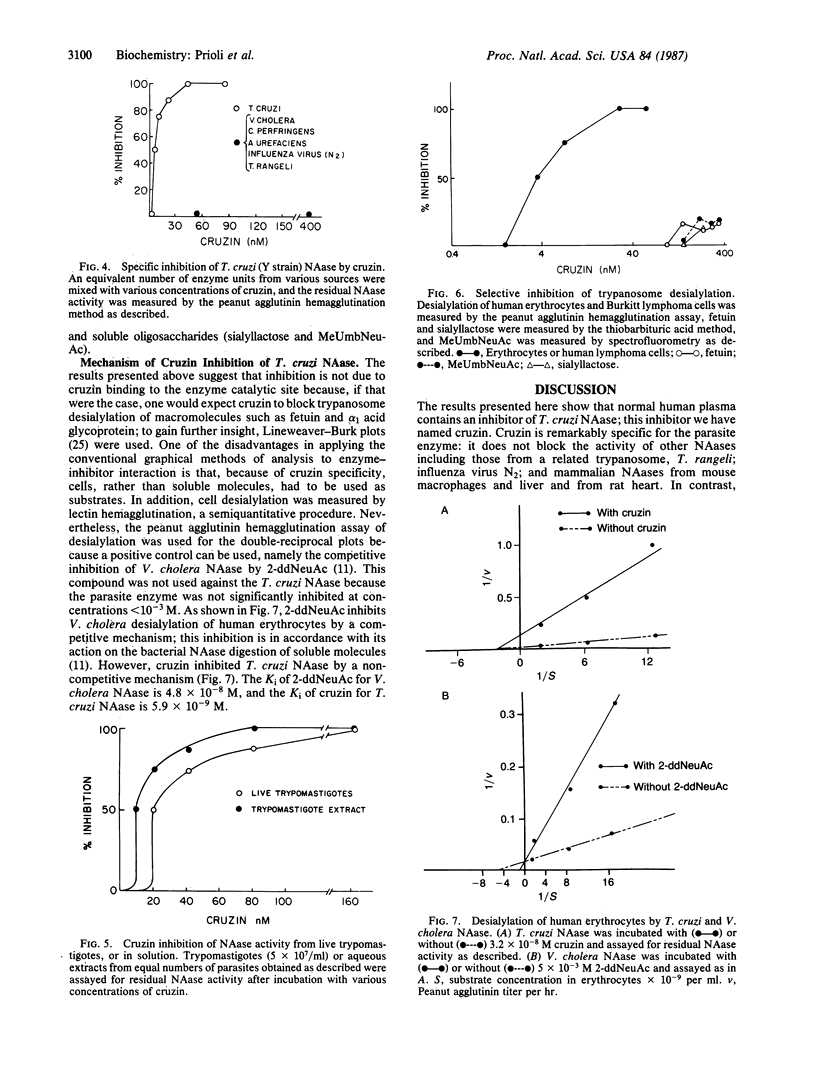
Abstract
Plasma of normal human individuals was shown to contain an inhibitor of Trypanosoma cruzi neuraminidase (NAase; acylneuraminyl hydrolase, sialidase, EC 3.2.1.18). The inhibitor has been purified to homogeneity by PEG precipitation, CM Affi-Gel Blue Sepharose chromatography, and gel filtration. The purified preparation inhibits T. cruzi NAase at a concentration as low as 10(-9) M and has no effect at concentrations at least 100 times higher on any of the other NAases tested, including those from influenza virus, the closely related trypanosome Trypanosoma rangeli, and mammalian NAases. The inhibitor is unique in that it prevents T. cruzi desialylation of intact mammalian cells but does not prevent desialylation of soluble glycoconjugates. In addition, the isolated material is effective in inhibiting the T. cruzi NAase whether the enzyme is on the parasite outer membrane or in solution. Molecular characterization indicates that the inhibitor is a glycoprotein with a Mr of 246,000 +/- 20,000 composed of subunits of Mr 28,000 +/- 2000. Its plasma concentration is at least 60 micrograms/ml. The mechanism of action has not been fully elucidated, but it appears to be noncompetitive. Attempts to match the isolated NAase inhibitor with known plasma glycoproteins have not been successful. In view of this and of the specificity of the inhibitor for T. cruzi, we have named the inhibitor "cruzin." This finding suggests a different approach in investigating the role that NAase plays in host-parasite interaction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshawe K. D., Currie G. A. Immunogenicity of L 1210 murine leukaemia cells after treatment with neuraminidase. Nature. 1968 Jun 29;218(5148):1254–1255. doi: 10.1038/2181254a0. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Rapid adhesion of nerve cells to muscle fibers from adult rats is mediated by a sialic acid-binding receptor. J Cell Biol. 1986 Jun;102(6):2273–2280. doi: 10.1083/jcb.102.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M., Cowing C. Presentation of antigen by B cells: functional dependence on radiation dose, interleukins, cellular activation, and differential glycosylation. J Immunol. 1985 Apr;134(4):2269–2275. [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Hünig T. The role of accessory cells in polyclonal T cell activation II. Induction of interleukin 2 responsiveness requires cell-cell contact. Eur J Immunol. 1983 Jul;13(7):596–601. doi: 10.1002/eji.1830130716. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Libby P., Alroy J., Pereira M. E. A neuraminidase from Trypanosoma cruzi removes sialic acid from the surface of mammalian myocardial and endothelial cells. J Clin Invest. 1986 Jan;77(1):127–135. doi: 10.1172/JCI112266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl P., Bodo G., Lindner J., Palese P. Einfluss von 2-Desoxy-2,3-dehydro-N-acetylneuraminsäure auf Myxovirus-Neuraminidasen und die Replikation von Influenza- und Newcastle Disease Virus. Z Naturforsch B. 1971 Aug;26(8):792–797. doi: 10.1515/znb-1971-0812. [DOI] [PubMed] [Google Scholar]
- Meindl P., Bodo G., Palese P., Schulman J., Tuppy H. Inhibition of neuraminidase activity by derivatives of 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. Virology. 1974 Apr;58(2):457–463. doi: 10.1016/0042-6822(74)90080-4. [DOI] [PubMed] [Google Scholar]
- Nydegger U. E., Fearon D. T., Austen K. F. Autosomal locus regulates inverse relationship between sialic acid content and capacity of mouse erythrocytes to activate human alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6078–6082. doi: 10.1073/pnas.75.12.6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. E. A developmentally regulated neuraminidase activity in Trypanosoma cruzi. Science. 1983 Mar 25;219(4591):1444–1446. doi: 10.1126/science.6338592. [DOI] [PubMed] [Google Scholar]
- Pereira M. E. A rapid and sensitive assay for neuraminidase using peanut lectin hemagglutination: application to Vibrio cholera and Trypanosoma cruzi. J Immunol Methods. 1983 Sep 30;63(1):25–34. doi: 10.1016/0022-1759(83)90206-5. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Hoff R. Heterogeneous distribution of neuraminidase activity in strains and clones of Trypanosoma cruzi and its possible association with parasite myotropism. Mol Biochem Parasitol. 1986 Aug;20(2):183–189. doi: 10.1016/0166-6851(86)90030-7. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Moss D. Neuraminidase activity in Trypanosoma rangeli. Mol Biochem Parasitol. 1985 Apr;15(1):95–103. doi: 10.1016/0166-6851(85)90031-3. [DOI] [PubMed] [Google Scholar]
- Potier M., Mameli L., Bélisle M., Dallaire L., Melançon S. B. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-D-N-acetylneuraminate) substrate. Anal Biochem. 1979 Apr 15;94(2):287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- Rosen S. D., Singer M. S., Yednock T. A., Stoolman L. M. Involvement of sialic acid on endothelial cells in organ-specific lymphocyte recirculation. Science. 1985 May 24;228(4702):1005–1007. doi: 10.1126/science.4001928. [DOI] [PubMed] [Google Scholar]
- Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- Schauer R., Wember M., Tschesche H. Isolation and characterization of an oligosaccharide- and glycoprotein-specific sialidase from human leucocytes. Hoppe Seylers Z Physiol Chem. 1984 Apr;365(4):419–426. doi: 10.1515/bchm2.1984.365.1.419. [DOI] [PubMed] [Google Scholar]







