Abstract
Excessive release of proinflammatory cytokines by innate immune cells is an important component of the pathogenic basis of malaria. Proinflammatory cytokines are a direct output of Toll-like receptor (TLR) activation during microbial infection. Thus, interference with TLR function is likely to render a better clinical outcome by preventing their aberrant activation and the excessive release of inflammatory mediators. Herein, we describe the protective effect and mechanism of action of E6446, a synthetic antagonist of nucleic acid-sensing TLRs, on experimental cerebral malaria (ECM) induced by Plasmodium berghei ANKA. We show that in vitro, low doses of E6446 specifically inhibited the activation of human and mouse TLR9. Tenfold higher concentrations of this compound also inhibited the human TLR8 response to single-stranded RNA. In vivo, therapy with E6446 diminished the activation of TLR9 and prevented the exacerbated cytokine response observed during acute Plasmodium infection. Furthermore, severe signs of ECM, such as limb paralysis, brain vascular leak, and death, were all prevented by oral treatment with E6446. Hence, we provide evidence that supports the involvement of nucleic acid-sensing TLRs in malaria pathogenesis and that interference with the activation of these receptors is a promising strategy to prevent deleterious inflammatory responses that mediate pathogenesis and severity of malaria.
Keywords: immunotherapy, innate immunity, nucleic acid recognition, inflammation
Malaria is still an important global concern affecting millions of people and putting 40% of the world's population at risk (1). Thanks to global governmental and nongovernmental initiatives, malaria vaccine development and testing are progressing rapidly. It is expected that such vaccines might diminish levels of severe malaria and give infants and small children a better chance of surviving the scourge (2). No efficacious vaccine for malaria exists yet, however, and most children are still vulnerable to the disease. We thus continue to rely on the development of effective therapeutical compounds to lessen symptoms and lethality in cases in which the infection takes hold.
Development of immunomodulatory chemotherapies is also an important strategy to improve the management of patients undergoing acute malaria episodes. Our view is supported by reports of people living in areas where transmission is holoendemic. In those areas, people are continuously infected by Plasmodium but the majority of infected adults rarely experience overt disease (3, 4). Although clinically immune, those individuals often remain persistently parasitemic. They usually go about their routines feeling essentially healthy despite harboring a parasite load that would almost universally prove lethal to a malaria-naive visitor. This vigor in the face of infection suggests that clinical manifestations of malaria are caused by an exaggerated host immune response to Plasmodium infection rather than the sole presence of blood parasites. Indeed, untreated or inadequately treated Plasmodium falciparum infections can persist for a long time in the human host (5), sometimes with no patent clinical symptoms.
Much of the overreactive immune responses result from activation of innate immune receptors such as the Toll-like receptors (TLRs), which are critical for proinflammatory cytokine production during microbial challenge. Since their discovery in the 1990s, a relatively large bulk of evidence implicating TLRs in the pathogenesis of multiple immune-mediated inflammatory disorders (IMIDs), such as systemic lupus erythematosus and rheumatoid arthritis, has accumulated. There is now sufficient validation around certain TLRs to justify them as therapeutical targets (6, 7). Curiously, antimalarial drugs were widely used for the treatment of IMIDs long before their inhibitory effect on intracellular TLRs was recognized (8).
The role of TLRs in the pathogenesis of malaria has been proposed by different studies. Although some conflicting data exist (9), the vast majority of studies (10–17), including our own (18–20), indicate that Plasmodium parasites possess intrinsic TLR agonists, which are important components of the proinflammatory responses and the immune-mediated symptoms in both rodent and human malaria. In addition, we observed a hyperresponsiveness of TLRs during acute episodes of human and rodent malaria (19). Long before TLRs were discovered and TLR4 was recognized as the receptor for LPS, it was shown that the amount of Escherichia coli LPS considered lethal to naive mice is decreased several hundredfold in mice infected with different Plasmodium species (21). This phenomenon remained unexplained until recently, when it was shown that malaria causes proinflammatory priming of innate immune responses (16, 19, 22). Our group has shown that TLR9 activation is a key mechanism in malaria-mediated inflammatory priming, because WT mice became extremely susceptible to low doses of LPS, whereas TLR9−/− mice were partially protected (19).
We therefore hypothesize that inhibiting activation of TLR9, or eventually other nucleic acid-sensing TLRs, by antibodies, peptides, or small molecules will render a better clinical outcome by preventing the excessive production of inflammatory mediators, a trait of acute malaria. With this in mind, we tested E6446, an unique synthetic antagonist for nucleic acid-sensing TLRs (Fig. 1), as an immunomodulatory strategy during acute episodes of malaria. The effect of oral treatment with E6446 was evaluated on an experimental cerebral malaria (ECM) model of Plasmodium berghei ANKA (PbA). We show that treatment with E6446 reduced the levels of proinflammatory cytokines produced during acute malaria and protected mice from ECM. Furthermore, mice with mutant nonfunctional UNC93b, a protein that is essential for the trafficking of nucleic acid-sensing TLRs from the endoplasmic reticulum to the endosomal compartment, are partially resistant to ECM. These findings provide evidence that the nucleic acid-sensing TLRs are crucial for the pathogenesis of PbA-induced ECM. Thus, E6446 inhibits the endosomal activation of nucleic acid-sensing TLRs in vivo and prevents ECM.
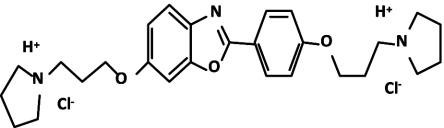
Fig. 1.
Structure of the E6446 compound. E6446 is a small, water-soluble, aromatic organic compound. Its bioactive structure is composed of benzoxazole with two-sided pyrrolidine rings.
Results
Target Inhibition of Nucleic Acid-Sensing TLRs by E6446.
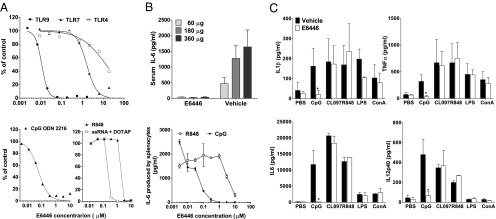
To test the effect of E6446 on TLR activation, we stimulated HEK293 cells stably transfected with TLR4/MD2, TLR7, or TLR9 and the ELAM-1–luciferase reporter gene under NF-κB promoter (23) with each of the corresponding ligands (LPS, R848, or CpG ODN 2006, respectively) in the presence of increasing concentrations of E6446. Measurement of ELAM-1–luciferase activity showed that E6446 specifically inhibited TLR9 activation with CpG ODN 2006, in the range of 0.01–0.03 μM. A 100-fold higher concentration (2–8 μM) of E6446 was required to inhibit TLR7/8 activated by the imidazoquinoline compound R848. Incubation of cells with even higher concentrations (30 μM) of E6446 was required to reduce 50% of TLR4 activation (Fig. 2A). Treatment of human peripheral blood mononuclear cells (PBMCs) with E6446 also diminished IL-6 production in response to the TLR9 (CpG ODN 2402) and TLR8 [single-stranded RNA (ssRNA)] agonists (24), in the range of 0.05 and 0.5, respectively. Approximately 5 μM was required to inhibit 50% of the response to R848, which also activates mouse TLR7 and human TLR8 (25) (Fig. 2A, Lower). Chloroquine, a widely used antimalarial drug, has immunomodulatory properties (26), specially by interfering with endosomal TLR function (27), and has been used in the treatment of autoimmune disorders (28). We thus compared the effect of E6446 with that of hydroxychloroquine. When tested in HEK-TLR9 cells stimulated with oligo 2006, E6446 was eightfold more potent than hydroxychloroquine (mean IC50 over several experiments of 0.01 vs. 0.08 μM for hydroxychloroquine). Similarly, in human PBMCs stimulated with oligo 2216, the IC50 for E6446 is 0.23 μM, whereas for hydroxychloroquine, it is 1.2 μM.
Fig. 2.
Targeting inhibition of nucleic acid-sensing TLRs by E6446. (A) (Upper) HEK293 cells stably transfected with plasmids carrying TLR4/MD2, TLR7, or TLR9 genes and the NF-κB reporter gene ELAM-1–luciferase were stimulated with the appropriate ligand (LPS with soluble CD14, R848, or oligo 2006) overnight. Next, Steady-Glo reagent (Promega, Inc.) was added to the wells, and the amount of luciferase activity in each sample was quantified in a Wallac Envision counter. (Lower) Ficoll-separated mononuclear cells were isolated from healthy volunteer donors, washed, and plated with stimulatory oligonucleotide CpG ODN 2216, R848, or ssRNA in the presence of the lyposomal trasfection reagent DOTAP in complete RPMI for 72 h. IL-6 levels in supernatant were quantified by ELISA (R&D Systems) and expressed as the percentage of levels found in nonstimulated cell supernatants. (B) (Upper) BALB/c mice were predosed with 60 mg/kg of E6446 1.5 h before challenge with the indicated dose of Oligo 1668 (Oligos, Etc.). At 2 h postchallenge, serum was collected and assayed for IL-6 by ELISA. (Lower) Spleen cells from mice were incubated with CpG ODN 1401 (1 μg/mL) or R818 (1 μM) for 72 h in the presence of increasing concentrations of E6446. (C) Mice were orally treated with 20 mg·kg−1·d−1 of E6446 during 5 d. Two hours after the last dose, spleen cells were harvested and challenged with the indicated stimuli. Cytokine levels in culture supernatants were analyzed by Searchlight multiplex. *P < 0.05.
To test the in vivo efficacy of E6446 on inhibition of TLR9, C57BL/6 mice were orally treated with 60 mg/kg of E6446 1.5 h before challenge with increasing concentrations of CpG ODN 1668. Oral treatment with E6446 completely inhibited IL-6 production in sera 2 h after challenge with oligonucleotide containing CpG motifs (Fig. 2B). Consistent with what we observed for human PBMCs, incubation of mouse spleen cells with increasing concentrations of E6446 diminished in vitro IL-6 production in response to CpG ODN 1668 (0.01–0.1 μM) and R848 (1–10 μM). The specific activity of E6446 on TLR9 activation was further confirmed in vivo. First, dose–response experiments showed that mice treated with 20 or 60 mg·kg−1·d−1 of E6446 presented dose-dependent inhibition of TLR9 signaling (Table S1). Next, BALB/c mice were orally treated with 20 mg·kg−1·d−1 of E6446 during 5 d. Two hours after the last dose, the spleen cells were harvested and cultured in the presence of the indicated TLR ligands or Con A. Multiplex analysis for various cytokines showed that 20 mg·kg−1·d−1 of E6446 caused target inhibition of responses to the TLR9 agonist (CpG ODN) but not to the synthetic nonnucleic acid agonists of TLR7 (i.e., CL097, R848) or LPS (Fig. 2C), further indicating the specificity of the E6446 compound.
E6446 Prevents Hyperresponsiveness of TLRs and LPS-Induced Septic Shock in Rodent Malaria.
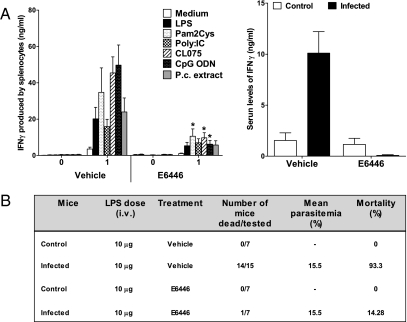
Because of its ability to block TLR activation by nucleic acids, we decided to evaluate the effect of E6446 on malaria pathogenesis. We first evaluated the efficacy of E6446 treatment on Plasmodium chabaudi infection. Although not usually lethal, we have previously shown that this murine model causes a proinflammatory priming of TLR responses rendering mice extremely susceptible to low doses of LPS (19). Treatment with 60 mg·kg−1·d−1 of E6446 diminished TLR responsiveness during acute malaria (Fig. 3A). Furthermore, when given at a higher concentration (120 mg·kg−1·d−1), which was not toxic, E6446 protected infected mice from LPS-induced shock (Fig. 3B). Of note, mice treated with 120 mg·kg−1·d−1 of E6446 presented even higher resistance (86% survival) to LPS-induced shock than TLR9−/− mice (38% survival) (19) compared with vehicle-treated mice (<10% survival). Based on our in vitro experiments (Fig. 2), it is conceivable that, in vivo, 120 mg·kg−1·d−1 of E6446 was able to inhibit activation of both TLR7 and TLR9. If this is the case, these results suggest that along with TLR9, TLR7 and TLR8 (in humans) may be involved in priming and enhancing the responsiveness of TLRs, systemic inflammatory reaction, and pathogenesis of malaria. Importantly, the highest serum drug concentration reached in vivo at a dose of 120 mg/kg is around 1 μM, which is 30- to 50-fold less than the E6446 concentration required to block in vitro activation of TLR4 by LPS. Furthermore, treatment with E6446 (120 mg·kg−1·d−1) did not prevent the death of noninfected mice after challenge with a lethal dose of LPS (500 μg) reinforcing the specific effect of E6446 for nucleic acid-sensing TLRs but not for TLR4, which is activated by LPS (Fig. 2A).
Fig. 3.
E6446 prevents priming of TLR proinflammatory responses. C57BL/6 mice were treated with 60 mg·kg−1·d−1 of E6446 or vehicle 1 d before and during 6 d postinfection with 105 P. chabaudi iRBCs. (A) At 7 d postinfection, spleens were harvested and cultured in the presence of LPS (1 μg/mL), Pam3cysK4 (1 μg/mL), Poly:IC (100 μg/mL), CL075 (100 ng/mL), CpG ODN (1 μg/mL), or P. chabaudi extract (100 μg/mL) for 48 h. Cytokine levels in cell culture supernatant or in sera were assessed by ELISA. P.c., P. chabaudi. (B) Mice were treated with 120 mg·kg−1·d−1 during 6 d postinfection with 105 P. chabaudi iRBCs. Mice were challenged i.v. at 7 d postinfection with 10 μg of LPS. Survival was monitored from 12–48 h after LPS challenge. An asterisk marks where comparisons reached statistically significant (P < 0.05).
E6446 Prevents ECM in Mice.
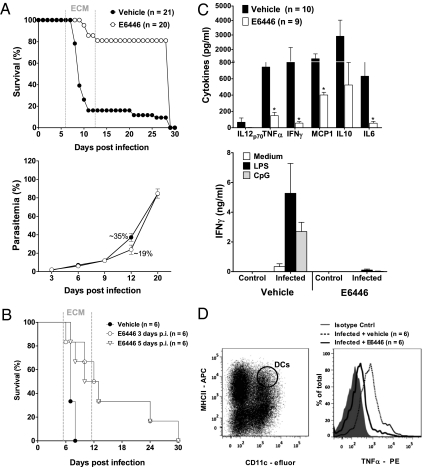
We next tested if blockade of cell signaling through nucleic acid-sensing TLRs has any impact on PbA-induced ECM. C57BL/6 mice were orally treated with 120 mg·kg−1·d−1 of E6446 or vehicle from day −1 to day 12 postinfection with 1 × 105 PbA-infected red blood cells (iRBCs). As expected for this malaria model (29), most mice treated with vehicle succumbed to PbA-mediated ECM by day 6–12 postinfection (Fig. 4A, Upper). Although no change in parasitemia was observed (Fig. 4A, Lower), treatment with E6446 had a pronounced effect on enhancing survival after infection with PbA.
Fig. 4.
E6446 protects mice against PbA-mediated CM. C57BL/6 mice were treated with 120 mg·kg−1·d−1 of E6446 1 d before and during 12 d postinfection with 105 PbA-infected erythrocytes (iRBCs). Mice treated with vehicle (acidified water) were infected and used as a control group. (A) Survival and parasitemia were compared between groups of mice at various days postinfection. (B) Effect of emergency treatment with E6446 on ECM. Mice were infected with 105 PbA iRBCs and treated with E6446 beginning at different days postinfection. p.i., postinfection. Survival was monitored throughout infection. (C) Cytokine levels were assessed in the sera (10 mice per group) or in supernatants of splenocytes cultured in the presence of LPS (1 μg/mL) or CpG ODN (1 μg/mL). (D) Analysis of intracellular staining of cytokines in CD11c+ MHCII+ splenic DCs from vehicle- or E6446-treated mice at 6 d postinfection. Results are from a sum of four different experiments that yielded similar results. An unpaired two-tailed Student's t test or Mann–Whitney U test was used to compare means between cytokine levels detected in the vehicle-treated or E6446-treated group of mice. A P value <0.05 was considered to be statistically significant.
To address the compelling efficacy of E6446 on an already established disease, we tested whether treatment with this compound could prevent the development of PbA ECM before the onset of symptoms. Mice were infected with PbA and treated with 120 mg/kg of E6446 from day 3, 5, or 7 up to day 12 postinfection. Although the efficacy of E6446 in preventing ECM decreased, mice treated with this compound had a better survival rate than vehicle-treated mice (Fig. 4B). E6446 failed to protect mice when administered after 6 d postinfection at the onset of the cerebral syndrome.
We next evaluated the effect of E6446 treatment on the cytokine profile during PbA infection. Mice treated with E6446 produced significantly lower levels of proinflammatory cytokines (Fig. 4C, Upper). Furthermore, PbA-mediated priming of TLR responses was also diminished by E6446 therapy (Fig. 4C, Lower). Nucleic acid-sensing TLRs (e.g., TLR9, TLR7) are highly expressed on dendritic cells (DCs) and were shown to be activated by Plasmodium products that play a relevant role in malaria pathogenesis (18, 30, 31). We thus sought to identify the effect of E6446 on cytokines produced by myeloid CD11c+ MHCII+ DCs using a flow cytometry (FACS) assay for intracellular cytokine staining. We found that in mice infected with PbA, the production of TNF-α was significantly increased at day 6 postinfection compared with noninfected mice. TNF-α production was abolished in cells from PbA-infected mice treated with E6446 (Fig. 4D).
To confirm the involvement of endosomal TLRs in ECM pathogenesis, we infected “three-deficient” (3d) mice with PbA. The 3d mice have a single mutation in the UNC93B1 gene and are not responsive to TLR3, TLR7, or TLR9 agonists (32). Importantly, our results in 3d mice resemble the results obtained in mice treated with E6446. These mice showed impaired production of proinflammatory cytokines and were more resistant to PbA-induced ECM, although presenting with similar parasitemia (Fig. S1). Hence, these results further support the hypothesis that E6446 therapy protects against PbA-induced ECM by blocking activation of nucleic acid-sensing TLRs.
Symptoms of ECM Are Attenuated by E6446 Therapy.
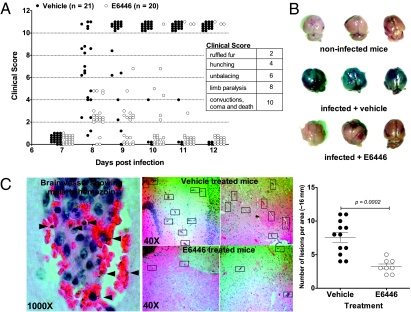
Brain seizures with severe debilitation are strong indicators of PbA ECM. We therefore performed neurological examinations in each mouse during acute PbA infection. We show that mice treated with E6446 attained a better clinical outcome; symptoms were attenuated compared with vehicle-treated mice (Fig. 5A). Proinflammatory cytokines, such as TNF-α and IL-1β, have been shown to affect blood–brain barrier (BBB) permeability, leading to leak and infiltration of mononuclear cells contributing to ECM (33). Because of its inhibitory effect on cytokine production in mouse cells (Fig. 2B) and in malaria infection (Figs. 3B and 4C), we next tested the effect of E6446 on the integrity of the BBB during PbA infection. We assessed cerebral vascular leakage after i.v. injection of 1% Evans blue in mice with end-stage ECM. Mice treated with vehicle displayed a distinct vascular leakage, shown by a blue staining of the brain, that was absent in E6446-treated mice (Fig. 5B). The morphological correlate of the vascular leak was investigated in brain tissue sections. Consistent with the macroscopic observation, brain vessels of infected mice treated with vehicle displayed typical microvascular damage with sequestration of iRBCs, including the presence of hemozoin, mononuclear cell adhesion on the endothelium, and hemorrhage into the parenchyma, which was much less frequent in E6446-treated mice. In general, PbA-infected mice treated with E6446 presented significantly lower numbers of brain intravascular inflammatory foci (Fig. 5C).
Fig. 5.
E6446 prevents breakdown of the BBB and neurological signs of malaria. (A) Each of the classic CM symptoms (ruffled fur, abnormal posture, unbalancing, limb paralysis, convulsion, coma, and death) was given a score (0, 2, 4, 8, or 10). Mice were then graphically ranked based on symptoms presented at each time point. Open and closed circles represent mice treated with either E6446 or vehicle. (B) After 7 d of infection, mice were injected i.v. with 0.2 mL of 1% Evans blue (Sigma–Aldrich) shortly before the death of vehicle-treated mice (120 mg·kg−1·d−1). One hour later, mice were killed and brain coloration was assessed. Naive mice were also injected with Evans blue and used as a control. (C) Brains from vehicle- or E6446-treated mice were harvested at day 7 postinfection and fixed in formalin; brain sections were then stained with H&E. Brain lesions were identified over oil immersion 1,000× magnification (Left) and counted over 40× magnification (Right) on an optical microscope. Statistical analysis was performed using an unpaired two-tailed Student's t test. A P value <0.05 was considered to be statistically significant.
Discussion
Severe malaria is a complex multisystem disorder in which multiple parasite and host factors contribute to disease severity and outcome. Nevertheless, years of studies on malaria have progressively shown an important role for proinflammatory cytokines in disease pathogenesis (34, 35). It is currently known that parasite sequestration is exacerbated by cytokine-mediated up-regulation of host endothelial receptors that bind to parasitized erythrocytes (i.e., intracellular adhesion molecule-1, vascular cellular adhesion molecule) (15, 33, 35, 36). Studies in mice with deficiency in several inflammatory mediators (e.g., TNF-α) (37), other cytokines, and chemokines (38) suggest that the ECM model used in this study is mainly a cytokine-associated encephalopathy. Furthermore, it is accepted that severe anemia, another important cause of disease severity common to P. falciparum and Plasmodium vivax malaria, does not occur as a result of destruction of iRBCs per se, because for every iRBC destroyed during malaria, ∼32 non-iRBCs are removed from the circulation (39). Cytokines such as TNF-α and IFN-γ suppress hematopoiesis and enhance uptake of noninfected erythrocytes (40), contributing to anemia (41–43). Thus, irrespective of the underlying events driving the pathogenesis of malaria, there is strong evidence for the direct or indirect implications of proinflammatory cytokines (34, 35).
One of the top priorities in current malaria research is the development of affordable strategies for preventing severe disease in Plasmodium-infected patients. Thus, understanding the complexity of malaria pathogenesis is essential to develop strategies to control disease severity, which include therapeutical drugs and prophylactic vaccines. Although acquired immunity following malarial infection has been thoroughly studied, innate immunity has not been subjected to similar scrutiny, let alone considered promising for therapeutical targets.
Indeed, high levels of TNF-α and other proinflammatory cytokines, such as IFN-γ and IL-6, have long been associated with acute malaria episodes, including cerebral malaria (CM) (34, 35). Therapy with anti-TNF mAbs did not protect children with CM, however (44). Importantly, TLRs are essential in driving the production of proinflammatory cytokines during malaria. Indeed, Coban et al. (13) have shown that MyD88−/− and TLR9−/− mice are more resistant to PbA-induced ECM. In addition, TLR9−/− mice infected with P. chabaudi are partially protected from i.v. injection of a low dose of LPS (19). Together, these results suggest that, in addition to TLR9 (12, 17–20), other MyD88-dependent TLRs are involved in the inflammatory response and pathogenesis of ECM. In this regard, the use of E6446 to treat malaria is highly appropriate. Instead of neutralizing TNF-α or other single cytokine activity, E6446 prevents cellular activation by nucleic acid-sensing TLRs, presenting a broader inhibitory effect on proinflammatory cytokine production. Our results show that treatment with E6446 prevented hyperresponsiveness of TLR responses as well as development of ECM during acute rodent malaria. Importantly, treatment with E6446 did not affect parasitemia, suggesting that the observed effect was attributable to modulation of excessive proinflammatory response during Plasmodium infection. Furthermore, the resistant phenotype of 3d mice, deficient in TLR3, TLR7, and TLR9 functions, strengthens our conclusions that nucleic acid-sensing TLRs are critical for the inflammatory response and pathogenesis of ECM.
As in many diseases associated with sepsis, the innate immune system appears to have a central role in causing its most deleterious sequelae during acute malaria episodes. Moreover, our results suggest that hyperresponsiveness of TLRs favors the direct activation of innate immune cells by Plasmodium components. Thus, the parasite-derived TLR agonists released during rupture of iRBCs would be partially responsible for TLR-induced cytokinemia as well as for the signs and/or symptoms observed during acute episodes of malaria, as is the case for paroxysm. Activation and priming of innate immune responses seem to occur early during malaria, which may explain why emergency treatment with E6446 failed to protect mice when started at end-stage disease. Indeed, chloroquine, which has been widely used in this model of ECM, is also ineffective in inhibiting further development of cerebral syndrome and saving mice with end-stage disease (45).
Until P. falciparum is eradicated, the goal must be to reduce the incidence of disease. It is through the study of pathogenesis in the laboratory and in the field that methods can be developed to reduce disease in the tropics. Therefore, E6446, a small molecule that acts as an antagonist for nucleic acid-sensing TLRs seems to be a real alternative to prevent severe and lethal malaria. The development of unique therapeutical strategies for prevention of clinical malaria is welcome, especially after recent reports that artemisinin-based therapies are failing a growing number of patients (46, 47). We therefore believe that E6446 or other immunomodulatory therapies can be used to prevent or delay the sepsis related-symptoms and CM observed during malaria infection, and thus provide additional time for the action of conventional antimalarial drugs to eradicate infection in patients who have malaria.
Methods
Mice.
C57BL/6 mice (originally purchased from Jackson Laboratories) 6–8 wk of age (Animal Unit, University of Massachusetts) were caged in groups of four animals. The animals were housed under standard conditions and had free access to a standard diet and tap water. Mice with deficiency to UNC93B1 (3d mice) were a gift from Bruce Beutler (The Scripps Research Institute, La Jolla, CA). All animal work was approved by the appropriate institutional animal care and use committee.
Drug and Treatment.
The compound E6446-02 (Eisai) was dissolved in water, and its concentrations were adjusted so that the final dose in body weight (mg/kg) was given in 0.1 mL. Mice treated with vehicle (water) were used as a control group. The animals were treated through oral gavage once daily at the dose stated for several days postinfection. The drug was administered 24 h before infection.
In Vitro Assays.
E6446 was assayed for suppression of freshly isolated human PBMCs or BALB/c mouse spleen IL-6 production in response to stimulation by oligonucleotide 1668, R848, or ssRNA. The compound was added to dissociated cells [5 × 105 per well in complete RPMI/10% (vol/vol)] before addition of stimulants. Cells were stimulated for 72 h, and supernatant was removed for ELISA analysis of IL-6 (Pestka Biomedical Laboratories and Quantikine Colorimetric ELISA kits; R&D Systems, Inc.).
Infection and Examination.
The P. chabaudi chabaudi AS strain and PbA (Swiss Tropical Institute) were used. The parasites kept in liquid nitrogen were thawed and passaged into C57BL/6 mice as parasite donors. For experimental infection, parasite-containing erythrocytes (iRBCs) were collected from donors in heparinized tubes by retroorbital bleeding and 1 × 105 iRBCs were injected i.p. into naive mice. These mice were carefully observed daily, and parasitemia was estimated by counting Giemsa-stained thin blood smears. For infections with the P. berghei model, neurological examination was performed by two independent observers using different parameters that included ruffled fur, abnormal postural responses, reduced reflexes and reduced grip strength, coma, and convulsions. Mice that demonstrated complete disability in all parameters or died between days 7 and 12 postinfection were considered as having CM. Mice were treated in accordance with procedures approved by the Animal Ethics Committee of the University of Massachusetts. Brains were removed and used for histological analysis after H&E staining.
Cytokine Analysis.
Cytokines were assessed in the sera or in supernatants of cultured splenocytes from C57BL/6 mice infected i.p. with 105 P. chabaudi or P. berghei iRBCs treated or not treated with E6446. Each experiment was repeated two to three times. Each animal was analyzed individually. Spleens were aseptically removed and macerated through a nylon mesh over the course of the experimental infection. After RBC lysis, splenocytes were resuspended in RPMI, 10% (vol/vol) FCS (Gibco), and 1% gentamicin (Schering Plough) at a density of 2.5 × 106 cells per milliliter. Splenocytes were subsequently cultured in 48-well plates for 48 h in a final volume of 1 mL in the presence of Con A (Sigma), higly purified LPS (Sigma–Aldrich), or CpG ODN (Invivogen). Measurements of cytokines were performed using commercially available ELISA kits (R&D Systems). Serum cytokines from P. chabaudi-infected mice were quantified with the CBA inflammation kit (Becton–Dickinson).
Intracellular Staining of Cytokines.
At day 6 postinfection with 105 PbA iRBCs, spleens from mice treated or not treated with E6446 were harvested and CD11c+ splenic DCs were isolated using CD11c microbeads (Miltenyi Biotec) according to the manufacturer's instructions. Cells (5 × 105) were Fc-blocked with 2.4G2 mAb (BD Pharmingen) and labeled with efluor-conjugated anti-CD11c, Cy5-conjugated anti-mouse MHCII, and phycoerythrin (PE)-conjugated anti-mouse CD40, CD86, and CD80 mAbs (BD Pharmingen). A nonrelated IgG mAb was used as a control for staining specificity. For intracellular analysis of cytokines, cells were fixed and permeabilized with BD Cytofix/Cytoperm (BD Pharmingen) and stained for intracellular TNF-α, IL-12 p40/p70 levels using PE-conjugated anti-mouse mAbs (BD Pharmingen). Data were analyzed using Flowjo software (TreeStar).
Statistical Analysis.
All data were analyzed using Graphpad Instat 4.0 software. Unless stated otherwise, all comparisons were performed using a two-tailed Student's t test. Mann–Whitney U testing was used for nonparametric analysis when data did not fit a Gaussian distribution. A P value ≤0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgments
We thank Bruce Beutler (Scripps Research Institute) for the 3d mice used in our experiments and Natalia Oliveira for her assistance in experiments with mice. R.T.G. is the recipient of a research fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico. This work was supported by National Institutes of Health Grants AI080907 (to R.T.G.) and AI079293 (to D.T.G.) and by the National Institute of Science and Technology for Vaccine Development (R.T.G.). B.S.F. received a scholarship from the Coordenação e Aperfeiçoamento de Pessoal de Nível Superior.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015406108/-/DCSupplemental.
References
- 1.Hay SI, et al. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;6:e1000048. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maher B. Malaria: The end of the beginning. Nature. 2008;451:1042–1046. doi: 10.1038/4511042a. [DOI] [PubMed] [Google Scholar]
- 3.Gatton ML, Cheng Q. Evaluation of the pyrogenic threshold for Plasmodium falciparum malaria in naive individuals. Am J Trop Med Hyg. 2002;66:467–473. doi: 10.4269/ajtmh.2002.66.467. [DOI] [PubMed] [Google Scholar]
- 4.Boutlis CS, Yeo TW, Anstey NM. Malaria tolerance—For whom the cell tolls? Trends Parasitol. 2006;22:371–377. doi: 10.1016/j.pt.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyles DE, Young MD. The duration of untreated or inadequately treated Plasmodium falciparum infections in the human host. J Natl Malar Soc. 1951;10:327–336. [PubMed] [Google Scholar]
- 6.O'Neill LA, Bryant CE, Doyle SL. Therapeutic targeting of Toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol Rev. 2009;61:177–197. doi: 10.1124/pr.109.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun S, Rao NL, Venable J, Thurmond R, Karlsson L. TLR7/9 antagonists as therapeutics for immune-mediated inflammatory disorders. Inflamm Allergy Drug Targets. 2007;6:223–235. doi: 10.2174/187152807783334300. [DOI] [PubMed] [Google Scholar]
- 8.Kalia S, Dutz JP. New concepts in antimalarial use and mode of action in dermatology. Dermatol Ther. 2007;20:160–174. doi: 10.1111/j.1529-8019.2007.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Togbe D, et al. Murine cerebral malaria development is independent of toll-like receptor signaling. Am J Pathol. 2007;170:1640–1648. doi: 10.2353/ajpath.2007.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adachi K, et al. Plasmodium berghei infection in mice induces liver injury by an IL-12- and toll-like receptor/myeloid differentiation factor 88-dependent mechanism. J Immunol. 2001;167:5928–5934. doi: 10.4049/jimmunol.167.10.5928. [DOI] [PubMed] [Google Scholar]
- 11.Krishnegowda G, et al. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: Cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem. 2005;280:8606–8616. doi: 10.1074/jbc.M413541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coban C, et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201:19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coban C, et al. Pathological role of Toll-like receptor signaling in cerebral malaria. Int Immunol. 2007;19:67–79. doi: 10.1093/intimm/dxl123. [DOI] [PubMed] [Google Scholar]
- 14.Ockenhouse CF, et al. Common and divergent immune response signaling pathways discovered in peripheral blood mononuclear cell gene expression patterns in presymptomatic and clinically apparent malaria. Infect Immun. 2006;74:5561–5573. doi: 10.1128/IAI.00408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tripathi AK, Sha W, Shulaev V, Stins MF, Sullivan DJ., Jr Plasmodium falciparum-infected erythrocytes induce NF-kappaB regulated inflammatory pathways in human cerebral endothelium. Blood. 2009;114:4243–4252. doi: 10.1182/blood-2009-06-226415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCall MB, et al. Plasmodium falciparum infection causes proinflammatory priming of human TLR responses. J Immunol. 2007;179:162–171. doi: 10.4049/jimmunol.179.1.162. [DOI] [PubMed] [Google Scholar]
- 17.Sam-Agudu NA, et al. TLR9 polymorphisms are associated with altered IFN-gamma levels in children with cerebral malaria. Am J Trop Med Hyg. 2010;82:548–555. doi: 10.4269/ajtmh.2010.09-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franklin BS, et al. MyD88-dependent activation of dendritic cells and CD4(+) T lymphocytes mediates symptoms, but is not required for the immunological control of parasites during rodent malaria. Microbes Infect. 2007;9:881–890. doi: 10.1016/j.micinf.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Franklin BS, et al. Malaria primes the innate immune response due to interferon-gamma induced enhancement of toll-like receptor expression and function. Proc Natl Acad Sci USA. 2009;106:5789–5794. doi: 10.1073/pnas.0809742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parroche P, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci USA. 2007;104:1919–1924. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark IA. Does endotoxin cause both the disease and parasite death in acute malaria and babesiosis? Lancet. 1978;2:75–77. doi: 10.1016/s0140-6736(78)91386-7. [DOI] [PubMed] [Google Scholar]
- 22.Hartgers FC, et al. Enhanced Toll-like receptor responsiveness associated with mitogen-activated protein kinase activation in Plasmodium falciparum-infected children. Infect Immun. 2008;76:5149–5157. doi: 10.1128/IAI.01579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 24.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 25.Jurk M, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 26.Fox RI. Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin Arthritis Rheum. 1993;23(Suppl 1):82–91. doi: 10.1016/s0049-0172(10)80012-5. [DOI] [PubMed] [Google Scholar]
- 27.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: An old drug against today's diseases? Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace DJ, Linker-Israeli M, Hyun S, Klinenberg JR, Stecher V. The effect of hydroxychloroquine therapy on serum levels of immunoregulatory molecules in patients with systemic lupus erythematosus. J Rheumatol. 1994;21:375–376. [PubMed] [Google Scholar]
- 29.Griffith JW, et al. Toll-like receptor modulation of murine cerebral malaria is dependent on the genetic background of the host. J Infect Dis. 2007;196:1553–1564. doi: 10.1086/522865. [DOI] [PubMed] [Google Scholar]
- 30.Langhorne J, et al. Dendritic cells, pro-inflammatory responses, and antigen presentation in a rodent malaria infection. Immunol Rev. 2004;201:35–47. doi: 10.1111/j.0105-2896.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 31.Ing R, Segura M, Thawani N, Tam M, Stevenson MM. Interaction of mouse dendritic cells and malaria-infected erythrocytes: Uptake, maturation, and antigen presentation. J Immunol. 2006;176:441–450. doi: 10.4049/jimmunol.176.1.441. [DOI] [PubMed] [Google Scholar]
- 32.Brinkmann MM, et al. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunt NH, et al. Immunopathogenesis of cerebral malaria. Int J Parasitol. 2006;36:569–582. doi: 10.1016/j.ijpara.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Clark IA, Budd AC, Alleva LM, Cowden WB. Human malarial disease: A consequence of inflammatory cytokine release. Malar J. 2006;5:85. doi: 10.1186/1475-2875-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunt NH, Grau GE. Cytokines: Accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491–499. doi: 10.1016/s1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 36.Haldar K, Murphy SC, Milner DA, Taylor TE. Malaria: Mechanisms of erythrocytic infection and pathological correlates of severe disease. Annu Rev Pathol. 2007;2:217–249. doi: 10.1146/annurev.pathol.2.010506.091913. [DOI] [PubMed] [Google Scholar]
- 37.Garcia I, et al. Transgenic mice expressing high levels of soluble TNF-R1 fusion protein are protected from lethal septic shock and cerebral malaria, and are highly sensitive to Listeria monocytogenes and Leishmania major infections. Eur J Immunol. 1995;25:2401–2407. doi: 10.1002/eji.1830250841. [DOI] [PubMed] [Google Scholar]
- 38.Campanella GS, et al. Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc Natl Acad Sci USA. 2008;105:4814–4819. doi: 10.1073/pnas.0801544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins WE, Jeffery GM, Roberts JM. A retrospective examination of anemia during infection of humans with Plasmodium vivax. Am J Trop Med Hyg. 2003;68:410–412. [PubMed] [Google Scholar]
- 40.Young NS. Aplastic anaemia. Lancet. 1995;346:228–232. doi: 10.1016/s0140-6736(95)91273-8. [DOI] [PubMed] [Google Scholar]
- 41.Thawani N, Tam M, Stevenson MM. STAT6-mediated suppression of erythropoiesis in an experimental model of malarial anemia. Haematologica. 2009;94:195–204. doi: 10.3324/haematol.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamikanra AA, Theron M, Kooij TW, Roberts DJ. Hemozoin (malarial pigment) directly promotes apoptosis of erythroid precursors. PLoS ONE. 2009;4:e8446. doi: 10.1371/journal.pone.0008446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDevitt MA, Xie J, Gordeuk V, Bucala R. The anemia of malaria infection: Role of inflammatory cytokines. Curr Hematol Rep. 2004;3:97–106. [PubMed] [Google Scholar]
- 44.van Hensbroek MB, et al. The effect of a monoclonal antibody to tumor necrosis factor on survival from childhood cerebral malaria. J Infect Dis. 1996;174:1091–1097. doi: 10.1093/infdis/174.5.1091. [DOI] [PubMed] [Google Scholar]
- 45.Hermsen C, van de Wiel T, Mommers E, Sauerwein R, Eling W. Depletion of CD4+ or CD8+ T-cells prevents Plasmodium berghei induced cerebral malaria in end-stage disease. Parasitology. 1997;114:7–12. doi: 10.1017/s0031182096008293. [DOI] [PubMed] [Google Scholar]
- 46.Noedl H, et al. Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 47.Dondorp AM, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.