Abstract
There has been a consistent gap in understanding how TNF-α neutralization affects the disease state of arthritis patients so rapidly, considering that joint inflammation in rheumatoid arthritis is a chronic condition with structural changes. We thus hypothesized that neutralization of TNF-α acts through the CNS before directly affecting joint inflammation. Through use of functional MRI (fMRI), we demonstrate that within 24 h after neutralization of TNF-α, nociceptive CNS activity in the thalamus and somatosensoric cortex, but also the activation of the limbic system, is blocked. Brain areas showing blood-oxygen level-dependent signals, a validated method to assess neuronal activity elicited by pain, were significantly reduced as early as 24 h after an infusion of a monoclonal antibody to TNF-α. In contrast, clinical and laboratory markers of inflammation, such as joint swelling and acute phase reactants, were not affected by anti-TNF-α at these early time points. Moreover, arthritic mice overexpressing human TNF-α showed an altered pain behavior and a more intensive, widespread, and prolonged brain activity upon nociceptive stimuli compared with wild-type mice. Similar to humans, these changes, as well as the rewiring of CNS activity resulting in tight clustering in the thalamus, were rapidly reversed after neutralization of TNF-α. These results suggest that neutralization of TNF-α affects nociceptive brain activity in the context of arthritis, long before it achieves anti-inflammatory effects in the joints.
Keywords: cytokines, antiinflammatory therapy
Arthritis is one of the most disabling chronic human diseases. Rheumatoid arthritis (RA) affects up to 1% of the population and is characterized by pain, swelling, and stiffness of joints, leading to a serious decay of life quality. Pain is the initial and prevailing symptom of the disease, leading to immobility, which in turn causes complications such as osteoporosis and cardiovascular disease. During the last 10 y the pharmacologic treatment of diseases such as RA has substantially improved because of the development of cytokine blocking agents (1).
Although inflamed joints express a multitude of mediators, including cytokines, chemokines, and growth factors, which contribute to the pathogenesis of arthritis, inhibition of TNF-α has emerged as a particularly successful therapeutic strategy (2, 3). The therapeutic success of TNF-α blockade in RA is unique and has been largely considered to result from rapid and efficient neutralization of joint inflammation based on breakdown of the inflammatory cytokine network in the affected joint, which results in an improvement of the signs and symptoms of the disease (4). It has, however, always been stunning, how fast the blockade of TNF-α improves the patient condition, in particular because diseases like RA are highly chronic, building up a vast amount of inflammatory tissue, and leading to irreversible damage of the cartilage and the bone. Thus, rapid resolution of this highly organized inflammatory tissue or tissue damage is very unlikely to explain the fast effect of TNF-α blockade. Even more importantly, neutralization of other inflammatory mediators, in particular IL-1, which is a central inducer of inflammation and structural damage in arthritis (5), appears to have less-pronounced and less-rapid effects on the symptoms of RA, although its role in protecting structural changes in the joints is substantial (6, 7).
These observations, and the fact that traditional antirheumatic drugs such as methotrexate achieve far slower clinical responses than TNF-α blockers, have led us to suspect that blockade of TNF-α could has additional effects that go beyond the sole inhibition of joint inflammation. We hypothesized that TNF-α blockade could influence processes in the CNS, which control the patient's perception of the disease state. Indeed, earlier observations in mice have suggested that TNF-α can induce a depressive-like behavior in mice (8). Pain processing and sensation are key mechanisms in the CNS elicited by arthritis, and pain is the dominant symptom of disease, which by far has the strongest impact on the disease burden of arthritis. Importantly, effects on pain also drive the standard read-out parameters for measuring therapeutic response in arthritis. Thus, if TNF-α blockade has unique pain-reducing properties, its efficacy in reducing clinical disease activity of RA will be high, as such disease-activity measures are strongly influenced by pain. In consequence, other treatment strategies, lacking such CNS effects, will less strongly affect the patient's disease state, even if showing similar anti-inflammatory or structure-sparing effects.
Interestingly, inflammation is considered to lower the threshold for pain perception in the CNS, leading to hyperalgesia (9). In contrast to the well-documented role of TNF-α as a proinflammatory cytokine, its role as a mediator of pain is incompletely characterized. It is known that both receptors for TNF-α are expressed in dorsal root ganglion neurons and that intra-articular injection of TNF-α blocking agents reduces nociception elicited by joint inflammation (10). TNF-α is also involved in development of mechanical hyperalgesia following injury (11, 12). Hence, we hypothesized that TNF-α leads to a major change in pain perception in the CNS during arthritis.
To search for the rapid effects of TNF-α blockade, we visualized cerebral nociception elicited by arthritis and its reversibility upon TNF-α blockade using blood-oxygen level-dependent (BOLD) functional MRI (fMRI) as early as 24 h after initiation of the treatment. The BOLD signal reflects changes in hemodynamics linked to increased or decreased neuronal metabolic activity in response to outer sensory stimulation, thus allowing localization of brain areas activated by stimuli (13, 14). As a proof-of-concept, we first evaluated whether TNF-α blockade rapidly reverses the hypernociception in patients with RA. We then performed an in-depth analysis of this rapid effect of TNF-α blockade in a mouse model of arthritis based on the overexpression of human TNF-α.
Results
Reduction of Nociceptive Responses in the CNS of Arthritis Patients by TNF-α Blockade Preceding Its Anti-Inflammatory Effects.
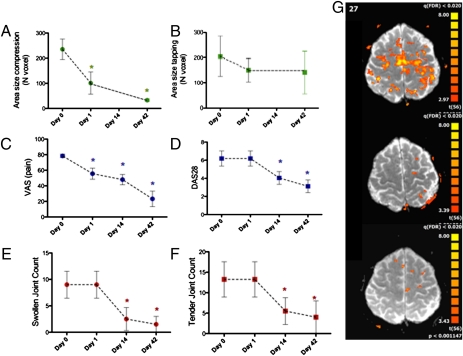
To test whether TNF-α blockade indeed affects the CNS, we first performed a proof-of-concept study in humans. We measured CNS activity in patients with RA by assessing the BOLD signal in the fMRI before and after exposure to an infusion of 3 mg/kg of the TNF-α blocker infliximab (IFX) (15, 16). The BOLD signal was induced by compression of the metacarpophalangeal joints of the dominantly affected hand, which is a standard assessment procedure for RA. Interestingly, the size of the brain area with enhanced BOLD activity was significantly reduced as early as 24 h after administration of the TNF-α blocker IFX. Moreover, it remained low up to 6 wk after the first infusion (Fig. 1A). In contrast, BOLD signals elicited by a standardized control procedure, such as finger tapping, were not affected by TNF-α blockade (Fig. 1B). Importantly, clinical measures of disease activity, such as joint swelling and joint tenderness, composite disease activity scores (DAS), such as DAS28, and acute phase reactants, such as blood sedimentation rate, serum C-reactive protein levels, and serum IL-6 levels, did not change measurably within 24 h after TNF-α blockade but improved later during the treatment (Fig. 1 C–E and Table S1). In contrast, subjective rating of pain intensity using the visual analog scale (VAS) was decreased within 24 h after TNF-α blockade (Fig. 1F). Thus, these data indeed indicate a fast effect of TNF-α blockade on the pain responses in the CNS even before a measurable anti-inflammatory effect is achieved.
Fig. 1.
Rapid reversal of pain induced BOLD signals after TNF-α blockade in patients with RA. (A and B) Area of BOLD signal based on fMRI scans elicited by joint compression (A) and finger tapping (B) in patients (n = 5) with RA before, 1, 14, and 42 d after administration of TNF-α blocking agent IFX. (C) VAS (reaching from 0 to 10) for arthritis-related pain before, 1, 14, and 42 d after administration of IFX; (D) DAS28 before, as well as 1, 14, and 42 d after administration of IFX; (E) swollen and (F) tender joint count based on the assessment of 28 joints. (G) Maps of BOLD activity in a single patient before (Top) as well as 1 (Middle) and 42 d (Bottom) after administration of IFX. Asterisks indicate significant difference to baseline (P < 0.05).
Reduced Activity in CNS Regions Involved in Pain Perception and in the Limbic System After TNF-α Blockade.
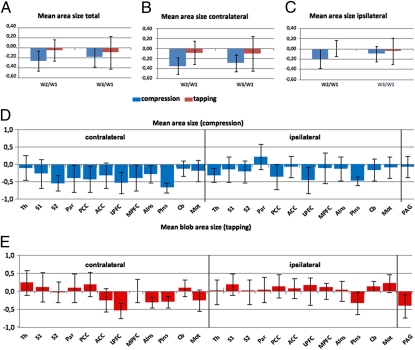
A more detailed analysis of the BOLD signal in arthritis patients showed that reduction of activated brain area size after TNF-α blockade could be attributed to the CNS regions contralateral to the affected joint (Fig. 2 A–C). No significant side difference was found for finger tapping, which was used as a control procedure (Fig. 2 A–C). TNF-α blockade did particularly change the activity of CNS structures, which are involved in pain perception, such as the thalamus and the secondary somatosensoric cortex (Fig. 2 D and E). Interestingly, centers of the limbic system were also affected, such as the cingulate and insular cortex. The cingulate cortex is particularly involved in forming and processing of emotions and memory, whereas the insular region is important for the subjective sense of the inner body, including the experience for pain and emotions (Fig. 2D). These findings suggest that not only pain responses are affected by TNF-α blockade but also CNS structures relevant for creating pain perception, emotions, and body experience.
Fig. 2.
Mapping of BOLD changes after TNF-α blocking therapy. (A–C) Changes in the area size of the BOLD signal elicited by joint compression (blue bars) or finger tapping (red bars) comparing day 0 with day 1 (W2/W1) and day 0 with day 42 (W3/W1) after IFX treatment: overall (A) and specific for the contralateral (B) and ipsilateral hemisphere (C). (D and E) Changes in the area size of the BOLD signal elicited by joint compression (D) or finger tapping (E) comparing day 0 with day 1 (W2/W1) at the following brain regions: thalamus (Th), secondary (S2) and primary (S1), somatosensoric cortex, parietal cortex (Par), posterior cingulate cortex (PCC), anterior cingulate cortex (ACC), lateral (LPFC) and medial (MPFC) prefrontal cortex, anterior (AIns) and posterior (PIns) insular cortex, cerebellum (Cb), motor cortex (M1), and periaqueductal gray (PAG).
Nociceptive Responses in Arthritic Mice Overexpressing Human TNF-α and Their Rapid Reversal by TNF-α Blockade.
To study the effect of TNF-α blockade on CNS pain responses elicited by arthritis in more detail, we used an animal model, which is characterized by an overexpression of human TNF-α in the peripheral joints because of knocking the human TNF-α gene into the mouse genome (17, 18). These TNF-α transgenic (TNFtg) mice spontaneously develop inflammatory arthritis, which leads to progressive functional impairment. The disease itself manifests as progressive swelling of the peripheral joints associated with decreased grip strength, mirroring progressively impaired joint function (Fig. S1). We first assessed the effect of TNF-α overexpression on behavioral pain responses and their reversibility upon TNF-α blockade by IFX. Spinal reflex arches, as measured by the tail-flick test, were not affected in early arthritis of TNFtg mice but were increased in latency at later disease stages (Fig. S2A). When testing mechanical hyperalgesia using von Frey filaments, the force (threshold) needed to elicit responses was significantly lower in early (6 wk) and later stages of the disease (10 wk) (Fig. 2B). The Hargraves test, assessing thermal hyperalgesia, showed a similar pattern, as observed with mechanical hyperalgesia (i.e., increased sensitivity to nociception in early and late stages of the disease of TNFtg mice) (Fig. S2C). In contrast, visceral nociception, measured by intraperitoneal injection of MgSO4, was significantly reduced rather than enhanced in TNFtg mice, both in early and late disease stages, suggesting a desensitization to visceral nociception in TNFtg mice (Fig. S2D). The Rotarod test measuring motor activity was not impaired at an early disease stage but significantly decreased at later stages (Fig. S3A). Similar data were obtained when the suspended-tail test was applied, which showed a significant decrease in overall activity in TNFtg mice but only in later stages of disease (Fig. S3B).
To test whether this nociceptive sensitization is reversible, we treated TNFtg mice with the TNF-α blocker IFX. Whereas the latency of spinal pain reflex arches, as measured by the tail-flick test, were normalized 72 h after TNF-α blockade (Fig. S2E, increased sensitivity to mechanical nociceptive stimuli in TNFtg mice was completely reversed as early as 24 h after injection (Fig. S2F). This profound and rapid antinociceptive effect of TNF-α blockade was further substantiated by the Hargreaves test, which normalized within 24 h after TNF-α blockade and remained at the level of WT mice over 72 h (Fig. S2G). Within this short time period after TNF-α blockade, no apparent change of clinic-analog parameters (such as paw swelling or grip strength) or histopathologic signs of arthritis (such as synovitis) could be observed. Moderate effects on visceral nociception were also observed, as the reduced sensitivity of TNFtg mice to visceral nociception was reversed to WT levels within 72 h after TNF-α blockade (Fig. S2H). TNF-α blockade also completely restored motor activity within the first 24 h (Rotarod test) (Fig. S3C). Similarly, but slightly less pronounced, decreased overall mobility and activity of TNFtg mice as measured by the suspended-tail test also improved upon TNF-α blockade(Fig. S3D).
Mapping of Nociceptive Brain Responses Elicited by Arthritis and Their Modulation by TNF-α Blockade.
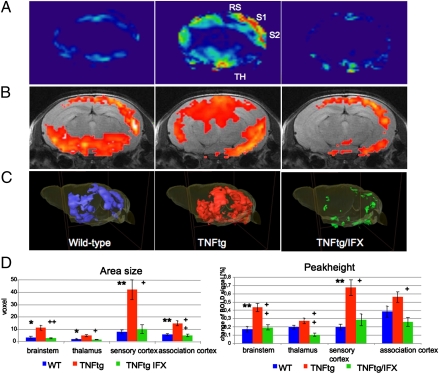
Comparative fMRI analysis of the brain-activity patterns elicited by heat stimulation of the hind paw revealed a significant increase of the BOLD activity in TNFtg mice in the so-called pain matrix (19, 20) of the brain compared with the WT mice (Fig. 3 A–C). TNF-α blockaded to a fast and complete reversal of the increased brain activity in TNFtg mice to the level of WT mice within 24 h. When quantifying the BOLD activity by measuring the area size and peak amplitude of the BOLD signal, we observed a consistent and significant increase in the somatosensoric cortex of TNFtg mice, which was completely abolished after TNF-α blockade (Fig. 3C). Smaller, but still significant, increases (Fig. 3C) were also observed in the thalamus, as well as in the association cortex in TNFtg compared with WT mice, which were again completely reversed upon blockade of TNF-α (Fig. 3D).
Fig. 3.
Reversible enhancement of central pain responses by TNF-α–mediated arthritis. Functional MRI of the brain of 10-wk-old WT and human TNFtg mice without and with human antiTNF-α antibody IFX (each n = 10). (A and B) 2D axial scans with heatmap (A) or superimposed on the corresponding anatomical image (B) show enhanced neuronal activity in the primary (S1) and secondary (S2) somatosensoric cortex, as well as in association cortex (RS) and the thalamus (Th) of TNFtg mice, which is reversible 24 h after IFX administration. (C) 3D reconstruction of functional brain activity. (D) Bar graphs showing area size (Left) and peak height (Right) of the BOLD signal in WT mice (blue) and TNFtg mice treated with either vehicle (red) or the human anti–TNF-α antibody IFX (green). Four key groups of brain regions (brainstem, thalamus, sensory cortex, and association cortex) involved in central nociception are shown. Asterisks indicate significant differences of WT to TNFtg plus those of TNFtg/IFX to TNFtg, with * and + indicating a P value of < 0.05 and ** and ++ a P value of < 0.025) (n = 10).
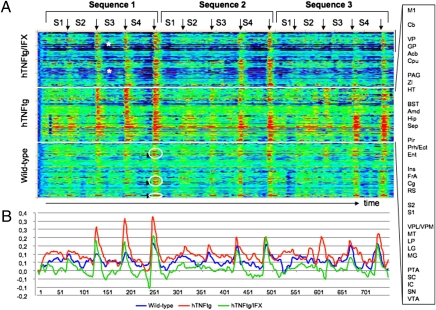
On the basis of these observations, we aimed at precisely mapping the interaction of the temporal structure of the BOLD signals in arthritis (Fig. 4A). Again, amplitudes of BOLD signals were generally higher in TNFtg mice than in WT mice. Moreover, even low-impact subthreshold stimulation led to increased brain activity in TNFtg mice, which was not found in WT mice (red color in Fig. 4A at S1/S2 and peaks in Fig. 4B). This pattern was completely reversed upon TNF-α blockade as early as 24 h after injection. Aside from higher amplitudes, brain activation was more widespread in TNFtg mice, affecting many more brain regions than in WT mice. In the latter, the nociceptive response was localized to several well-defined brain areas (vertical pattern in Fig. 4A, white circles). This spreading of brain activation in TNFtg mice was partially reversed upon blockade of TNF-α. In addition, the increased BOLD activity was maintained even during the intervals between stimulations and did not completely regress to baseline levels (horizontal red lines in Fig. 4A; red line in Fig. 4B is permanently high above baseline). TNF-α blockade rapidly reversed this prolonged activation, and the BOLD signal in the time periods between the stimulations dropped dramatically, at times below the baseline level. This effect was particularly pronounced in somatosensoric cortical and limbic regions of the brain (asterisks at dark blue intervals in Fig. 4A and green line Fig. 4B).
Fig. 4.
Temporal profiles and reversibility of TNF-α–induced changes of central pain response. (A) Temporal profiles showing the intensity of BOLD signals obtained by fMRI of 10-wk-old WT mice and human TNFtg mice treated either with vehicle or with human anti–TNF-α antibody IFX. Colors indicate peak height of the BOLD signal from very low (dark blue) to very high (red). The x axis indicates time with at total of three sequences, each of which comprises four pain stimuli (S1 to S4) with increasing intensity (40, 45, 50, 55 °C). The y axis reflects 32 different brain regions as follows: motor cortex (M1), cerebellum (Cb), ventral pallidum (VP), globus pallidus (GP), nucleus accumbens (Acb), striatum (CPu), periaqueductal gray (PAG), zona incerta (ZI), hypothalamus (HT), bed nucleus of stria terminalis (BST), amygdala (Amd), hippocampus (Hip), septal area (Sep), piriform cortex (Pir), perirhinal/ectorhinal cortex (Prh/Ect), entorhinal cortex (Ent), insular cortex (Ins), frontal association cortex (FrA), cingulate cortex (Cg), retrosplenial cortex (RS), secondary (S2) and primary somatosensory cortex (S1), ventral posterolateral/posteromedial thalamic nucleus (VPL/VPM), medial thalamus (MT), lateral posterior thalamic nucleus (LP), lateral (LG) and medial geniculate nucleus (MG), pretectal area (PTA), superior (SC) and inferior colliculus (IC), substantia nigra (SN), ventral tegmental area (VTA). (B) Curves showing average time-dependent changes of the amplitudes of BOLD signals in WT (blue), hTNFtg mice (red), and the latter treated with the human anti–TNF-α antibody IFX (green).
Rewiring of the Pain Matrix in the CNS by Chronic Arthritis and Its Reversibility by TNF-α Blockade.
Higher-order changes in brain function may not only be reflected in changes of response properties of single brain structures. Instead, dynamic changes of the complex network connectivity (i.e., within the pain matrix) might occur. Graph theory has proven to have a high impact on the quantitative analysis of complex networks (i.e., brain network organization) (19, 20). We therefore wondered if graph theoretical analysis explaining the functional connectivity of CNS regions provides insight into the dynamic of rewiring of the pain matrix in arthritis and after neutralization of TNF-α. After removing the global (mainly task-driven) signals, cross-correlation of the fMRI time profiles of all structures, which were significantly activated, revealed distinct patterns of interactions within the pain matrix (Fig. S4A, lower triangular half). There was an increased degree of connectivity, clustering, and modularity in arthritic TNFtg mice compared with WT mice (Fig. S4A and Table S2). These changes largely resulted from the formation of a tight cluster of the thalamus, the periaqueductal gray, and the amygdala (Kamada-Kawai plots, Fig. S4B). TNF-α blockade led to a rapid partial dissolution of this cluster, resulting in a more diffuse network, indexed by an overall decreased connectivity, clustering, and modularity similar to that observed in WT mice.
Discussion
In this study we show that neutralization of the proinflammatory cytokine TNF-α rapidly affects CNS pain responses elicited by arthritis. Administration of TNF-α blockers rapidly abrogates CNS activity linked to nociceptive stimuli in patients with arthritis, as well as in mice with TNF-α–driven arthritis. This effect occurs immediately after antibody administration and well before anti-inflammatory effects of TNF-α blockade are observed. Our data suggest that TNF-α appears to trigger hypernociception because of changed pain processing in the CNS and a strong linkage to activation of limbic areas involved in pain experience, emotions, and body sensation.
Cytokine blockade has dramatically changed the treatment of inflammatory diseases, particularly of inflammatory arthritides, such as RA. Despite RA being the embodiment of a chronic disease, patients with RA experience a very rapid improvement of disease-related symptoms upon blockade of TNF-α. Although, it takes several weeks to observe a consistent and objective reduction of joint inflammation (16), many patients report on a rapid effect of TNF-α blockade on their subjective disease state. However, the basis of this rapid effect has not been addressed convincingly so far, although CNS effects of TNF-α blockade have always been suspected to play a role in this process. We were stunned by the fact that TNF-α blockade triggered a profound and significant change of CNS activity in RA patients as early as 24 h after its administration, compared with baseline CNS activity. In contrast, joint swelling, as well as composite DAS (DAS28) did not change significantly within the first 24 h, although they improved later. These observations suggest that functional CNS changes clearly precede the improvement of inflammatory signs of arthritis in human patients. Nociceptive signals elicited by joint compression did induce the activity in CNS structures, which are typically involved in pain perception, such as the thalamus and the somatosensoric cortex, but also in parts of the limbic system, such as the cingulum and the insular region, which are relevant for the inner body sensation as well as negative emotional experience, including pain experience. Suppression of neuronal activity in these centers may likely explain the rapid improvement of the symptom state of patients with RA after TNF-α blockade, because the perception of nociceptive stimuli in the CNS is impaired and activation of CNS centers involved in shaping the emotional state of the individual—as well as body perception—is blocked.
In murine arthritis triggered by TNF-α overexpression, we observed that behavioral measurements of nociceptive responses showed a sensitization to somatic nociception (hyperalgesia) during arthritis. Moreover, investigation of arthritic mice by fMRI unequivocally demonstrated an altered pattern of brain activation, as measured by an enhanced BOLD activity. Even low-impact subthreshold stimulation led to increased BOLD signals, which could not be depicted in nonarthritic controls. In support of this notion, TNF-α blockade caused a significant and rapid reduction of these nociceptive BOLD signals in the somatosensoric and the association cortex, as well as the thalamus, within 24 h. Upon neutralization of TNF-α, both thermal and mechanical hyperalgesia induced by arthritis were completely reversed within 24 h, an observation that supports the results obtained in RA patients. Within this short period no apparent effect on clinical analog parameters (such as paw swelling) or histopathologic signs of arthritis (such as synovitis) could be observed. This result indicates that the CNS effect of TNF-α blockade precedes its anti-inflammatory effect.
Interestingly, we even observed a decrease in the BOLD signal below the normal levels observed in nonarthritic WT mice. This effect was again particularly pronounced in somatosensoric cortical and limbic regions of the brain. One explanation for this decrease of the BOLD signal below the baseline level could be an increased activity of the cerebral inhibitory network developed during this chronic pain state (21). In this context, it is worth mentioning that brain network analysis detected a tight cluster consisting of thalamic structures, the periaquaeductal gray, and limbic areas, such as the amygdala in TNFtg mice. This finding supports the concept of an increased efficacy of the cerebral inhibitory network in arthritis. This rewiring may have developed as an adaptation to chronic pain preventing the abnormally increased flow of nociceptive signals to higher CNS structures, especially to the cortex. Importantly, TNF-α blockade rapidly induced partial dissolution of this cluster; this happened within 24 h after TNF-α blockade, in parallel with the reduction of the nociceptive BOLD activity and the restoration of the normal nociceptive behavior.
In summary, these data show that TNF-α leads to more intensive, widespread, and prolonged brain activity upon nociceptive stimulation, which is reversible upon neutralization of TNF-α. This reversal of pain sensitization occurs rapidly and is not primarily linked to the anti-inflammatory effects of TNF-α blockade. In patients with RA, a very similar rapid and profound down-regulation of nociceptive brain activity can be observed, which did precede the alleviation of joint inflammation. Although subclinical anti-inflammatory effects of TNF-α blockade within the first 24 h after exposure to TNF-α blockade cannot be ruled out completely, the lack of a drop in acute phase reactant and cytokine levels within this short time interval does at least not support such a concept. Our findings emphasize that changes of nociceptive responses in the brain precede the anti-inflammatory effect of TNF-α blockers. Importantly, these findings may be a good explanation for the rapid improvement of the symptom state induced by TNF-α blocking therapy. These data also suggest that anticytokine therapy targeting TNF-α may achieve its (fast or rapid) clinical benefit remote from the inflamed joint through influencing CNS processes. Our findings also extend previous observations on the effects of TNF-α in the CNS (i.e., the regulation of expression of clock genes), which may explain the high prevalence of fatigue in inflammatory diseases (22). Finally, our findings also raise the possibility that the assessment of responsiveness of BOLD activity in the CNS of RA patients and patients with other forms of inflammatory arthritis may be used as a surrogate marker for predicting clinical responses to therapeutic intervention in a much faster way than it is currently realized.
Materials and Methods
Mice.
The human TNF-α and TNFtg mice (strain Tg197) were previously described (17). Treatment with IFX (Centocor), a neutralizing chimeric human-murine monoclonal antibody directed against human TNF-α, was done by a single intravenous injection of a dose of 10 mg/kg antibody 24 h before testing (15). Clinical evaluation was performed weekly, starting at 4 wk after birth. Arthritis was evaluated in a blinded manner, as described previously (18). All animal experiments were approved by the local ethics committee of the University of Erlangen-Nuremberg.
Tests for Nociceptive Behavior.
The tail-flick test was conducted using a tail-flick analgesia meter (Columbus Instruments), Hargreaves test by IITC Plantar Analgesia Meter, and measurement of paw-withdrawal latency upon heat stimulation. The mechanical nociception test was performed by applying an ascending series of von Frey hairs (23). In visceral nociception tests, mice were intraperitoneally injected with MgSO4 (120 mg/kg) (23). Depression was tested by the tail-suspension test and locomotor activitiy was tested by Ugo Basile 7750 accelerating Rotarod (Ugo Basile) (24).
Functional MRI in Mice.
WT and TNFtg mice (n = 10 per group, 10 wk) were anesthetized with isoflurane and placed on a cradle inside the magnetic resonance tomograph (Bruker BioSpec 47/40, quadrature head coil) (25). The contact heat stimuli sequences (40, 45, 50, and 55 °C, plateau for 5 s, ramp 15 s) were presented at the right hind paw with 3-min and 25-s intervals, three times, using a custom-made computer-controlled Peltier heating device. A series of 750 sets of functional images (matrix 64 × 64, field of view 15 × 15 mm, slice thickness 0.5 mm, axial, 22 slices) were sampled using gradient echo-based Echo Planar Imaging Technique (single shot: TR = 4,000 ms, TEef = 24.38 ms, NEX = 2) within 50 min. Finally, 22 corresponding anatomical T2 reference images (RARE, slice thickness 0.5 mm, field of view 15 × 15 mm, matrix 256 × 128, TR = 2,000 ms, TEef =56 ms) were taken as previously described in detail (26).
Patients.
Five female patients with RA, failing on standard treatment with disease-modifying antirheumatic drugs and having active disease with joint tenderness and swelling, received the TNF-α blocker IFX at a dose of 3 mg/kg as an intravenous infusion. Mean (± SD) age was 56.3 ± 8.2 y and mean (± SD) disease duration was 8.5 ± 3.3 y. The number of tender and swollen joints, joint pain (VAS ranging from 0 to 10), overall disease activity calculated by the DAS based on 28 joints (DAS28) (27), C-reactive protein, and IL-6 levels were assessed at baseline and 1, 14, and 42 d after the infusion.
Functional MRI in Humans.
All anatomical and fMRI data were acquired on a 3T scanner (Magnetom Trio; Siemens) using a standard eight-channel phased-array head coil. The ethics committee of the University Clinic of Erlangen approved all procedures and written informed consent was obtained from all patients. For anatomic datasets, we used a T1-weighted MPRAGE-sequence (field-of-view = 256 mm, matrix size = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm3, slices = 176, slice thickness = 1 mm, TR = 1,900 ms, TE = 1.13 ms). For each subject, two experiments with different stimulation conditions (first, finger-tapping and second, compression of the metacarpophalangeal joints) were performed. In each of them, 93 whole-brain images were obtained with a gradient-echo, echo-planar scanning sequence (TR = 3,000 ms, TE = 30 ms, flip angle 90°; field-of-view, 220 mm2, acquisition matrix 64 × 64, 36 axial slices, slice thickness 3 mm, gap 0.75 mm).
Functional MRI Analysis.
Functional analysis was performed for mice and humans using Brain Voyager QX (Version 10.3) and our own software MagnAn (25), as previously described (28). In summary, after preprocessing [motion-corrected using sinc interpolation Gaussian spatial (human: FWHM = 4 mm, mouse: 0.469 mm) and temporal (FWHM = 3 volumes) smoothing], general linear modeling analysis with separate predictors for each stimulus was performed. The statistical parametric mapping obtained were corrected for multiple comparisons, false-discovery rate threshold at z-score level of 3.3, and different groups of activated voxels were labeled as belonging to certain brain structures based on (i) the mouse atlas from Paxinos (29) or (ii) the Mai atlas of the human brain (30). The voxels, which were significantly activated by the above criterion, were counted as the activated volume per given brain region. The mean corresponding peak activity was determined for each stimulation temperature for mice and for tapping and compression in humans, averaged over all activated brain structures and finally over all subjects of one experimental group, respectively, to provide a global group comparison.
Graph Theoretical Analysis.
Functional connectivity patterns were computed as cross-correlations of the residuals after the global signal mean was removed by linear regression and represented as correlation matrices. Similarity across these correlation matrices was calculated as Spearman rank correlation. Modularity and clustering coefficients, as well as several centrality measures, were computed from the weighted pattern of positive correlations present between brain regions (31). The network modularity index was obtained by optimally subdividing the network into modules, such that most connections are made within modules and only few connections exist between modules (32, 33). Clustering was computed from the weighted functional-connectivity matrix (34) and scaled relative to a population of 100 random networks with identical degree distribution. Node-strength centrality was measured as the sum of each node's positive cross-correlations. The networks are visualized using a force-based algorithm after Kamada-Kawai for achieving that all edges are of more or less equal length, and there exist as few crossing edges as possible (35).
Statistical Analysis.
Data are presented as mean ± SEM. Group mean values were compared by the Student's t tests in a task-specific single-test framework to assess significant differences between the different mouse strains.
Supplementary Material
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft FG 661/TP4 and SPP1468-Immunobone (to A.H. and G.S.); the Bundesministerium für Bildung und Forschung projects 01EM0514, 01GQ0731, 0314102, Ankyloss and Immunopain (to G.S. and A.H.); the Masterswitch, Kinacept, and Adipoa projects of the European Union (to G.S.); the Interdisciplinary Centre for Clinical Research and the Erlanger Leistungsbezogene Anschubfinanzierung und Nachwuchsförderung (ELAN) fund of the University of Erlangen-Nuremberg (to G.S. and A.H.); K.B. is Doerenkamp Professor for Innovations in Animal and Consumer Protection.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 3461.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011774108/-/DCSupplemental.
References
- 1.Smolen JS, Steiner G. Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov. 2003;2:473–488. doi: 10.1038/nrd1109. [DOI] [PubMed] [Google Scholar]
- 2.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 3.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 4.Brennan FM, Jackson A, Chantry D, Maini R, Feldmann M. Inhibitory effect of TNFa antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989;334:244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello CA. IL-1: Discoveries, controversies and future directions. Eur J Immunol. 2010;40:599–606. doi: 10.1002/eji.201040319. [DOI] [PubMed] [Google Scholar]
- 6.Singh JA, et al. Biologics for rheumatoid arthritis: An overview of Cochrane reviews. Cochrane Database Syst Rev. 2009;4:CD007848. doi: 10.1002/14651858.CD007848.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Berg WB. Uncoupling of inflammatory and destructive mechanisms in arthritis. Semin Arthritis Rheum. 2001;30(5) Suppl 2:7–16. doi: 10.1053/sarh.2001.23704. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor JC, et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinold H, et al. Spinal inflammatory hyperalgesia is mediated by prostaglandin E receptors of the EP2 subtype. J Clin Invest. 2005;115:673–679. doi: 10.1172/JCI200523618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boettger MK, et al. Antinociceptive effects of tumor necrosis factor alpha neutralization in a rat model of antigen-induced arthritis: evidence of a neuronal target. Arthritis Rheum. 2008;58:2368–2378. doi: 10.1002/art.23608. [DOI] [PubMed] [Google Scholar]
- 11.Marchand F, et al. Effects of Etanercept and Minocycline in a rat model of spinal cord injury. Eur J Pain. 2009;13:673–681. doi: 10.1016/j.ejpain.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Schäfers M, et al. Selective stimulation of either tumor necrosis factor receptor differentially induces pain behavior in vivo and ectopic activity in sensory neurons in vitro. Neuroscience. 2008;157:414–423. doi: 10.1016/j.neuroscience.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thulborn KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta. 1982;714:265–270. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- 15.Knight DM, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993;30:1443–1453. doi: 10.1016/0161-5890(93)90106-l. [DOI] [PubMed] [Google Scholar]
- 16.Maini R, et al. ATTRACT Study Group Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: A randomised phase III trial. Lancet. 1999;354:1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 17.Keffer J, et al. Transgenic mice expressing human tumour necrosis factor: A predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diarra D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 19.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Borsook D, Becerra L. Phenotyping central nervous system circuitry in chronic pain using functional MRI: considerations and potential implications in the clinic. Curr Pain Headache Rep. 2007;11:201–207. doi: 10.1007/s11916-007-0191-7. [DOI] [PubMed] [Google Scholar]
- 21.Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- 22.Cavadini G, et al. TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad USA. 2007;104:12843–12848. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whishaw IQ. In: The Behavior of the Laboratory Rat: A Handbook with Tests. Whishaw IQ, Kolb B, editors. Oxford: Oxford University Press; 1999. pp. 478–486. [Google Scholar]
- 24.Jones BJ, Roberts DJ. The quantiative measurement of motor inco-ordination in naive mice using an acelerating rotarod. J Pharm Pharmacol. 1968;20:302–304. doi: 10.1111/j.2042-7158.1968.tb09743.x. [DOI] [PubMed] [Google Scholar]
- 25.Hess A, Sergejeva M, Budinsky L, Zeilhofer HU, Brune K. Imaging of hyperalgesia in rats by functional MRI. Eur J Pain. 2007;11:109–119. doi: 10.1016/j.ejpain.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Hennig J, Nauerth A, Friedburg H. RARE imaging: A fast imaging method for clinical MR. Magn Reson Med. 1986;3:823–833. doi: 10.1002/mrm.1910030602. [DOI] [PubMed] [Google Scholar]
- 27.Prevoo ML, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 28.Knabl J, et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotactic Coordinates. Burlington, MA: Elsevier Academic Press; 2001. [Google Scholar]
- 30.Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. Burlington, MA: Elsevier Academic Press; 2008. [Google Scholar]
- 31.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Girvan M, Newman ME. Community structure in social and biological networks. Proc Natl Acad Sci USA. 2002;99:7821–7826. doi: 10.1073/pnas.122653799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman ME. Analysis of weighted networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70:056131. doi: 10.1103/PhysRevE.70.056131. [DOI] [PubMed] [Google Scholar]
- 34.Onnela JP, Saramäki J, Kertész J, Kaski K. Intensity and coherence of motifs in weighted complex networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71:065103. doi: 10.1103/PhysRevE.71.065103. [DOI] [PubMed] [Google Scholar]
- 35.Kamada T, Kawai S. An algorithm for drawing general undirected graphs. Inf Process Lett. 1989;31:7–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.