Abstract
Neuromyelitis optica (NMO) is an inflammatory disease affecting the optic nerve and spinal cord, in which autoantibodies against aquaporin 4 (AQP4) water channel protein probably play a pathogenic role. Here we show that a B-cell subpopulation, exhibiting the CD19intCD27highCD38highCD180− phenotype, is selectively increased in the peripheral blood of NMO patients and that anti-AQP4 antibodies (AQP4-Abs) are mainly produced by these cells in the blood of these patients. These B cells showed the morphological as well as the phenotypical characteristics of plasmablasts (PB) and were further expanded during NMO relapse. We also demonstrate that interleukin 6 (IL-6), shown to be increased in NMO, enhanced the survival of PB as well as their AQP4-Ab secretion, whereas the blockade of IL-6 receptor (IL-6R) signaling by anti–IL-6R antibody reduced the survival of PB in vitro. These results indicate that the IL-6–dependent B-cell subpopulation is involved in the pathogenesis of NMO, thereby providing a therapeutic strategy for targeting IL-6R signaling.
Keywords: neuroinflamatory disease, autoimmunity, multiple sclerosis, central nervous system, IL-6 receptor blockade
Neuromyelitis optica (NMO) is an inflammatory demyelinating disorder characterized by recurrent attacks of severe optic neuritis and myelitis. Unlike the conventional form of multiple sclerosis (CMS), the lesions of NMO tend to spare the cerebral white matter, especially during the early stage (1), and even a single episode of attack can cause serious neurological deficits such as total blindness and paraplegia. Accordingly, accumulation of irreversible damage to the central nervous system (CNS) along with rapid progression of disability is more frequently found in NMO compared with CMS (2).
NMO can be distinguished from CMS by clinical, neuroimaging, and serological criteria (3). It is now known that serum anti-aquaporin 4 (AQP4) autoantibodies can be used as a disease marker of NMO (1, 2). AQP4 is the most abundantly expressed water channel protein in the CNS and is highly expressed in the perimicrovessel astrocyte foot processes, glia limitans, and ependyma (4). Emerging clinical and pathological observations suggest that anti-AQP4 antibodies (AQP4-Abs) play a key role in the pathogenesis of NMO. Prior studies have documented a significant correlation of serum AQP4-Ab levels with the therapeutic efficacy of plasma exchange during clinical exacerbations of NMO (2, 5). In the CNS lesions of NMO, reduced expression of AQP4 on astrocytes is evident even during the early stage (6), which is followed by the occurrence of vasculocentric destruction of astrocytes associated with perivascular deposition of complement and IgG (7).
On the other hand, recent studies have suggested that AQP4-Abs alone are incapable of causing the clinical and pathological features of NMO. In fact, Hinson et al. emphasized the role of cellular immunity in combination with AQP4-Abs by showing that the attack severity of NMO was not correlated with serum AQP4-Ab levels (8). It was also demonstrated that direct injection of IgGs derived from NMO patients into the brains of naïve mice did not cause NMO-like lesions, although brain tissue destruction associated with leukocyte infiltration was elicited by coinjecting human complement (9). Other groups have shown that the passive transfer of IgGs from NMO patients to rats challenged with induction of experimental autoimmune encephalomyelitis (EAE) may cause a decrease in the expression of AQP4 in astrocytes along with worsening of clinical EAE (10–12). In contrast, the transfer of IgGs to unimmunized rats did not cause any pathology. These results suggest that induction of AQP4-Ab–mediated pathology in NMO depends on the presence of complement, leukocytes, and T cells.
Although AQP4-Ab–secreting cells are a potential target for therapy, detailed characteristics of AQP4-Ab–producing cells have not been clarified yet. Because some NMO patients have elevated serum anti-nuclear and anti–SS-A/SS-B Abs (1), as found in patients with systemic lupus erythematosus (SLE) or Sjögren syndrome, NMO might share common pathological mechanisms with these autoimmune diseases. Kikuchi et al. previously reported that CD180− B cells are activated B cells capable of producing autoantibodies in SLE (13). CD180 is a member of the leucine-rich repeat family of molecules with homology to Toll-like receptor 4 (14), which is highly expressed by naïve and memory B cells but not by plasma cells (15). Odendahl et al. demonstrated that CD27highCD38+ B cells, capable of producing high-affinity IgG (16), are increased in the peripheral blood of SLE patients with some correlation to disease activity (17). Considering the phenotypes of autoantibody-producing cells reported in SLE, we analyzed the expression of CD27, CD38, and CD180 on CD19+ B cells in the peripheral blood of NMO patients. We found that CD27highCD38highCD180− B cells were significantly increased in AQP4-Ab seropositive patients diagnosed with NMO or NMO spectrum disorder (1) compared with healthy subjects (HS) or CMS patients. Notably, this B-cell subpopulation was found to be a major source of AQP4-Abs in the peripheral blood of AQP4-Ab seropositive patients and depended on interleukin-6 receptor (IL-6R) signaling for survival.
Results
CD27highCD38highCD180− B Cells Were Increased in the Peripheral Blood of NMO Patients.
Although AQP4-Abs are identified as IgGs (18), no prior study has focused on proportional changes of B-cell subsets in NMO. We therefore performed multicolor flow cytometric analysis of peripheral blood mononuclear cells (PBMC) derived from patients and controls. After starting the study, we soon noticed a remarkable expansion of a distinct B-cell subset in some patients with NMO. The expanded B cells were identified as a population of CD27high, CD38high, and CD180−, and showed lower expression of CD19 than other B cells (Fig. 1A). Notably, this population did not express the B-cell marker CD20 (Fig. S1). First, we collected samples from patients in remission and analyzed the pooled data. We found that the proportion of this subpopulation among CD19+ B cells was significantly increased in AQP4-Ab seropositive patients with NMO or NMO spectrum disorder (Fig. 1B) compared with HS or CMS patients. There was no significant difference in the proportion of this B-cell subpopulation between those with typical NMO and those with NMO spectrum disorder. Furthermore, the frequency of this B-cell subpopulation was correlated with the serum AQP4-Ab titer (Fig. S2). Comparison of paired samples obtained from the same patients during relapse and in remission showed that the CD27highCD38highCD180− B cells further increased during relapse (Fig. 1C). In contrast, the frequencies of CD27− naïve B cells (nB) and CD27+CD38−-low memory B cells (mB) were not altered in AQP4-Ab seropositive patients compared with controls (Fig. S3). The large majority of seropositive patients were treated with corticosteroids. However, the frequency of CD27highCD38highCD180− cells among CD19+ B cells was not correlated with the daily corticosteroid dose given to patients (Fig. S4). Moreover, the increase in cells in NMO patients was still evident compared with that in CMS patients similarly treated with corticosteroids (Fig. S5). Taken together, the selective increase in CD27highCD38highCD180− B cells in seropositive patients was thought to reflect their role in the pathogenesis of NMO but not to be an effect of the corticosteroid treatment.
Fig. 1.
CD27highCD38highCD180− B cells increased in NMO patients. (A) A flow cytometric scheme for the analysis of B-cell subpopulations. PBMC from HS and NMO in remission were stained with fluorescence-conjugated anti-CD19, -CD27, -CD38, and -CD180 mAbs. CD19+CD27+ cells were partitioned (Upper) and analyzed for expression of CD38 and CD180 (Lower). Values represent the percentage of CD38highCD180− cells within CD19+CD27+ cells. (B) Analysis of the pooled data derived from patients in clinical remission. This shows the percentages of CD27highCD38highCD180− cells within CD19+ cells from HS, seropositive patients, and CMS patients (**P < 0.01; Tukey's post hoc test). (C) Comparison of remission and relapse of NMO. Data obtained from the same patients are connected with lines (*P < 0.05; Wilcoxon signed rank test).
Expanded Cells Resemble Early Plasma Cells in Gene Expression and Morphology.
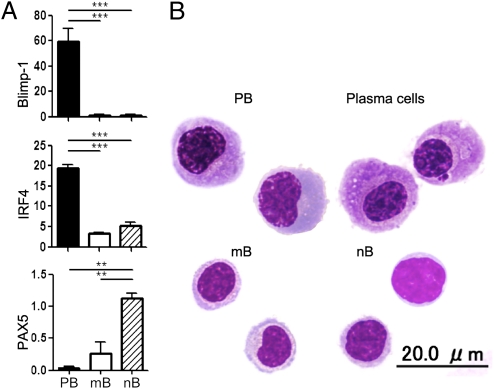
To gain insights into the developmental stage of the CD27highCD38highCD180− B cells, we quantified the mRNA expression of B-cell–associated transcription factors in sorted cell populations. Compared with nB and mB, this population showed much higher expression of B-lymphocyte-induced maturation protein 1 (Blimp-1) and IFN regulatory factor 4 (IRF4), which are essential for the regulation of plasma cell differentiation (19, 20) (Fig. 2A). In contrast, the expression of paired box gene 5 (PAX5), known to be down-regulated in early plasma cell differentiation (21), was reciprocally reduced in the B-cell subset. This gene expression pattern is very similar to that of plasma cells. However, it was notable that the cells of interest expressed CD19, which is not detected in mature plasma cells. Moreover, only 40% of this population expressed the most reliable plasma cell marker CD138 (22). Morphological analysis also confirmed the similarity of this population to plasma cells: they exhibit eccentric nucleus, perinuclear hof region, and abundant cytoplasm. However, they possess a larger nucleus with a lower extent of chromatin clumping compared with CD138+ plasma cells derived from HS (Fig. 2B). Notably, the CD138+ population among CD27highCD38highCD180− cells in NMO patients was morphologically indistinguishable from the CD138− population in NMO patients or HS, indicating the immature characteristic of CD27highCD38highCD180− cells (Fig. S6). These phenotypical and morphological characteristics as well as the results of the quantitative real-time PCR (qRT-PCR) analysis indicate that this B-cell population is equivalent to plasmablasts (PB) (22–26). Hereafter, we use the term “PB” to distinguish this population from other B cells.
Fig. 2.
Resemblance of CD19intCD27highCD38highCD180− cells to plasma cells. (A) mRNA expression of Blimp-1, IRF4, and PAX5. B-cell subpopulations [CD27highCD38highCD180− (PB), CD27− naïve (nB), CD27+CD38−-low memory (mB)] were sorted by FACS and total RNA was extracted for qRT-PCR analysis. RNA levels were normalized to ACTB for each sample (**P < 0.01; ***P < 0.001; Tukey's post hoc test). (B) May–Grünwald–Giemsa staining of B-cell subpopulations. PB (Upper Left), mB (Lower Left), and nB (Lower Right) from NMO are presented along with morphologically identified plasma cells (CD19intCD27highCD38highCD138+) from HS (Upper Right).
Expression of B-Cell Cytokine Receptors on PB.
Prior studies have identified cytokines that are critically involved in the differentiation and/or survival of plasma cells, including IL-6 and B-cell–activating factor (BAFF). IL-6 induces B-cell differentiation into plasma cells, maintains early plasma cell survival, and enhances plasma cell IgG secretion (24). Besides, IL-6 is elevated in the cerebrospinal fluid (CSF) or peripheral blood of NMO patients compared with that of CMS patients and HS (27, 28). In a rodent autoimmunity model, IL-6 deficiency caused impaired autoantibody secretion by B cells (29). Given the potential role of IL-6 in NMO, we performed flow cytometry analysis for the expression of IL-6R. Results showed remarkable expression of IL-6R on PB, although it was only marginal or absent on mB and nB (Fig. S7). Because BAFF and A proliferation-inducing ligand (APRIL) can also promote the survival of PB (25, 26), we next evaluated the expression of the receptors for BAFF and APRIL, BAFF receptor (BAFF-R), B-cell maturation antigen (BCMA), and transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI). Expression of BCMA and TACI was selectively up-regulated in PB in parallel with IL-6R. In contrast, BAFF-R was up-regulated in mB and nB, but not in PB (Fig. S7).
PB Is a Selective Source of AQP4-Abs in Peripheral Blood.
We were interested to know whether PB were capable of producing AQP4-Abs upon stimulation with cytokines and, therefore, examined the ability of IL-6, BAFF, and APRIL to enhance AQP4-Ab secretion by PB. We cultured the isolated PB for 6 d in the presence or absence of each cytokine, and evaluated the presence of AQP4-Abs in the supernatants by measuring IgG binding to Chinese hamster ovary (CHO) cells transfected with the human AQP4 vector (CHOAQP4) or the vector control (CHOVC). We found that IL-6, but not BAFF or APRIL, could significantly enhance AQP4-Ab secretion from PB (Fig. S8), as assessed by specific IgG binding to CHOAQP4. Further study focusing on IL-6 showed that exogenous IL-6 promoted the production of AQP4-specific IgGs from PB (Fig. 3A), but not from the other B-cell subpopulations. Similar results were obtained from six independent experiments (Fig. S9), indicating that PB could be major AQP4-Ab producers in PBMC. In the absence of addition of IL-6, supernatants from PB did not show any significant reactivity to CHOAQP4. To further analyze the AQP4-Ab–secreting potential of each B-cell subpopulation, we next stimulated the cells with a combination of IL-6, IL-21, and anti-CD40 that efficiently induces B-cell differentiation and IgG production (30). This polyclonal stimulation induced the secretion of similar amounts of IgGs from mB and PB. However, only the supernatant of PB specifically reacted to CHOAQP4 cell transfectants, indicating that AQP4-Ab–producing B cells were highly enriched in PB (Fig. 3B).
Fig. 3.
Production of AQP4-Abs by PB. (A) Using flow cytometry, we examined whether AQP4-Abs could be produced by PB, mB, or nB cells. FACS-sorted cells were cultured with IL-6 (1 ng/mL) for 6 d and supernatants were collected. Supernatant IgGs reactive to CHOAQP4 (open histogram) and CHOVC cells (closed histogram) were detected by anti-human IgG secondary antibody. The supernatant from PB (Left), but not from mB or nB, contains IgGs reactive to CHOAQP4, indicating that only PB secrete AQP4-Abs after stimulation with IL-6. (B) Memory B cells (mB) produce IgGs but not AQP4-Abs. B-cell subpopulations were cultured in the presence of IL-6 (1 ng/mL), IL-21 (50 ng/mL), and anti-CD40 mAb (1 μg/mL) for 6 d. IgGs in the culture supernatants were measured by sandwich ELISA (Left) (each assay was performed in quadruplicate). Data from three patients are expressed as mean ± SD. The activity of AQP4-Abs in the culture supernatants from PB and mB was also measured by flow cytometry (Right). Aliquots of CHOAQP4 cells (AQP4) and CHOVC cells (VC) (n = 4 for each) were stained with the supernatant of PB or mB from every patient. Data are expressed as median fluorescence intensity values from the results of three patients (*P < 0.05; Tukey's post hoc test). ND, not detected; NS, not significant.
Survival and Functions of PB Depend on IL-6 Signaling.
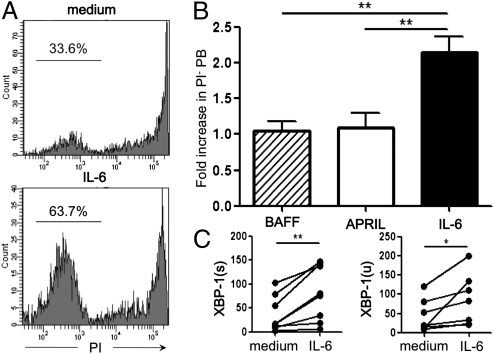
We evaluated the influence of IL-6, BAFF, and APRIL on the survival of PB after 2 d of in vitro culture (Fig. 4A). Among the added cytokines, only IL-6 was found to significantly promote the survival of PB (Fig. 4B). We also assessed the expression levels of X-box-binding protein 1 (XBP-1) in PB by qRT-PCR after 24 h of culture with or without IL-6. XBP-1 is a transcription factor critical for IgG secretion (31), and the splicing process of XBP-1 mRNA yields a more active and stable protein. We found that the expression of both unspliced [XBP-1(u)] and spliced [XBP-1(s)] forms of XBP-1 mRNA was augmented in PB by the addition of IL-6. These results suggest that IL-6 promoted the survival of PB and enhanced IgG secretion from PB, leading to an increased production of AQP4-Abs in NMO patients (Fig. 4C). In addition, we found that the frequency of PB tended to be increased when serum IL-6 levels were higher than the mean ± 2× SEM of those in HS [PB/PBMC (%) for IL-6 high group 0.62 ± 0.47 (%); PB/PBMC (%) for IL-6 low group 0.15 ± 0.05 (mean ± SD)]. These observations prompted us to address whether the blockade of IL-6R signaling could exhibit any influence on PB. We cultured PBMC derived from AQP4-Ab seropositive patients in the presence of 20% autologous serum and examined the effect of adding anti–IL-6R antibody by counting the number of surviving PB. We found that the frequency of PB among total B cells decreased significantly in the presence of anti–IL-6R mAb (Fig. 5 A and B). Among six patients examined, the PB reduction was remarkable in three patients, but was only marginal in the other patients. Notably, the former group of patients showed higher IL-6 levels in the serum (4.69, 6.47, and 25.5 pg/mL for each patient), compared with the latter (1.42, 1.43, and 2.91 pg/mL). The frequency of other B-cell subpopulations did not change with the addition of anti–IL-6R mAb. These results led us to postulate that in vivo administration of anti–IL-6R mAb may ameliorate NMO.
Fig. 4.
Effect of exogenous IL-6 on PB. (A) IL-6 promotes the survival of PB. FACS-sorted PB were cultured in the presence or absence of recombinant IL-6 (1 ng/mL) for 2 d. PI staining of cultured PB showed that exogenous IL-6 increased the percentage of surviving cells (Lower) compared with cells cultured in the medium alone (Upper). Values shown are percentages of unstained cells. (B) Comparison of IL-6 with BAFF and APRIL. Here we show that only IL-6 could promote cell survival. Data are expressed as fold increase of % PI− cells following the addition of each cytokine. At least four independent experiments were performed to obtain the results (**P < 0.01; Tukey's post hoc test). (C) Effect of IL-6 on XBP-1 expression. FACS-sorted PB were cultured with or without IL-6 for 24 h, and total RNA was extracted from the cells to quantify expression levels of XBP-1(u) and XBP-1(s) by qRT-PCR. Each line connects values obtained from seven independent experiments (*P < 0.05; **P < 0.01; Wilcoxon signed-rank test).
Fig. 5.
IL-6R blockade selectively inhibits the survival of PB. (A) PBMC were cultured in a medium containing 20% autologous serum in the presence of IL-6R–blocking antibody or its isotype control mAb for 2 d. The cells were stained and analyzed as described in the experiment in Fig. 1A. Data represent the percentages of PB within CD19+CD27+ cells. A representative pair of six independent experiments is shown. (B) The percentage of PB within CD19+ B cells (PB%) was determined for each pair of cultures either with anti–IL-6R mAb (IL-6R–blocking) or isotype control mAb (isotype) before and after starting the culture. Then, the PB survival ratio was calculated for each culture by dividing the PB% at the end of the culture by the PB% obtained before starting the culture. Lines connect the PB survival ratios of six independent experimental pairs to clarify that IL-6R blockade reduces PB survival (*P < 0.05; Wilcoxon signed-rank test).
Discussion
A growing body of evidence suggests that AQP4-Abs play a pathogenic role in NMO (6, 7, 10–12). Here we report that a B-cell subpopulation bearing the CD19intCD27highCD38highCD180− phenotype is responsible for the selective production of AQP4-Abs. The cells that we call PB are vulnerable to IL-6R blockade by anti–IL-6R mAb, leading us to propose anti–IL-6R mAb as a therapeutic option for NMO. Bennett et al. recently reported that plasma cells in CSF are a potential source of pathogenic AQP4-Abs (10). However, this study has not excluded a possible role of AQP4-Abs produced in the peripheral blood. It has been repeatedly shown that the passive transfer of pathogenic autoantibodies, including AQP4-Abs (10–12, 32), augments the formation of inflammatory lesions in EAE. Therefore, once T-cell–mediated inflammation takes place in the CNS, pathogenic autoantibodies produced outside the CNS are able to enter the CNS compartment. It is also notable that AQP4-Abs are more abundant in the peripheral blood of NMO patients than in their CSF (33). Taken together, we speculate that PB that are expanded in the peripheral blood during relapse may play a critical role in the pathogenesis of NMO by producing AQP4-Abs, although more work is necessary to explore whether PB can enter the CNS.
It is generally thought that circulating IgGs are mainly secreted by long-lived plasma cells residing in healthy bone marrow. It remains unclear how PB secreting AQP4-Abs can differentiate and survive in the peripheral circulation. It has been previously shown that autoantibodies producing plasma cells accumulate in peripheral lymphoid organs (34). It would be interesting to investigate which organs blood PB move to during the course of NMO. The levels of IL-6 in the serum and CSF are elevated in NMO compared with HS or CMS patients (27, 28). In this regard, it is of note that blocking IL-6R signaling was found to dramatically reduce the survival of PB ex vivo, which was dependent on the presence of autologous serum containing IL-6. These results suggest that the increase of PB in AQP4-Ab seropositive patients could be attributed to the increased IL-6 in the serum. We also demonstrated that improved PB survival in the presence of exogenous IL-6 was accompanied by up-regulated expression of XBP-1. It is noteworthy that wild-type and XBP-1−/− B cells start to produce more IL-6 after forced overexpression of XBP-1(s), which results in the operation of a positive feedback loop controlling IgG secretion (31). Treatment with anti–IL-6R is promising because IL-6R blockade could terminate this vicious loop that controls the production of autoantibodies.
It has been reported that NMO patients have higher levels of BAFF in the serum or CSF compared with CMS patients (35). BAFF is also known to support plasma cell differentiation and survival of PB induced in vitro (25). However, in our ex vivo study, BAFF did not promote the survival of PB, indicating that PB were not a target for BAFF. We speculate that BAFF might specifically act on an early process of plasma cell differentiation and does not have an influence on cells like PB that have entered a later stage.
IL-6R blockade by humanized mAb against IL-6R (tocilizumab) has proven to be useful for treating immune-mediated diseases, including rheumatoid arthritis (36) and Castleman's disease (37). Here we propose that IL-6R–blocking antibody treatment should be considered as a therapeutic option for NMO. Currently, most NMO patients are being treated with corticosteroids in combination with immunosuppressive drugs and plasma exchange (38). Anti-CD20 mAb, which causes B-cell depletion, has also been used for serious cases of NMO. Because the level of B-cell depletion appears to correlate with the suppressive effects of anti-CD20 in NMO (39), it has been argued that B cells are essential for the pathogenesis of NMO, either via acting as antigen-presenting cells or as autoantibody producers. Weber et al. recently reported that activated antigen-specific B cells serve as antigen-presenting cells and polarize proinflammatory T cells in EAE (40), supporting the view that the therapeutic effects of anti-CD20 might be attributable to the depletion of antigen-presenting B cells. Notably, they also cautioned that elimination of CD20+ cells might deplete nonactivated cells as well as regulatory B cells possessing anti-inflammatory potentials. Although the effect of anti-CD20 on AQP4-Ab–secreting cells has not been reported, it is likely that the majority of PB are not affected because they do not express CD20. Consistent with our prediction, anti-CD20 treatment was not effective in aggressive cases of NMO (41, 42). It appears that selective depletion of activated antigen-specific B cells could be a more promising strategy to improve the efficacy of B-cell–targeted therapies for NMO. In this regard, PB-targeting therapy is a promising approach. Given the efficacy of IL-6R blockade in reducing the number of PB ex vivo, we find it very interesting to explore the effect of anti–IL-6R mAb on NMO.
Materials and Methods
Patients and Controls.
A cohort of 24 AQP4-Ab seropositive patients was recruited at the Multiple Sclerosis Clinic of the National Center of Neurology and Psychiatry (NCNP). Among these, 16 met the revised NMO diagnostic criteria (3). The other 8 were diagnosed with NMO spectrum disorder (1) because they did not develop both myelitis and optic neuritis (optic neuritis alone in 6 cases; myelitis alone in 2 cases). Seventeen age- and sex-matched CMS patients and 20 HS were enrolled as controls. Serum AQP4-Ab levels were measured by a previously reported protocol by courtesy of Kazuo Fujihara at Tohoku University (Sendai, Japan) (33). All CMS patients had relapsing-remitting MS and fulfilled McDonald diagnostic criteria (43).
At the time of blood sampling, 21 seropositive patients were receiving corticosteroids (prednisolone 5–25 mg/d). Seven of these patients were also being treated with azathioprine (12.5–100 mg/d) or tacrolimus (3 mg/d). Six CMS patients were receiving low-dose corticosteroids without immunosuppressants. None of the seropositive or CMS patients had received IFN-β, i.v. corticosteroids, plasma exchange, or i.v. immunoglobulins for at least 1 mo before blood sampling. Blood sampling during relapse was performed in six seropositive NMO patients before they received intensive therapy starting with i.v. corticosteroids. Five of these patients were followed up further and blood was collected again after they entered remission. Anti-nuclear and/or anti–SS-A Abs were detected in some of the seropositive patients, but none met the diagnostic criteria for SLE or Sjögren syndrome. Demographic features of the patients are presented in Table 1. The study was approved by the Ethics Committee of the NCNP.
Table 1.
Demographic features
| HS | Seropositive patients | CMS patients | |
| Number | 20 | 24 | 17 |
| Age | 44.7 ± 2.8 | 47.9 ± 3.2 | 41.3 ± 3.0 |
| Male:female | 5:15 | 1:23 | 5:12 |
| Disease duration | 12.0 ± 1.6 | 9.4 ± 2.4 | |
| Age of symptom onset | 36.1 ± 3.0 | 31.9 ± 3.4 | |
| Relapses in last 2 y | 1.4 ± 0.3 | 0.7 ± 0.2 | |
| EDSS score in disease remission | 5.0 ± 0.5 | 2.1 ± 0.6 | |
| Other autoantibodies | ANA 13, SS-A 5 | ND |
Demographic features for HS, AQP4-Ab seropositive patients, and CMS patients. Values are expressed as number or mean ± SEM. ANA, anti-nuclear antibody; ND, not detected; SS-A, anti–SS-A antibody; EDSS, expanded disability status scale.
Reagents.
The following Abs were used in this study: mAbs against CD38, CD19, CD27, CD20, and PE-streptavidin (Beckman Coulter); mAbs against CD180 and BAFF-R (BD Biosciences); mAbs against IL-6R and TACI as well as Abs against BCMA and CD40 (R&D Systems); rabbit anti-human AQP4 antibody (Santa Cruz Biotechnology); FITC-anti-rabbit IgG (Jackson ImmunoResearch Laboratories); and FITC-anti-human IgG antibody (MP Biomedicals). Recombinant proteins of BAFF (ProSpec), APRIL (Abnova), IL-6 (PeproTech), and IL-21 (Invitrogen) were purchased. Propidium iodide (PI) was obtained from Sigma-Aldrich. RPMI 1640 supplemented with 10% FBS, 2 mM l-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin (Life Technologies) was used for cell culture.
Flow Cytometry, Cytology, and Cell Culture.
PBMC were separated using density centrifugation on Ficoll-Paque PLUS (GE Healthcare Biosciences). B cells were analyzed and sorted by FACSAria (BD Biosciences). Each B-cell subset was stained with May–Grünwald–Giemsa. To evaluate AQP4-Ab production in vitro, each B-cell subset (1 or 2 × 104) was cultured for 6 d in the medium alone, in the presence of IL-6 (1 ng/mL) or in the presence of IL-6 (1 ng/mL), IL-21 (50 ng/mL), and anti-CD40 (1 μg/mL). Culture supernatants were harvested and analyzed for AQP4-Ab production as described below. To examine the effect of cytokines on the survival of PB, the cells (4 × 103) were cultured in the medium alone or in the presence of BAFF (100 ng/mL), APRIL (300 ng/mL), or IL-6 (1 ng/mL) in 96-well U-bottom plates for 2 d and stained with PI to assess cell survival. In parallel, the cells were cultured for 1 d and harvested to evaluate mRNA expression by qRT-PCR. To assess the effect of IL-6 signaling blockade, PBMC (5 × 105) were preincubated with anti–IL-6R Abs (1 μg/mL) at 4 °C for 20 min, cultured in AIM-V medium (Invitrogen) containing 20% of heat-inactivated serum obtained from each patient in 96-well flat-bottom plates for 2 d, and analyzed by flow cytometry.
Quantitative RT-PCR Analysis.
mRNA from each cell subset was isolated according to the manufacturer's instructions using an RNAeasy Kit (Qiagen). RNA was further treated with DNase using the RNase-Free DNase Set (Qiagen) and reverse-transcribed to cDNA using a cDNA synthesis kit (Takara Bio). PCR was performed using iQ SYBR Green Supermix (Takara Bio) on a LightCycler (Roche). RNA levels were normalized to endogenous β-actin (ACTB) for each sample. Primers used are listed in Table S1.
Measurement of Ig Isotypes and Serum IL-6.
Secreted IgG in the culture supernatant was quantitated by sandwich ELISA using affinity-purified goat anti-human IgG-Fc (Bethyl Laboratories). Bound IgG was measured according to the manufacturer's instructions. Serum IL-6 was measured by ELISA (R&D Systems) according to the manufacturer's instructions.
AQP4-Ab Detection Assay.
Human AQP4-expressing cells were established to detect AQP4-Abs by flow cytometry. A human AQP4 (hAQP4) M23 splice variant from a clone collection (Invitrogen) was amplified by PCR and subcloned into a pIRES-DsRed-Express vector (Clontech). CHO cells (American Type Culture Collection) were transfected with this hAQP4 M23 vector (CHOAQP4) or vector control (CHOVC) using FuGENE 6 Transfection Reagent (Roche). After 2 wk of geneticin (Invitrogen) selection, stable clones were established by single-cell sorting. The expression of hAQP4 in the established clones was confirmed using anti-human AQP4 antibody and FITC-anti-rabbit IgG antibody. Reactivity of AQP4-Abs to CHOAQP4 was confirmed using seropositive NMO patients’ sera diluted at 1:1,000 and FITC-anti-human IgG antibody. To measure the AQP4-Ab activity in culture supernatants, these supernatants were concentrated up to 10 times using an Amicon Ultra 0.5 mL 100K device (Millipore), and 10 μL of the solution was added to 3 × 104 CHOAQP4 and CHOVC cells. After incubation on ice for 20 min, cells were washed with sterile PBS containing 1% BSA and stained with FITC-anti-human IgG antibody. After a 10-min incubation on ice, the cells were washed and fixed for 15 min in 2% paraformaldehyde. Then the cells were washed and analyzed by flow cytometry.
Data Analysis.
Histogram overlay analysis was performed using Cell Quest software (BD Biosciences). Statistics were calculated using Prism (GraphPad Software). Wilcoxon signed-rank test, Mann–Whitney U test, ANOVA, or Spearman's correlation test were also used when appropriate. Post hoc tests were used as a multiple comparison test after confirmation of equal variances by ANOVA.
Supplementary Material
Acknowledgments
We thank T. Takahashi and K. Fujihara for establishing the diagnosis of NMO. We also thank S. Hirose for advice on morphological analysis. We also thank M. Murata, director of the Neurology Department, NCNP Hospital for kindly supporting our sample collection. This work was supported by a Health and Labour Sciences Research Grant on Intractable Diseases (Neuroimmunological Diseases) from the Ministry of Health, Labour and Welfare of Japan, and a Grant-in-Aid for Scientific Research (S) from the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017385108/-/DCSupplemental.
References
- 1.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 2.Jarius S, et al. Mechanisms of disease: Aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol. 2008;4:202–214. doi: 10.1038/ncpneuro0764. [DOI] [PubMed] [Google Scholar]
- 3.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 4.Tait MJ, Saadoun S, Bell BA, Papadopoulos MC. Water movements in the brain: Role of aquaporins. Trends Neurosci. 2008;31:37–43. doi: 10.1016/j.tins.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Jarius S, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131:3072–3080. doi: 10.1093/brain/awn240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roemer SF, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- 7.Misu T, et al. Loss of aquaporin 4 in lesions of neuromyelitis optica: Distinction from multiple sclerosis. Brain. 2007;130:1224–1234. doi: 10.1093/brain/awm047. [DOI] [PubMed] [Google Scholar]
- 8.Hinson SR, et al. Prediction of neuromyelitis optica attack severity by quantitation of complement-mediated injury to aquaporin-4-expressing cells. Arch Neurol. 2009;66:1164–1167. doi: 10.1001/archneurol.2009.188. [DOI] [PubMed] [Google Scholar]
- 9.Saadoun S, et al. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349–361. doi: 10.1093/brain/awp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett JL, et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66:617–629. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradl M, et al. Neuromyelitis optica: Pathogenicity of patient immunoglobulin in vivo. Ann Neurol. 2009;66:630–643. doi: 10.1002/ana.21837. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita M, et al. Neuromyelitis optica: Passive transfer to rats by human immunoglobulin. Biochem Biophys Res Commun. 2009;386:623–627. doi: 10.1016/j.bbrc.2009.06.085. [DOI] [PubMed] [Google Scholar]
- 13.Kikuchi Y, et al. RP105-lacking B cells from lupus patients are responsible for the production of immunoglobulins and autoantibodies. Arthritis Rheum. 2002;46:3259–3265. doi: 10.1002/art.10672. [DOI] [PubMed] [Google Scholar]
- 14.Divanovic S, et al. Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat Immunol. 2005;6:571–578. doi: 10.1038/ni1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Good KL, Avery DT, Tangye SG. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J Immunol. 2009;182:890–901. doi: 10.4049/jimmunol.182.2.890. [DOI] [PubMed] [Google Scholar]
- 16.Wrammert J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odendahl M, et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol. 2000;165:5970–5979. doi: 10.4049/jimmunol.165.10.5970. [DOI] [PubMed] [Google Scholar]
- 18.Hinson SR, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221–2231. doi: 10.1212/01.WNL.0000289761.64862.ce. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro-Shelef M, et al. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 20.Klein U, et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol. 2006;7:773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 21.Kallies A, et al. Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity. 2007;26:555–566. doi: 10.1016/j.immuni.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Jourdan M, et al. An in vitro model of differentiation of memory B cells into plasmablasts and plasma cells including detailed phenotypic and molecular characterization. Blood. 2009;114:5173–5181. doi: 10.1182/blood-2009-07-235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 24.Kawano MM, Mihara K, Huang N, Tsujimoto T, Kuramoto A. Differentiation of early plasma cells on bone marrow stromal cells requires interleukin-6 for escaping from apoptosis. Blood. 1995;85:487–494. [PubMed] [Google Scholar]
- 25.Avery DT, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112:286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belnoue E, et al. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111:2755–2764. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- 27.Içöz S, et al. Enhanced IL-6 production in aquaporin-4 antibody positive neuromyelitis optica patients. Int J Neurosci. 2010;120:71–75. doi: 10.3109/00207450903428970. [DOI] [PubMed] [Google Scholar]
- 28.Yanagawa K, et al. Pathologic and immunologic profiles of a limited form of neuromyelitis optica with myelitis. Neurology. 2009;73:1628–1637. doi: 10.1212/WNL.0b013e3181c1deb9. [DOI] [PubMed] [Google Scholar]
- 29.Tsantikos E, et al. Autoimmune disease in Lyn-deficient mice is dependent on an inflammatory environment established by IL-6. J Immunol. 2010;184:1348–1360. doi: 10.4049/jimmunol.0901878. [DOI] [PubMed] [Google Scholar]
- 30.Ettinger R, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 31.Iwakoshi NN, Lee AH, Glimcher LH. The X-box binding protein-1 transcription factor is required for plasma cell differentiation and the unfolded protein response. Immunol Rev. 2003;194:29–38. doi: 10.1034/j.1600-065x.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 32.Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988;130:443–454. [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi T, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: A study on antibody titre. Brain. 2007;130:1235–1243. doi: 10.1093/brain/awm062. [DOI] [PubMed] [Google Scholar]
- 34.Hoyer BF, et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada K, Matsushita T, Kira J, Tsuji S. B-cell activating factor of the TNF family is upregulated in neuromyelitis optica. Neurology. 2010;74:177–178. doi: 10.1212/WNL.0b013e3181c919ee. [DOI] [PubMed] [Google Scholar]
- 36.Nishimoto N, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: A multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:1761–1769. doi: 10.1002/art.20303. [DOI] [PubMed] [Google Scholar]
- 37.Nishimoto N, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106:2627–2632. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- 38.Okamoto T, et al. Treatment of neuromyelitis optica: Current debate. Ther Adv Neurol Disord. 2008;1:5–12. doi: 10.1177/1756285608093978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cree BA, et al. An open label study of the effects of rituximab in neuromyelitis optica. Neurology. 2005;64:1270–1272. doi: 10.1212/01.WNL.0000159399.81861.D5. [DOI] [PubMed] [Google Scholar]
- 40.Weber MS, et al. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol. 2010;68:369–383. doi: 10.1002/ana.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capobianco M, et al. Variable responses to rituximab treatment in neuromyelitis optica (Devic's disease) Neurol Sci. 2007;28:209–211. doi: 10.1007/s10072-007-0823-z. [DOI] [PubMed] [Google Scholar]
- 42.Nasir S, Kerr DA, Birnbaum J. Nineteen episodes of recurrent myelitis in a woman with neuromyelitis optica and systemic lupus erythematosus. Arch Neurol. 2009;66:1160–1163. doi: 10.1001/archneurol.2009.194. [DOI] [PubMed] [Google Scholar]
- 43.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.