately after the caspase cleavage site at D497. Constitutive overexpression of this truncated GTAp63 in stably selected, untreated H1299 transfectants strongly suppressed clonogenic survival (Fig. S3C). Taken together, these data lead us to conclude that in response to DNA damage, GTAp63 works by amplifying proapoptotic mechanisms after being cleaved by initially activated caspases. In the absence of genotoxic stress, however, GTAp63 is neutral, explaining its very high constitutive expression in unstressed spermatogenic precursors in the testis.
Fig. 3.
GTAp63 induces apoptosis in response to genotoxic stress. (A) Schematic of the GTAp63α domain structure, including the DNA binding domain (DBD), the oligomerization domain (oligo), and the SAM domain. Point mutations that were introduced for functional analysis are indicated. Mutation C306R disrupts DNA binding (28), whereas mutation D497A prevents caspase cleavage (20). A stop codon at position 498 results in the synthesis of a truncated, constitutively active GTAp63 that mimics the fragment generated by caspase cleavage in response to DNA damage (used in Fig. S3C). Note, the numbering of positions refers to conventional TAp63, starting at the second ATG (see Fig. 1C, TAp63), to match the published numbering (28). (B) In response to cisplatin treatment, caspase-cleavable wild-type GTAp63 decreases long-term clonogenic survival. H1299 cells (p53 null) were stably transfected with empty vector (pIRESneo, Clontech; control) or expression constructs for wild-type GTAp63α or its mutants. The cells were treated with cisplatin for 24 h, followed by drug removal and culturing for 7 d. Emerging clones were stained with Crystal violet. (C) Quantitation of the plate area covered by cell clones from B. Columns represent mean values and the SEM. P values were calculated using Student's t test. (D) Apoptosis in response to DNA damage depends on GTAp63. H1299 cells stably expressing GTAp63 or its mutants were treated with cisplatin for 24 h. Activation of caspases was detected by staining with fluorescently labeled DEVD, indicating mid- and late-stage apoptosis. The mean proportion of apoptotic cells and its SE are indicated. The corresponding raw data are shown in Fig. S3B. (E) Induction of proapoptotic genes by wild-type GTAp63 in response to cisplatin. GTAp63α or the indicated mutants were stably expressed in H1299 cells. Cells were treated with cisplatin or DMSO for 24 h, followed by quantitative RT-PCR to determine the levels of p53/p63-responsive genes. Wild-type GTAp63 induces the proapoptotic genes PUMA/BBC3, Noxa/PMAIP1, and CD95L/TNF6SF6, but DNA-binding and noncleavable mutants do not. On the other hand, the cell-cycle regulatory genes CDKN1A/p21 and GADD45A are not or only minimally induced by GTAp63. P values were calculated as described in C.
Treatment of cells with cisplatin led to selective induction of the proapoptotic p53-response genes PUMA/BBC3, NOXA/PMAIP1, and CD95L/TNF6SF6 as a function of wild-type GTAp63 expression, again depending on the DNA binding domain and the caspase cleavage site (Fig. 3E). In contrast, the up-regulation of cell-cycle arrest genes (i.e., p21/CDKN1A and GADD45A) by cisplatin showed little or no dependence on GTAp63 (Fig. 3E), a phenomenon comparable to recently identified p53 mutants with selectively enhanced binding to proapoptotic gene promoters (21). In summary, we conclude that GTAp63 is activated by DNA damage and subsequent caspase cleavage to induce p53-responsive, proapoptotic genes. However, GTAp63 enhanced apoptosis, even when transcription was blocked by actinomycin D (Fig. S3 D and E). Moreover, cleaved GTAp63 was also found to associate with mitochondria, possibly providing an additional proapoptotic function (Figs. S3 F and G), as described for p53 (6).
GTAp63 Expression Is Lost in Testicular Cancer Cells via Transcriptional Silencing, but Fully Restored by Histone Deacetylase Inhibition.
Given the homology of p63 with the tumor suppressor p53, we reasoned that the high expression of GTAp63 in germ-cell precursors of normal testis might contribute to suppression of malignancy in this tissue. This theory implies that GTAp63 expression should be frequently lost in testicular cancer.
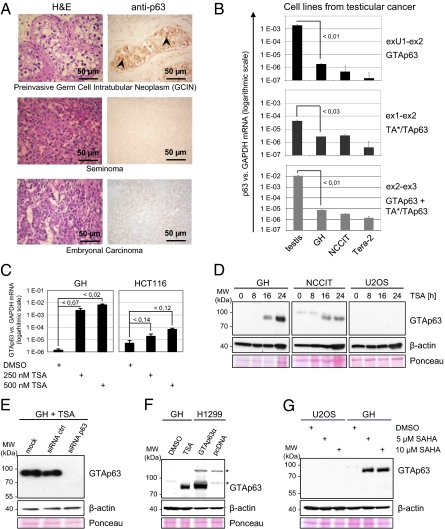
To test this hypothesis, we immunostained 98 cases of human testicular cancers with a specific polyclonal p63 antibody that recognizes a common region shared by all forms of human p63. Indeed, in contrast to normal testis, the majority of invasive malignant germ-cell tumors have lost p63 expression (examples in Fig. 4A and Fig. S4A, and summarizied in Table 1). Seminomas revealed loss of p63 expression in 77% (46 of 60) of cases, and their rare female counterpart, ovarian dysgerminoma, did so in 60% (3 of 5) of cases. Moreover, 100% (27 of 27) of testicular embryonal carcinoma showed loss of p63 expression. In contrast, the preinvasive tumor stage classified as germ cell intratubular neoplasm mostly retained p63 expression (5 of 6 cases). In sum, we conclude that the development of invasive testicular germ-cell neoplasms correlates with loss of p63 expression, consistent with a tumor suppressor role of GTAp63.
Fig. 4.
GTAp63 is frequently lost in testicular germ-cell cancers, but expression can be fully rescued by HDAC inhibitors. (A) Frequent loss of p63 expression in invasive human testicular germ cell cancers. Representative examples of seminomas and embryonal carcinomas (the two most important testicular cancer types) are shown. Staining with H&E and polyclonal p63 antibody 9424, as in Fig. 2B. The faint cellular outlines are nonspecific. More examples are in Fig. S4A. The results obtained from all 98 tumors are summarized in Table 1. (Upper) In contrast, preinvasive germ-cell intratubular neoplasms (GCIN) mostly preserve expression of nuclear p63 (arrowheads). Seminiferous tubules are completely filled with atypical GCIN cells that lack normal differentiation and spermatogenesis. (Left) Large atypical tumor cells arranged in sheets separated by loose fibrous stroma. H&E staining. (Right) Shows that p63 is lost. (B) Compared with normal human testis, GTAp63 mRNA expression is strongly reduced in all three randomly chosen cell lines from human testicular germ-cell cancers. P values were calculated as described in Fig. 3C. (C) GTAp63 is lost in testicular cancer cell lines via transcriptional silencing but fully restored by HDAC inhibition. Real-time RT-PCR to quantify GTAp63 mRNA, normalized for GAPDH. GH cells (teratocarcinoma) were treated with TSA for 24 h. This treatment increased the level of GTAp63 mRNA up to 3,000-fold (logarithmic scale). No comparable increase was seen for conventional TAp63 or TAp73 in the same cells (Fig. S4B). Moreover, only a very modest increase of GTAp63 (20-fold) was seen in colon carcinoma cells (HCT116). P values were calculated as described in Fig. 3C. (D) Restoration of p63 protein in human testicular cancer cells upon HDAC inhibition. GH and NCCIT cells (teratocarcinomas) were treated with 500 nM TSA, followed by 4A4 immunoblot detection of p63. In contrast, U2OS human osteosarcoma cells treated in parallel exhibit no detectable GTAp63. (E) Silencing of p63 via siRNA abolishes the GTAp63 protein detectable in TSA-treated GH cells, confirming its identity. (F) The single band detectable by anti-p63 antibodies in TSA-treated GH cells corresponds to the molecular weight of recombinant GTAp63 expressed in H1299 cells (a lung adenocarcinoma line with no detectable endogenous p63). (G) SAHA also induces GTAp63 protein in testicular cancer cells but not in osteosarcoma cells. GH cells (teratocarcinoma) and U2OS cells (osteosarcoma) were treated with SAHA for 24 h, followed by immunoblot detection of p63. A band corresponding to the molecular weight of recombinant GTAp63 was only detected in SAHA-treated GH cells.
Table 1.
p63 detection in human cancers
| Tumor type | No. of cases | p63-negative | p63-positive | % p63-negative |
| Seminoma | 60 | 46 | 14 | 77% |
| Embryonal carcinoma | 27 | 27 | 0 | 100% |
| Ovarian dysgerminoma | 5 | 3 | 2 | 60% |
| Germ cell intratubular neoplasms | 6 | 1 | 5 | 17% |
| TOTAL | 98 | 77 | 21 | 79% |
Three randomly chosen human testicular cancer cell lines expressed far less GTAp63 mRNA compared with normal testis tissue (Fig. 4B, note the logarithmic scale). The histone deacetylase (HDAC) inhibitor trichostatin A (TSA) led to a strong and specific restoration of GTAp63 mRNA levels by over 3,000-fold (Fig. 4C). In contrast, TSA induced only a relatively feeble increase of conventional TAp63 (only 14-fold) and no change of TAp73 levels. (Fig. S4B and ref. 20). HDAC inhibition led to only moderately augmented GTAp63 levels in nontesticular HCT116 cancer cells (Fig. 4C). Upon TSA treatment, the restoration of GTAp63 mRNA levels was accompanied by the appearance of a single p63 protein species in testicular cancer but not in U2OS osteosarcoma cells (Fig. 4D), which was ablated by siRNA against p63 (Fig. 4E). The electrophoretic mobility of the TSA-restored p63 protein species was identical to recombinant GTAp63 (Fig. 4F). A similar testicular cancer-specific restoration was also observed with the HDAC inhibitor SAHA (suberoylanilide hydroxamic acid), currently in clinical use (Fig. 4G), but not with an inhibitor of DNA methylation, 5-azacytidine (Fig. S4C). Together, these findings are compatible with the notion that GTAp63 expression in testicular cancer is not genetically lost but “only” transcriptionally repressed through the action of HDACs, and therefore fully restorable. Notably, although HDAC-mediated repression is a common phenomenon in cancer, the magnitude of induction of GTAp63 by TSA is to our knowledge the most dramatic increase in gene expression reported so far for HDAC inhibitors for any gene.
Discussion
Here we identify unique transactivating isoforms of p63 termed GTAp63 that are specifically expressed in stem and precursor cells of the human male germ line. Phylogenetically, this expression was supported by insertion of an endogenous retrovirus with its requisite LTR during recent primate evolution. Upon DNA damage, GTAp63 induces apoptosis, thus maintaining the integrity of the genome that is transmitted to the next generation. Hence, this is one of the very few positive cases where the most imminent danger that the genome faces during evolution—that is, the spread of ERVs—has ultimately fortified the expression of a central guardian to preserve germ-line integrity during the long reproductive period of humans and their immediate relatives. Consistent with this model, GTAp63 appears to act as a tumor suppressor in human testicular germ-cell cancers, frequently losing its expression epigenetically. Importantly, testicular GTAp63 function can be pharmacologically rescued in testicular cancer cells.
ERV9 LTR-Driven p63 Expression in Germ Cells.
p63 is the evolutionarily most ancient member of the p53 family. Its function in the germ line of Caenorhabditis elegans (22) and the sea anemone Nematostella vectensis (23) strongly suggests that germ-line maintenance may represent the original function of p63 gene products, long before tumor suppression was an issue. Such protective mechanisms appear to be particularly necessary in male spermatogonia and spermatocytes, given their extreme replication activity compared with any other cell type (humans produce approximately 50–200 million spermatozoa per individual per day). Of note, the insertion of a retroviral LTR occurred only recently in primate evolution, coinciding with the time when the Hominidae lineage separated from other primates, a leap associated with a boost in longevity and complexity, most obvious in cerebral development. These features are likely to increase the sensitivity of the system to even low numbers of mutations, raising the demand for stricter control to preserve the species.
A similar—albeit, less central—example of recent evolution of the p53 system is provided by the p53-induced gene PIG3 (alias TP53I3) (24). Here, a microsatellite sequence serves as a p53-responsive promoter element, and p53-responsiveness for this gene was achieved only in Hominoidea (humans, great apes but also small apes) (Fig. 1E) (25, 26).
Interestingly, in Hodgkin's lymphoma expression of the proto-oncogene CSF1R was recently shown to be driven by an LTR from the ERV THE1B (16). Conversely, the insertion of the ERV9 LTR upstream of TP63 represents to our knowledge a unique example of an ERV-derived sequence that controls expression of a gene that functions in germ-line protection and tumor suppression.
ERVs can be considered selfish genes that spread through genomes contingent upon their ability to replicate in germ cells, rather than in somatic cells. It follows that promoter activity of these viruses in the germ line provides them with an evolutionary advantage. The activity of the ERV9 LTR as a promoter in germ cells is therefore not surprising. Indeed, ERV9 LTR enables the expression of a green fluorescent reporter protein in the germ line of transgenic zebrafish (Danio renio) (30), suggesting an evolutionary conserved mechanism of germ-line–specific promoter activity. In summary, our results strongly suggest that this germ-line–specific promoter activity was positively reappropriated by the host cell into guarding and maintaining the genome by integrating this LTR upstream of TP63.
GTAp63: A Testicular Tumor Suppressor Candidate.
The p63 mRNA and protein are lost in the vast majority of human testicular germ-cell tumors. Taken together with the proapoptotic function of p63, this finding strongly suggests that p63 performs the functions of a p53-like tumor suppressor in this cell type. Silencing of p63 expression in testicular tumor cells is completely restored by HDAC inhibition. This finding was previously reported in tumor cells of other origin (20), albeit not even close to the magnitude seen in testicular cancer cells. Patients with testicular cancer may thus benefit from pharmacological HDAC inhibition.
GTAp63, when cleaved by caspases, provides a positive-feedback loop to proapoptotic stimuli, which appears to constitute at least one major reason why germ cells are exquisitely sensitive to DNA damage. This sensitivity avoids testicular cancer. Even more importantly, however, it greatly enhances genetic fidelity within the male germ line and ensures error-free passaging of the genome to the next generation.
Materials and Methods
Cell Culture, PCR, Immunoblot Analysis, Flow Cytometry and Immunohistochemistry, and Isolation of Mitochondria.
Cell culture, PCR and immunoblot analysis, flow cytometry and immunohistochemistry, and isolation of mitochondria are described in SI Materials and Methods. Primers are listed in Table S1.
RACE.
RACE was performed using the Rapid cDNA Amplification Kit (Clontech). For details see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank R. Löwer for the gift of testicular cancer cell lines, members of the German Primate Research Center (Göttingen) for primate DNA, and C. Hippel and A. Dickmanns for excellent technical assistance. This work was supported by the German Cancer Aid/Dr. Mildred Scheel Stiftung, the European Union 6th Framework Program (Integrated Project Active p53), the German Research Foundation (Deutsche Forschungsgemeinschaft), US National Cancer Institute Grant 5R01CA93853, the Wilhelm Sander Stiftung, the Statens Sundhedsvidenskabelige Forskningsråd of Denmark, the Danish Cancer Society (Kræftens Bekæmpelse), and the Novo Nordisk Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016201108/-/DCSupplemental.
References
- 1.Arnheim N, Calabrese P. Understanding what determines the frequency and pattern of human germline mutations. Nat Rev Genet. 2009;10:478–488. doi: 10.1038/nrg2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang A, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 3.Yang A, McKeon F. P63 and P73: P53 mimics, menaces and more. Nat Rev Mol Cell Biol. 2000;1:199–207. doi: 10.1038/35043127. [DOI] [PubMed] [Google Scholar]
- 4.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 5.Vousden KH, Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Mihara M, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 7.Dumont P, Leu JI, Della Pietra AC, 3rd, George DL, Murphy M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet. 2003;33:357–365. doi: 10.1038/ng1093. [DOI] [PubMed] [Google Scholar]
- 8.Sayan AE, et al. P73 and caspase-cleaved p73 fragments localize to mitochondria and augment TRAIL-induced apoptosis. Oncogene. 2008;27:4363–4372. doi: 10.1038/onc.2008.64. [DOI] [PubMed] [Google Scholar]
- 9.Yang A, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 10.Suh EK, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–628. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- 11.Nakamuta N, Kobayashi S. Expression of p63 in the testis of mouse embryos. J Vet Med Sci. 2003;65:853–856. doi: 10.1292/jvms.65.853. [DOI] [PubMed] [Google Scholar]
- 12.Petre-Lazar B, et al. The role of p63 in germ cell apoptosis in the developing testis. J Cell Physiol. 2007;210:87–98. doi: 10.1002/jcp.20829. [DOI] [PubMed] [Google Scholar]
- 13.Petre-Lazar B, et al. p63 expression pattern in foetal and neonatal gonocytes after irradiation and role in the resulting apoptosis by using p63 knockout mice. Int J Radiat Biol. 2006;82:771–780. doi: 10.1080/09553000600960019. [DOI] [PubMed] [Google Scholar]
- 14.Cohen CJ, Lock WM, Mager DL. Endogenous retroviral LTRs as promoters for human genes: A critical assessment. Gene. 2009;448(2):105–114. doi: 10.1016/j.gene.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Jern P, Coffin JM. Effects of retroviruses on host genome function. Annu Rev Genet. 2008;42:709–732. doi: 10.1146/annurev.genet.42.110807.091501. [DOI] [PubMed] [Google Scholar]
- 16.Lamprecht B, et al. Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nat Med. 2010;16:571–579. doi: 10.1038/nm.2129. [DOI] [PubMed] [Google Scholar]
- 17.Svensson AC, et al. Chromosomal distribution, localization and expression of the human endogenous retrovirus ERV9. Cytogenet Cell Genet. 2001;92(1-2):89–96. doi: 10.1159/000056875. [DOI] [PubMed] [Google Scholar]
- 18.López-Sánchez P, Costas JC, Naveira HF. Paleogenomic record of the extinction of human endogenous retrovirus ERV9. J Virol. 2005;79:6997–7004. doi: 10.1128/JVI.79.11.6997-7004.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costas J, Naveira H. Evolutionary history of the human endogenous retrovirus family ERV9. Mol Biol Evol. 2000;17:320–330. doi: 10.1093/oxfordjournals.molbev.a026312. [DOI] [PubMed] [Google Scholar]
- 20.Sayan BS, et al. Cleavage of the transactivation-inhibitory domain of p63 by caspases enhances apoptosis. Proc Natl Acad Sci USA. 2007;104:10871–10876. doi: 10.1073/pnas.0700761104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlereth K, et al. DNA binding cooperativity of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol Cell. 2010;38:356–368. doi: 10.1016/j.molcel.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 22.Derry WB, et al. Regulation of developmental rate and germ cell proliferation in Caenorhabditis elegans by the p53 gene network. Cell Death Differ. 2007;14:662–670. doi: 10.1038/sj.cdd.4402075. [DOI] [PubMed] [Google Scholar]
- 23.Pankow S, Bamberger C. The p53 tumor suppressor-like protein nvp63 mediates selective germ cell death in the sea anemone Nematostella vectensis. PLoS ONE. 2007;2:e782. doi: 10.1371/journal.pone.0000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JH, et al. The p53-inducible gene 3 (PIG3) contributes to early cellular response to DNA damage. Oncogene. 2010;29:1431–1450. doi: 10.1038/onc.2009.438. [DOI] [PubMed] [Google Scholar]
- 25.Contente A, Dittmer A, Koch MC, Roth J, Dobbelstein M. A polymorphic microsatellite that mediates induction of PIG3 by p53. Nat Genet. 2002;30:315–320. doi: 10.1038/ng836. [DOI] [PubMed] [Google Scholar]
- 26.Contente A, Zischler H, Einspanier A, Dobbelstein M. A promoter that acquired p53 responsiveness during primate evolution. Cancer Res. 2003;63:1756–1758. [PubMed] [Google Scholar]
- 27.Pi W, et al. The LTR enhancer of ERV-9 human endogenous retrovirus is active in oocytes and progenitor cells in transgenic zebrafish and humans. Proc Natl Acad Sci USA. 2004;101:805–810. doi: 10.1073/pnas.0307698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Celli J, et al. Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell. 1999;99(2):143–153. doi: 10.1016/s0092-8674(00)81646-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.