Abstract
Retinal prostheses aim to restore functional vision to those blinded by outer retinal diseases using electric stimulation of surviving neurons. Previous work indicates that repetitive stimulation with stimuli that activate the synaptic network reduces the sensitivity of retinal neurons to further stimulation. Such desensitization may contribute to the fading of visual percepts over time reported by human subjects. Here, we show that desensitization may be more complex than previously considered. We recorded spike trains from rabbit retinal ganglion cells and found that desensitization persists in the presence of inhibitory blockers (strychnine and picrotoxin), indicating amacrine cell inhibition is not solely responsible for reducing sensitivity in response to electric stimulation. The threshold for direct activation of the ganglion cell changes little during the simultaneous desensitization of the synaptically mediated response, indicating that desensitization likely occurs upstream of the spike generator. In addition to the rapid desensitization acting over hundreds of milliseconds (τ = 176.4 ± 8.8ms), we report the presence of a slow acting desensitization with a time course of seconds (τ = 14.0 ± 1.1sec). The time course of the two components of desensitization that we found are similar to the two phases of brightness fading seen in human subjects. This suggests that the reduction in ganglion cell firing due to desensitization may be responsible for the fading of visual percepts over time in response to prosthetic stimulation.
Introduction
Outer retinal diseases such as age-related macular degeneration and retinitis pigmentosa preferentially affect the photoreceptors, diminishing the ability of the eye to capture photons of light. However, a significant number of neurons remain viable in the inner retina 1. This includes retinal ganglion cells, whose axons transmit visual information to the brain in the form of spike trains. In retinal prostheses, spiking is elicited in retinal ganglion cells using electric stimulation with the goal of restoring functional vision to patients blinded by such diseases 1-3. Human trials have demonstrated the ability to elicit spatially patterned vision 4 across a range of brightness levels 5. However, the quality of these percepts varies considerably and many significant challenges remain to be addressed. For example, the ability to elicit temporally stable visual percepts remains limited 6.
In order to improve clinical outcomes with retinal prostheses, it may be necessary to more precisely control the spatial and temporal pattern of ganglion cell spiking that is elicited by prosthetic stimulation. The ability to control spiking depends on whether the neuronal response is elicited by direct activation of the ganglion cell, or through activation of neurons presynaptic to the ganglion cell (e.g. bipolar cells). Direct activation of the ganglion cell has the ability to elicit spike trains at very high rates (250-500Hz) 7-9. However, direct activation is also likely to cause incidental activation of passing axons on the inner retinal surface. This will expand the spatial region of ganglion cell activation, and may smear the elicited percept. Alternatively, the activation of presynaptic neurons is advantageous in that it provides better spatial control over neural activation by avoiding ganglion cell axons. Unfortunately, activation through the synaptic network limits the ability to control the temporal pattern of ganglion cell spiking. For example, in response to repetitive stimulation, ganglion cells respond robustly to the first pulse, but the response decreases for subsequent pulses 10,11. We refer to this reduction in ganglion cell sensitivity to repetitive stimulation as desensitization. Such desensitization has not been fully characterized and remains poorly understood.
It is important to gain a better understanding of desensitization since it may limit the temporal resolution of elicited vision in human trials. For example, in response to repetitive stimulation of the retina, blind subjects report a percept that is initially bright, but then fades in time with both fast (hundreds of milliseconds) and slow (several seconds) temporal components 6. Whether this psychophysical effect is the result of ganglion cell desensitization or from processes in higher visual centers remains unknown. One likely contributor to desensitization in the retina is amacrine cell inhibition; in response to electric stimulation, amacrine cells induce prolonged inhibitory currents in bipolar and ganglion cells 7,12. This results in a prolonged hyperpolarization of bipolar and ganglion cells that would be expected to reduce the sensitivity of the bipolar cell and/or ganglion cell to further stimulation for some period of time. Also, amacrine cells are thought to be activated secondary to activation of bipolar cells, suggesting amacrine cell inhibitions will necessarily accompany excitatory input from bipolar cells. However, the direct involvement of amacrine cells in desensitization has not been demonstrated.
Here, we report several new findings related to desensitization. In addition to the rapid desensitization that has been reported previously, we report the presence of a much slower mechanism. The dual time course of ganglion cell desensitization reported here is consistent with the dual time course of brightness fading in blind subjects, raising the possibility that brightness fading is a retinal phenomenon 6. Finally, we propose a new stimulation strategy that utilizes desensitization to facilitate direct activation of the ganglion cell at one-spike-per-pulse without interference from synaptically mediated spikes.
Methods
Animal preparation and retina isolation
The care and use of animals followed all federal and institutional guidelines, and all protocols were approved by the Institutional Animal Care and Use Committees of the Boston VA Healthcare System and/or the Subcommittee of Research Animal Care of the Massachusetts General Hospital. Seventeen New Zealand White Rabbits (~2.5kg) were anesthetized with injections of xylazine/ketamine and subsequently euthanized with an intracardial injection of pentobarbital sodium. Immediately after death, the eyes were removed. All procedures following eye removal were performed under dim red illumination. The front of the eye was removed, the vitreous was eliminated. The retina was separated from the retinal pigment epithelium and mounted, photoreceptor side down, to a 10-mm square piece of Millipore filter paper (0.45 μm HA Membrane Filter) that was mounted with vacuum grease to the recording chamber (~1.0 ml volume). A 2-mm circle in the center of the Millipore paper allowed light from below to be projected onto the photoreceptors. Extra pieces of retina were stored in 100mL of Ames medium at room temperature (pH 7.4) continuously bubbled with 95% O2 and 5% CO2, and were stored for no longer than 3hrs prior to use.
Electrophysiology and light responses
Patch pipettes were used to make small holes in the inner limiting membrane, and ganglion cells were targeted under visual control. Spiking was recorded with a cell-attached patch electrode (4-8MΩ) filled with superfusate. Two silver-chloride coated silver wires served as the ground and were positioned at opposite edges of the recording chamber each approximately 15 mm from the targeted cell. The retina was continuously perfused at 4mL/min with Ames (pH 7.4) at 36°C, equilibrated with 95% O2 and 5% CO2. Pharmacological agents were applied to the bath by switching a 3-way stopcock to a 200mL reservoir of Ames containing a cocktail of 10μM strychnine and 100μM picrotoxin. Approximately 3min was allowed for the drugs to wash in and take effect.
The light stimulus was controlled by VisionWorks software, and data acquisition and stimulus triggering was controlled by custom software written in LabView (National Instruments) and Matlab (Mathworks). Light stimuli were projected on to the retina from below through an LCD projector (InFocus) and focused onto the photoreceptor outer segments with a steady, photopic background. Light stimuli consisted of stationary flashed squares (size range: 100-1000μm), 1-sec duration, centered at the soma. Stimulus intensity was 50-75% above background light level. Moving bars (300 × 1,800μm moving at 600μm/sec) were used to classify directionally selective (DS) cells. Other than noting whether targeted ganglion cells were ON, OFF, or ON-OFF DS, they were not further classified.
Electric Stimulation
Electric stimulation was delivered via a 10kΩ Platinum-Iridium electrode (MicroProbes); the exposed area was conical with an approximate height of 125μm and base diameter of 15μm, giving a surface area of ~5,900μm2, comparable to a 40μm disk electrode. Pulse stimuli were controlled by Multi-Channel Systems STG2004 hardware and software. Two silver-chloride coated silver wires served as the return for the stimulation electrode; each was positioned approximately 8mm from the targeted cell and approximately 12mm from each other. The height of the stimulating electrode remained fixed at 25μm above the inner limiting membrane. The stimulating electrode was placed directly over the soma.
Stimuli were cathodal-first, biphasic-pulses that were rectangular in shape, containing symmetric cathodal and anodal phases. Upon obtaining a cell, the threshold for the synaptically mediated response was determined by delivering 1-ms pulses (no interphase delay) every 2sec at varying stimulus amplitude. Threshold was defined as the lowest stimulus amplitude to elicit 2 or more spikes to at least 50% of the pulses. The average threshold was 27.7 ± 8.5μA (mean ± standard deviation). After obtaining threshold, trains of 10 pulses were delivered at 2x threshold at pulse rates of 2, 4, 8, 16Hz. The stimulus was repeated 3-5 times for each pulse rate with at least 5sec between consecutive presentations. For long duration stimuli, 1ms-pulse trains of 16Hz were delivered for 300 pulses (~19sec) at 2x threshold. The stimulus was repeated 4-5 times with at least 1min between consecutive presentations.
In another set of experiments, 1ms-pulse trains of 16Hz were presented for 150 pulses (~8.5sec) at 2x threshold where 10ms after the 140th pulse, a brief burst of short-duration pulses (200-300μsec) was delivered. The short-duration pulses were cathodal-first and the interval between the cathodal and anodal phases was 3ms so as to allow visualization of action potentials elicited by direct activation of the ganglion cell. The delay between the anodal phase and the cathodal phase of the next pulse in the burst was 10ms, giving a period of 13.4-13.6ms (or 73.5-74.6Hz). All tests for statistical significance are paired t-test using a significance level of 5% (α = 0.05). Also, we used standard deviation when showing the response of a single cell to multiple stimulus trials, and we use standard error when we take the average across all cells, unless stated otherwise.
Results
Ganglion Cell Desensitization in Response to Stimulation with Pulse Trains
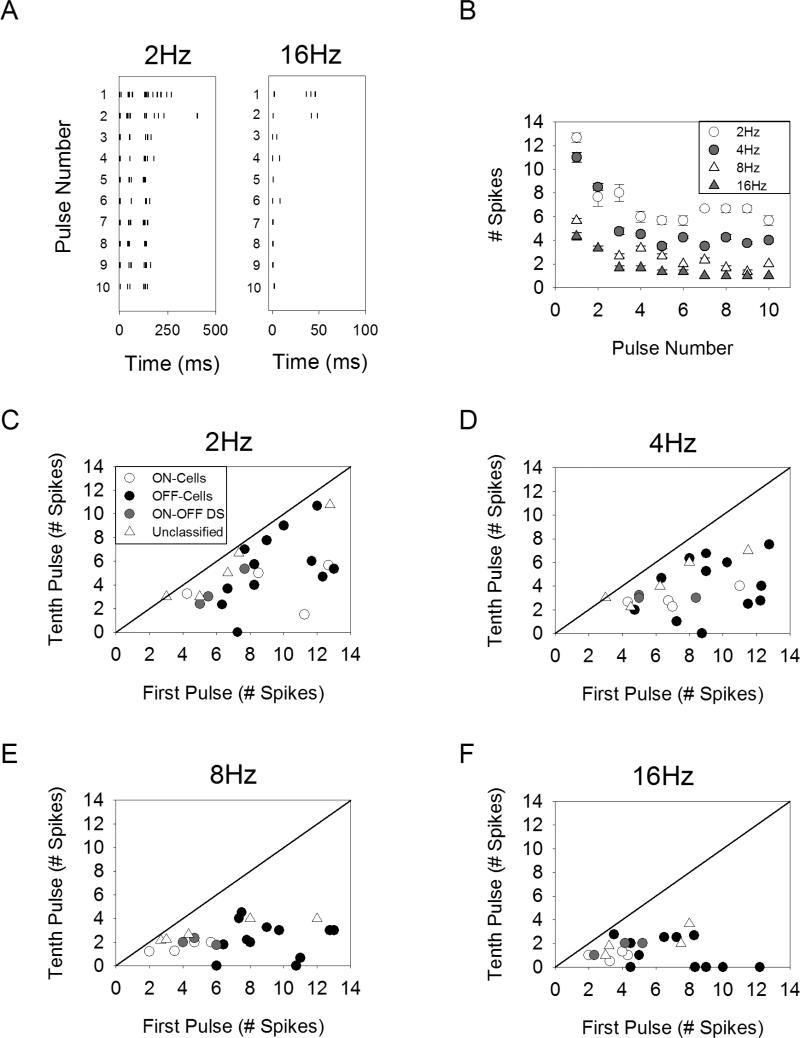
Spike trains were recorded from retinal ganglion cells using cell-attached patch clamp recordings from the isolated rabbit retina. Electric stimulation was delivered with a 10kΩ Platinum-Iridium electrode positioned 25μm directly above the ganglion cell body. The response of a typical cell to a train of ten pulses of 1-ms duration is shown in Figure 1A-B. For all stimulation rates tested, the response to the tenth pulse contained fewer spikes than that of the first pulse, indicating that the sensitivity of the ganglion cells becomes suppressed. We refer to this response suppression as desensitization.
Figure 1.
Desensitization of ganglion cell responses to stimulation with pulse trains. A. Ganglion cell spiking response to electric stimulation with ten pulses of 1-ms duration delivered at 2Hz (left) and 16Hz (right). B. The average number of spikes (4 stimulus repetitions) in response to each pulse is shown for pulse rates of 2-16Hz for a representative cell. C-F. Each point represents a different cell, plotting the average number of spikes elicited in response to the first pulse versus the tenth pulse.
There was a surprising level of cell-to-cell variability both in the level of desensitization and the overall range of response levels seen for a given stimulus amplitude. The response to the first versus tenth pulses is plotted for all cells (Figure 1C-F); points falling below the line of unity indicate desensitization. While some cells show no desensitization, others are completely desensitized by the tenth pulse. This is true even between ganglion cells of the same class; for example, in response to 4Hz stimulation the population of OFF-cells exhibit a wide range of desensitization. We did not further classify the ganglion cells into specific types (e.g. OFF-alpha) and therefore our data does not allow us to determine if there is a similar level of desensitization within cells of a given type. The range of response levels observed across cells was also large. While threshold is known to vary from cell-to-cell 13, the stimulus amplitude was set to twice threshold for all cells (see Methods), and yet the response to the first pulse still spanned a range of 3.0-13.0 spikes.
In order to quantify the level of desensitization, the normalized response was averaged across all cells (n=24) and is shown for pulse rates of 2, 4, 8, and 16Hz (Figure 2A). As pulse rate is increased, the response plateaus at lower levels relative to the response of the first pulse, indicating the level of desensitization increases with pulse rate. . The use of standard error can give the impression that there is little cell-to-cell variability (Figure 2A). In fact, there is a significant level of cell-to-cell variability (compare Figure 1C-F versus Figure 2A). We plotted the ratio of the response to the tenth pulse to that of the first pulse (using standard deviation to illustrate variability) (Figure 2B). Comparing the distribution across pulse rates, the ratios were statistically different for all pairwise comparisons between pulse rates (p<0.02 for all comparisons, paired t-test), expect for 8Hz versus 16Hz. These results indicate that (1) the level of desensitization increases with pulse rate, and (2) the level of desensitization varies from cell to cell.
Figure 2.
Desensitization increases with the rate of stimulation . A. The normalized spiking response averaged across all cells (n=24). B. The ratio of the number of spikes elicited by the tenth pulse to the number of spikes elicited by the first pulse for each pulse rate. Error bars in A indicate standard error, while error bars in B indicate standard deviation (see text).
Increasing the pulse rate caused the overall response level to be decreased (i.e. the number of spike elicited for 2Hz stimulation was larger than that of 16Hz stimulation for each individual pulse) (Figure 1A-B). This is partly because the inter-pulse interval decreases for high pulse rates. For example, for 8Hz stimulation, the inter-pulse interval is 125ms, and therefore those spikes occurring with a relatively long latency of 200ms will not occur until after the second pulse has been delivered. In such a scenario, the response to the second or third pulse would be expected to exceed the response to the first pulse. Interestingly, this is not the case – the number of spikes decreases monotonically with pulse number for all rates tested (Figure 2A). Therefore, it appears as though the longer latency spikes are suppressed as a result of repetitive stimulation. This can be seen in Figure 1A, where the longer latency appear only for the first two pulses, but the spikes with short latency continue to fire at 1-2 spikes/pulse. This suggests that the effect of desensitization may be different on the short latency versus the long latency spikes; this is examined in detail below.
Desensitization of the Early and Late Phase Response Components
We examined the temporal response components of the synaptically mediated response in order to explore whether the level of desensitization varies as a function of response latency. We found that the response to epi-retinal stimulation contains discrete clusters of spikes (Figure 3A-B), consistent with the response to sub-retinal stimulation 14. For the cell shown in Figure 3A, the first cluster of spikes occurs within 7ms, followed by a second and third cluster centered around 50ms and 140ms, respectively. An examination of the early phase response on an expanded timescale reveals sub-clusters can exist within the broader clusters (Figure 3B). The earliest spikes occurred within 4ms of the stimulus onset. These spikes were abolished by application of cadmium chloride (n=4/4) (data not shown), confirming they were synaptically mediated and do not arise as a result of direct activation of the ganglion cell.
Figure 3.
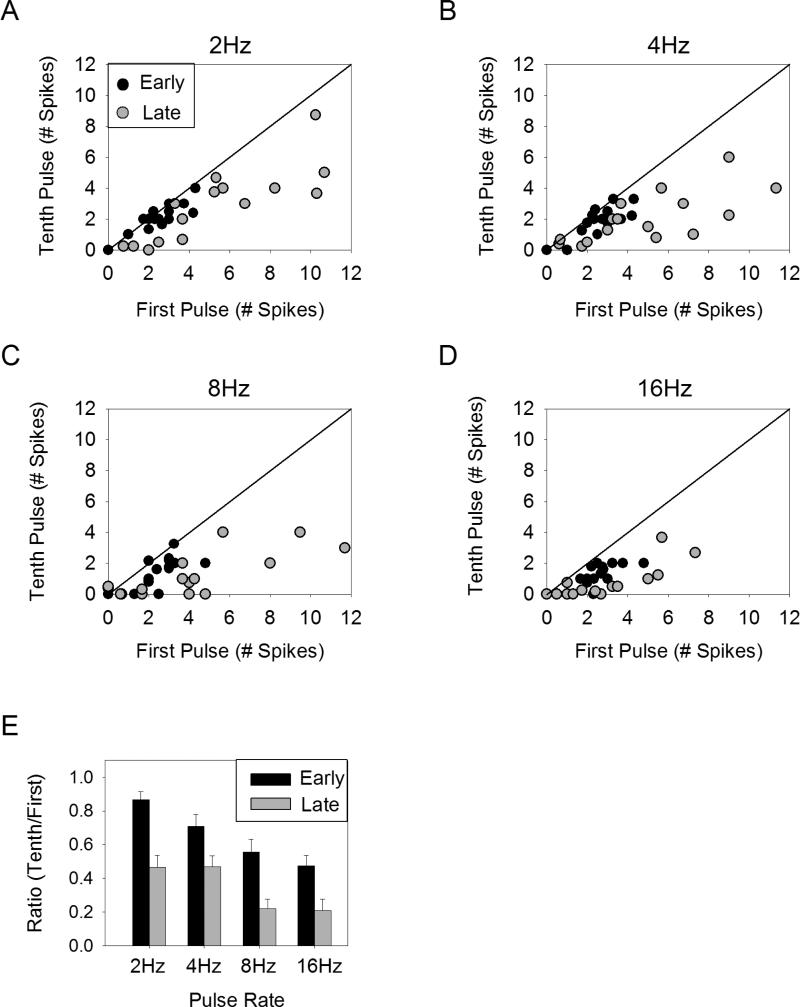
Synaptically mediated responses contain discrete spiking clusters. A. Spiking response to a pulse train of 2Hz. The response to three stimulation repetitions (30 pulses in all) are overlaid, revealing separate clusters of spikes. Latency was measured relative to stimulus onset. B. An expanded view of the early phase response (box in panel A). C-D. The number of spikes recorded from a representative cell is plotted as a function of pulse number for all rates. The spiking response was split into early phase spikes (<15ms latency) and late phase spikes (>15ms latency). Error bars indicate standard deviation.
The response to ten pulses was divided into early phase spikes, occurring within 15ms of the stimulation onset, and late phase spikes, occurring with latencies >15ms. The choice of 15ms was made by observing that early phase spikes generally fell within this range. The response of a typical cell is shown for both the early (Figure 3C) and late (Figure 3D) phase response components. Notice that the late phase spikes show significant response suppression even for 2Hz stimulation, while the early phase spikes show little desensitization regardless of pulse rate.
The level of desensitization for the early versus the late phase components was examined across the population by plotting the number of spikes elicited by the first pulse against the spikes elicited by the tenth pulse for all cells tested (n=16) (Figure 4A-D). For 2Hz and 4Hz stimulation, the early phase response falls along the line of unity, while the late phase response falls below the line of unity. For 8Hz and 16Hz, both the early and late phase components are slightly below the line of unity. This indicates that there is some desensitization in both the early and late phase responses, but the early phase desensitization is considerably less. In order to quantify the level of desensitization, the number of spikes elicited by the tenth pulse was normalized to that of the first pulse (Figure 4E). This ratio was significantly larger for the early phase as compared to the late phase response for all pulse rates tested (p-value range 0.0008-0.049 for all comparisons, paired t-test). This suggests the early phase response exhibits less desensitization than the late phase response.
Figure 4.
Desensitization of early versus late phase spiking response. A-D. The number of spikes following the first pulse is plotted versus the number of spikes following the tenth pulse on a cell-by-cell basis for early (<15ms latency) (black circles) and late (>15ms latency) (gray circles) phases of the response. E. The average ratio of the number of spikes elicited by the tenth pulse to the number of spikes elicited by the first pulse is shown for the early and late phase response components. Error bars indicate standard error.
In addition to comparing the level of desensitization for the early and late phase response components, we were interested in whether either of these response components could respond more reliably to higher stimulation rates. In order to test this, we analyzed only those cells that were capable of responding with at least 1 spike/pulse for all ten pulses to 16Hz (n=16/24). We found that in response to stimulation at 16Hz, the tenth pulse elicited at least 1 spike for the early phase response in 13/16 cells, while for the late phase response only 4/16 cells responded with at least 1 spike to the tenth pulse. This can be seen in Figure 4D, where the early phase response to the tenth pulse tends to be 1-2 spikes, while the late phase response to the tenth pulse is often 0 spikes. Therefore, the early phase response shows (1) less desensitization and (2) responds more reliably to high pulse rates than the late phase response.
Desensitization Persists in the Presence of Inhibitory Blockers
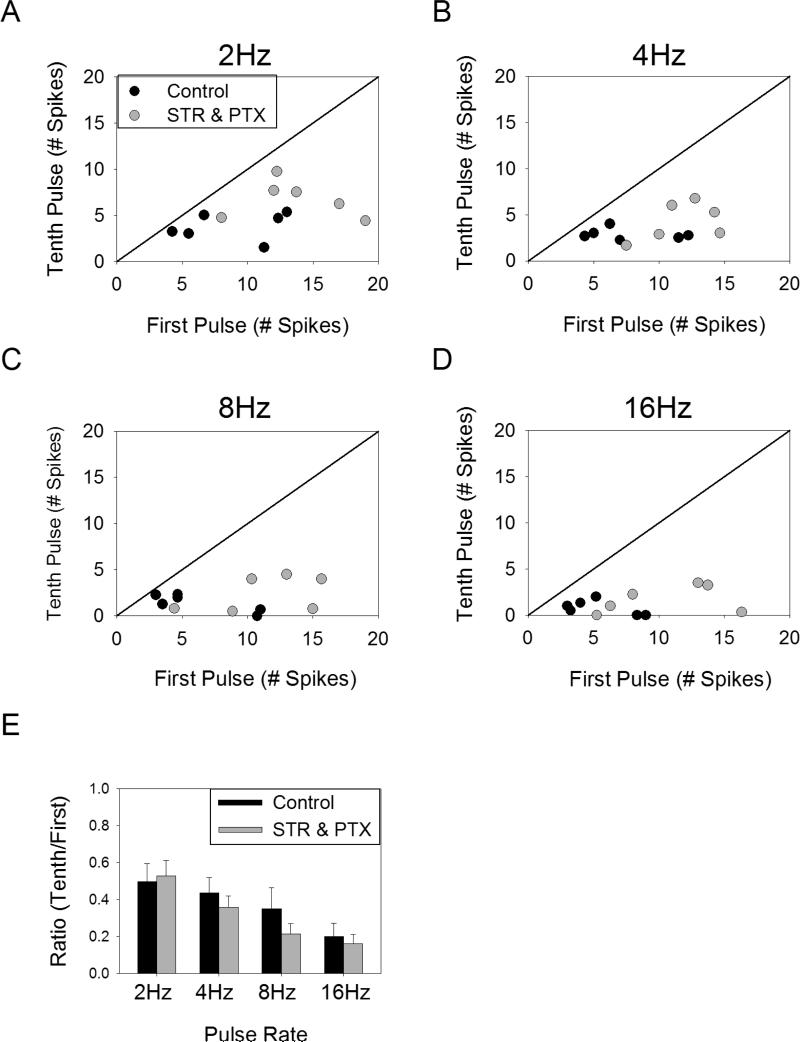
Inhibition from amacrine cells has been proposed as a potential mechanism of desensitization 7,14. In order to determine the contribution of inhibition to the observed desensitization, we pharmacologically blocked glycine and GABAA/C receptors using strychnine (10μM) and picrotoxin (100μM), respectively. The results from a typical experiment are shown in Figure 5. In the control condition the response to the later pulses was reduced relative to the first pulse for all pulse rates tested. After applying the cocktail of inhibitory blockers, there was still a significant suppression of the response to later pulses, suggesting there is a mechanism of desensitization that acts independently from amacrine cell inhibition.
Figure 5.
Desensitization persists in the presence of inhibitory blockers. The mean number of spikes elicited in response to ten pulses of 1ms duration delivered at rates of 2-16Hz is plotted for control conditions (A) and in the presence of strychnine (10μM) and picrotoxin (100μM) for a typical cell (B). Error bars indicate standard deviation.
The effect of inhibitory blockers across all cells tested (n=6) was examined by plotting the number of spikes elicited by the first pulse versus the number of spikes elicited by the tenth pulse both with and without the blockers present (Figure 6A-D). In the presence of blockers, all points remained below the line of unity, indicating that blocking amacrine cell inhibition does not eliminate desensitization. Comparing the effect of the blockers on the ratio of the response to the tenth pulse to the first pulse (Figure 6E), there was no significant effect (p > 0.091 for all comparisons, paired t-test).
Figure 6.
Effect of inhibitory blockers on desensitization. A-D. The average number of spikes elicited in response to the first pulse versus the tenth pulse across all cells (n=6) for stimulation at 2-16Hz in control conditions (black circles) and in the presence of strychnine (10μM) and picrotoxin (100μM) (gray circles). E. The ratio of the number of spikes elicited by the tenth pulse to the number of spikes elicited by the first pulse is shown for each pulse rate, both with and without inhibitory blockers. Error bars indicate standard error.
While this data indicates the presence of a desensitizing mechanism that does not involve amacrine cell inhibition, we cannot rule out the possibility that amacrine cell inhibition plays a role in desensitization. This is because the addition of inhibitory blockers increased the overall response level (Figure 6A-D). This increase in response level may have caused an increase in the desensitization arising from an undefined mechanism (e.g. vesicle depletion), thus offsetting any decrease in desensitization resulting from blocked amacrine cell inhibition. In other words, the presence of blockers could potentially cause (1) more vesicle depletion (for example) and (2) less amacrine cell inhibition, effectively canceling out and causing our estimate of desensitization to remain unchanged (Figure 6E). Nevertheless, this data indicates that amacrine cell inhibition is not the sole means by which retinal ganglion cells become desensitized.
Desensitization Precedes the Spike Generator
Our results so far indicate that in response to repetitive pulsatile stimulation, the synaptically mediated response becomes desensitized, and that this desensitization is mediated by one or more mechanisms that are distinct from amacrine cell inhibition. One possibility is that desensitization could involve the spike generating mechanism (i.e. the transient Na+ and delayed rectifier K+ channels that generate spikes). In order to test this, we created a stimulus paradigm that could test the level of desensitization of the spike generator. Standard 1ms pulses were delivered at 16Hz (Figure 7A, black) in order to desensitize the synaptic response. An example of the desensitization is shown in Figure 7B, where the initial 3 pulses elicit a spiking response and the subsequent pulses elicit no spiking. After ~8sec of stimulation at 16Hz, four short-duration pulses were superimposed on the pulse train (Figure 7A, gray) to estimate the threshold for direct activation (Figure 7C). Previous studies have shown that short-duration pulses directly activate the ganglion cell with minimal synaptic contribution 7. Therefore, this tests whether the spike generator mechanism has become desensitized as a result of the 16Hz pulse train. If the threshold for direct activation is not altered significantly by the 16Hz pulse train, then the desensitization of the synaptically mediated response is likely mediated upstream of the spike generating mechanism.
Figure 7.
Effect of desensitization on threshold for direct activation. A. Illustration of the stimulus with a 16Hz pulse train of 1ms (black) superimposed with four short-duration pulses (200μsec) (gray) that elicit spikes through direct activation. B. Example of spiking response to 16Hz pulse train of 1ms, giving an initial burst of spikes and quickly desensitizing to give no response by the fourth pulse. C. Example of the response to the stimulus illustrated in A, where the desensitizing pulse train of 16Hz was presented for over 8sec before the onset of the short duration pulses. The stimulus artifacts of the 16Hz pulses (asterisks) and the short duration pulses (arrows) can be seen. D. Zooming in on the gray box in C, a spike is elicited by direct activation for 65μA input current, but not for 63μA. E. The effect of the desensitizing pulse train on the threshold for direct activation is shown (n=4).
We applied this stimulus paradigm only to those cells that did not exhibit sustained, synaptically mediated spiking to 16Hz stimulation. The reason for focusing on this subset of cells is that the lack of sustained spiking indicates that sensitivity was reduced by at least a factor of two (since the 16Hz pulse train was delivered at twice threshold). Therefore, if the threshold for direct activation is increased by at least a factor of two, then the spike generator may be responsible for the desensitization of the synaptic response.
The threshold for direct activation of the ganglion cell with four short-duration pulses was defined as the minimal stimulus amplitude at which half of the pulses elicited spikes. Threshold for direct activation was measured after ~8sec of stimulation with the 16Hz pulse train, and this threshold was compared to the threshold at baseline (i.e. no 16Hz pulse train). An example of the response to four short-duration pulses in the presence of the 16Hz pulse train is shown in Figure 7C. The stimulus artifacts from the 1ms pulses can be seen (asterisks), but there were no synaptically mediated spikes elicited by theses stimulus pulses. The stimulus artifacts of the short-duration pulses are indicated by arrows. An expanded view of the response to short-duration pulses of two stimulus amplitudes is shown (Figure 7D). A spike was elicited when the stimulus amplitude was set to 65μA, but no spike was elicited for 63μA, suggesting the threshold for direct activation is in the range of 63-65μA. The threshold for direct activation at baseline (no 16Hz pulse train) was only slightly lower (54μA). Similar results were seen across all cells tested (n=4); the average increase in threshold for direct activation during 16Hz stimulation relative to baseline was 29.9 ± 4.7%. Therefore, the change in threshold for direct activation is relatively small compared to the at least two-fold change in sensitivity of the synaptically mediated response. The ability to elicit spikes via direct activation suggests the spike generator has been only weakly desensitized and therefore cannot be responsible for the much larger desensitization of the synaptically mediated response that we observed. The fact that the threshold for direct activation was increased slightly during 16Hz stimulation could result from either a slight desensitization of the spike generator (e.g. inactivation of voltage-gated sodium channels), or from hyperpolarization of the membrane as a result of synaptic input. Whatever the cause, the desensitization of the spike generator appears to be minor compared to desensitization of the synaptically mediated response.
Desensitization Occurs on Multiple Timescales
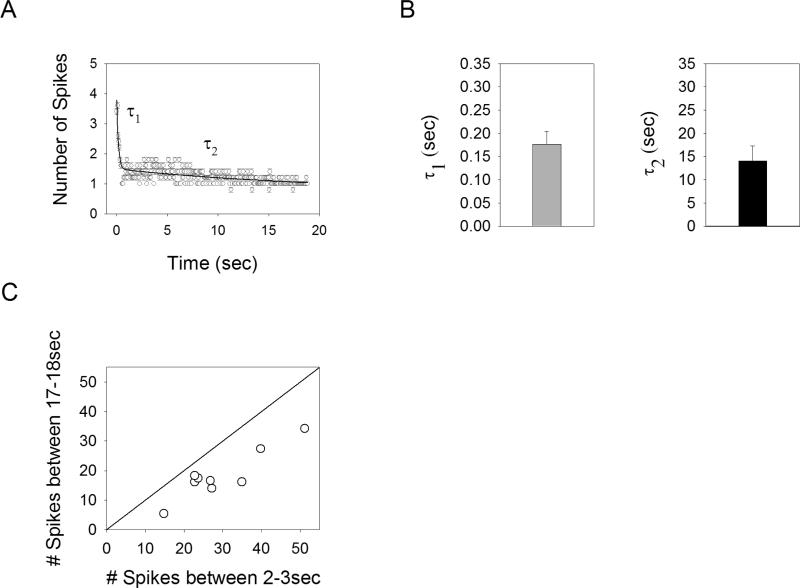
Human clinical trials report that in response to electric stimulation of the retina, brightness decays with a rapid component (hundreds of milliseconds), as well as over a much longer timescale (many seconds) 6. While there are many possible sites along the visual pathway that may be responsible for this decay in brightness, one possibility is that desensitization of retinal neurons plays a role. To explore whether a slow acting mechanism of desensitization was present in the retina, we delivered a relatively long duration (19sec) pulse train of 16Hz (1ms pulse duration). The response of an example cell is shown in Figure 8A. The number of spikes elicited in response to each pulse undergoes a significant drop within the first second, followed by a much slower reduction that persists for the duration of the stimulus. This suggests there are two separate mechanisms of desensitization, one acting rapidly and one acting over many seconds.
Figure 8.
Desensitization occurs on multiple timescales. A. Average number of spikes elicited per pulse for a long duration pulse train (16Hz, 1ms pulses, 19sec). A double exponential is fit to the data (solid line). B. Time constants derived from the best-fit double exponentials averaged across all cells (n=9) for the rapid (τ1) and slow (τ2) desensitization. C The average number of spikes elicited in the window of 17 to 18sec after the onset of the long duration pulse train versus the number of spikes in a window of 2 to 3sec after stimulus onset. A line of unity slope is shown.
In order to estimate the timescales of these two mechanisms, the responses to the long duration stimuli were fit with double exponential functions (solid line in Figure 8A). The reason for using a double exponential function is that a single exponential would not be sufficient to capture the slow reduction in sensitivity over many seconds. For example, for a single exponential with a time constant of 200ms, the response will have reached within 1% of steady-state after 1sec, and there would be negligible differences between the time windows of 2-3sec and 17-18sec. This is clearly not the case; the number of spikes was reduced in the time window of 17-18sec vs. 2-3sec for all cells tested (n=9), giving an average reduction of 38.1 ± 1.6% (Figure 8C). This reduction in response level can not be explained by a single mechanism of desensitization, and is likely the result of two separate mechanisms. The average time constants for the best-fit double exponential were found to be τ1 = 176.4 ± 8.8ms and τ2 = 14.0 ± 1.1sec (Figure 8B). Therefore, the two mechanisms of desensitization have response kinetics that differ by a factor of ~100.
For ganglion cells that responded persistently to long duration pulse trains at 16Hz, the latencies of the elicited spikes had some similar features that could be seen across cells. Three example cells are shown in Figure 9A-C, where the latency of all spikes elicited by 16Hz stimulation is plotted for the duration of the stimulus. Notice that after 19sec of stimulation, all cells continued to elicit early phase spikes (<15ms) at 1-2 spikes/pulse. These early phase spikes occurred for almost all cells (n=8/9), consistent with earlier results (Figure 4) that indicate the early phase spikes respond more reliably to high pulse rates than later phase spikes. However, there was some evidence that later spikes with latencies in the range of 40-55ms could respond persistently to long duration stimulation – at least in some cells (Figure 9C, n=2/9). The early spikes contained two distinctive components: spikes occurring within 6ms of stimulus offset (n=6/9, Figure 9A, 9C) and spikes occurring in the range of 6-15ms (n=5/9, Figure 9A, 9B). Interestingly, the latencies of the elicited spikes tended to slowly increase for the spikes occurring within 6-15ms, mirroring the slow acting desensitization. Conversely, the very early spikes (<6ms) did not show a drift of latency over time.
Figure 9.
Latency for all spikes elicited in response to a 1-ms pulse train at 16Hz for 19sec (all responses to 4 trials shown overlaid). A-C. Three example cells exhibit various combinations of the immediate spiking (<6ms), early spiking (6-15ms), and late spiking (40-55ms). The y-axis in C is different than that of A and B.
Discussion
The ability to control the temporal pattern of ganglion cell spiking may be critical to the ultimate success of retinal prostheses. Here, we report the presence of two mechanisms of desensitization in response to activation through the synaptic network, one acting rapidly (τ = 176.4 ± 8.8ms) that persists when amacrine cell inhibition is blocked, the other acting much more slowly (τ = 14.0 ± 1.1sec). We also show that the synaptically mediated response contains multiple components and that the level of desensitization is different in each; early phase spikes (<15ms latency) show less desensitization than late phase spikes (>15ms latency). Also, the threshold for direct activation of the ganglion cell changes little during desensitization of the synaptically mediated response, suggesting that the source of desensitization likely precedes the spike generator. Determination of the precise mechanism(s) by which the desensitization occurs will require further study.
Implications for Stimulation Strategies in Retinal Prostheses
The goal of an effective stimulation strategy is to precisely control ganglion cell spiking patterns with electric stimulation. Two general stimulation strategies that have been put forth are, (1) selectively activate the ganglion cells without simultaneously activating the synaptic network 7, or (2) selectively activate the bipolar cells without eliciting spikes through direct activation of the ganglion cells 15. With respect to the first approach, it is possible to selectively activate ganglion cells without eliciting a synaptically mediated response using epi-retinal stimulation with short-duration pulses 7,16. A major advantage of this approach is that direct activation of ganglion cells can be achieved at very high rates without any apparent desensitization 7-9. However, such selective activation is restricted to a limited range of stimulus amplitudes since increasing the amplitude to a factor of ~2.5 times threshold elicits synaptically mediated spikes (but see 16). Given that the threshold between individual ganglion cells has been shown to differ by a factor of >2.5 17, it is unlikely that selective activation of large populations of ganglion cells is possible without producing incidental activation of presynaptic bipolar cells. Another disadvantage of using direct activation of the ganglion cells is that the ganglion cell axons are highly sensitive to electric stimulation 18. The incidental activation of passing axons on the inner retinal surface will expand the region over which neurons are activated and may smear the elicited percept.
The second strategy is to selectively activate the bipolar cells without activating the ganglion cell directly. The advantage of this approach is thought to arise from utilization of existing inner retinal circuitry, presumably creating spiking patterns that better resemble those that that arise under normal physiological conditions. In addition, avoiding the activation of ganglion cell axons would provide better spatial control over the pattern of elicited activity. Unfortunately, a major disadvantage of eliciting synaptically mediated responses with a retinal prosthesis is that there is a significant level of desensitization. This prevents precise control over the temporal pattern of elicited spikes (Figure 1-2). Furthermore, methods to selectively activate bipolar cells do not currently exist. Even for sub-retinal stimulation, where the bipolar cells are closer to the stimulating electrode than the ganglion cells, the threshold for direct versus synaptic activation does not differ significantly, even over a wide range of pulse durations 14.
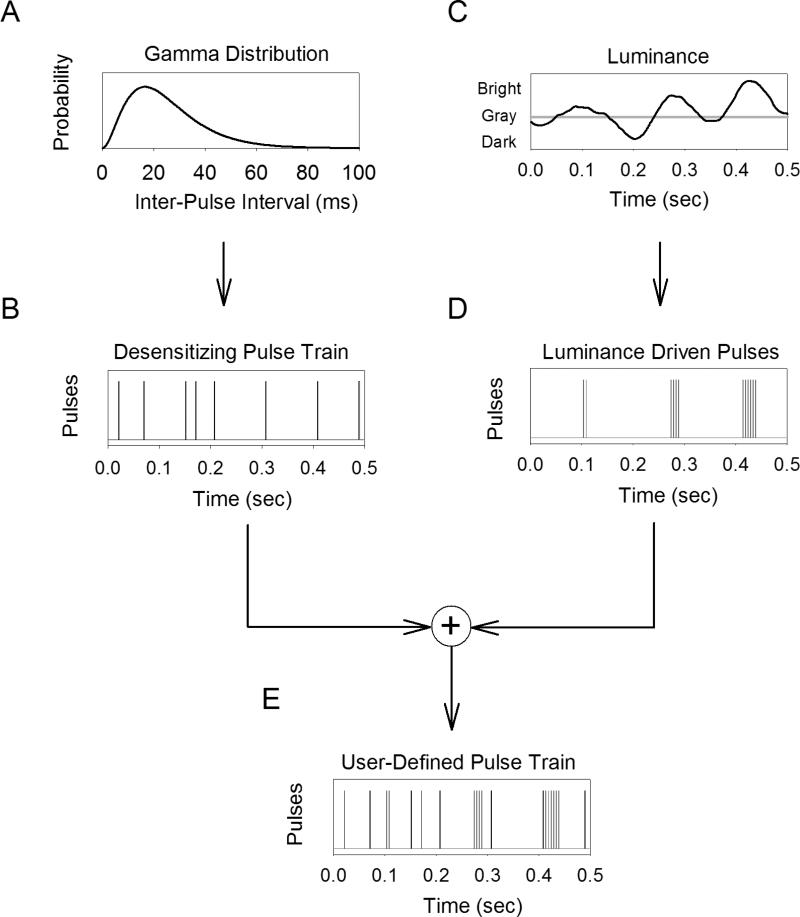
We propose an alternative stimulation strategy for retinal prostheses that overcomes some of the challenges discussed above (Figure 10). Consider a local region of the retina where the luminance within the region varies in time (Figure 10C). In order to encode this luminance in ganglion cell spike trains with prosthetic stimulation, a user-defined pulse train must be delivered to a nearby electrode. The stimulation method consists of two components: (1) a desensitizing pulse train that is continuously delivered regardless of incoming luminance (Figure 10A-B), and (2) bursts of pulses that are delivered as a function of incoming luminance (Figure 10C-D). The resulting pulse train that is delivered is the sum of the two individual components (Figure 10E). The key to this strategy is that the presence of a desensitizing pulse train abolishes the synaptically mediated response. Therefore, the luminance-driven pulses will elicit one-spike-per-pulse through direct activation of the ganglion cell with little or no contribution from synaptically mediated spikes. Conversely, if the desensitizing pulse train was not present, the luminance-driven pulses are likely to elicit synaptically mediated spikes, the exact number of which will be difficult to predict due to desensitization.
Figure 10.
A stimulation strategy consisting of a train of 1ms pulses that cause desensitization (A-B) combined with bursts of short-duration pulses that encode luminance fluctuations (C-D). The purpose of the desensitizing pulse train is to attenuate or abolish the synaptically mediated response and it is delivered independent of luminance. Fluctuations in luminance are encoded in ganglion cell firing rate by delivering bursts of short-duration pulses when the luminance exceeds some threshold (e.g. background light level). The 1ms pulses elicit one-spike-per-pulse through direct activation, and this can be used to replicate the spontaneous discharge characteristics of ganglion cells (i.e. following gamma distributed inter-spike intervals). These pulses elicit one-spike-per-pulse through direct activation, where brightness level is encoded by increasing the number of pulses per burst. The result is a user-defined pulse train that is the sum of both the desensitizing pulse train and luminance-driven pulses (E).
An important part of this stimulation strategy is the temporal pattern of pulses in the desensitizing pulse train. The desensitizing pulse train will itself is expected to elicit 1-2 spikes per pulse through both direct activation and through the early phase of the synaptically mediated spikes (Figure 7). Therefore, the temporal pattern of pulses can be made to best replicate the spontaneous discharge of a typical ganglion cell. For example, primate ganglion cells (ON and OFF, midget and parasol) fire spontaneously at ~20Hz with inter-spike intervals that are fit well by a gamma-distribution function 19. Therefore, if the interval between each consecutive pulse is drawn from a gamma-distribution function (Figure 10A) then the resulting spikes will have similar discharge statistics as a spontaneously firing ganglion cell under natural conditions. Assuming that spontaneous spikes do not convey visual information under natural conditions 20,21, the spikes induced by the desensitizing pulse train will not contribute to visual perception. In fact, there is evidence that visual information is conveyed in bursts of spikes with relatively short inter-spike intervals 22; much shorter than those present in the desensitizing pulse train or during spontaneous spiking. Unfortunately, the stimulus artifact obscured many of the spikes elicited by direct activation from the 1-ms pulses that make up the desensitizing pulse train, preventing us from evaluating the effectiveness of this stimulation strategy.
The ganglion cells recorded in the present study did not typically exhibit spontaneous spiking, and therefore the effect of a desensitizing pulse train will have on the spontaneous discharge could not be tested. There is evidence that spontaneous spiking in ON cells is due to a basal level of glutamate release onto the ganglion cells 23. In such a scenario, it is possible that synaptic desensitization will decrease the mean firing rate of the neuron. This could be useful, for example, in degenerate retina where the firing rate is pathologically elevated 24. A limitation to the strategy presented here is that direct activation of the ganglion cell with the luminance-driven pulses will produce incidental activation of ganglion cells axons. This will reduce control over the spatial pattern of activation, and presumably smear the elicited percept. Therefore, the benefits of improved temporal resolution must be weighed against the impact of reduced spatial resolution.
Amacrine Cell-Independent Desensitization
Prior to our study, several lines of evidence had suggested amacrine cell inhibition played a key role in the desensitization of the synaptic response. First, electric stimulation have the retina has been shown to activate amacrine cells, inducing feedback (Margalit and Thoreson, 2006) and feedforward inhibition (Fried et al., 2006) in bipolar and ganglion cells, respectively 12. The hyperpolarizing effect that inhibition has on bipolar cell terminals and on ganglion cell dendrites would be expected to reduce the sensitivity of the synaptically mediated response to subsequent electric stimulation. Second, the time course of the amacrine cell response to electric stimulation fits well with the time course of desensitization; the inhibitory currents in the ganglion cell begin later than the excitatory currents and persist for hundreds of milliseconds 7,12. This is consistent with the response to repetitive pulsatile stimulation, where the response to the first pulse is large and the response to pulses delivered within the next several hundred milliseconds is suppressed (Figure 1).
Surprisingly we found that desensitization persists in the presence of inhibitory blockers. This indicates the presence of a desensitizing mechanism that is distinct from amacrine cell inhibition; although our results do not preclude the possibility that amacrine cells also contribute to desensitization. Further, the fact that applying inhibitory blockers caused an overall increase in the spiking response to electric stimulation indicates that GABAergic and/or glycinergic mediated inhibition modulates the ganglion cell response to electric stimulation (Figure 6).
Our results suggest the mechanism of rapid desensitization precedes the spike generator (i.e. Na+ and K+ channels mediating action potentials) (Figure 7), but it is possible that other voltage-gated channels in the ganglion cell membrane are involved. For example, retinal ganglion cells have been shown to exhibit spike rate adaptation 25, generally attributed to some type of K+ channel, such as Ca2+-activated or Na+-activated K+ channels 26 or slowly activating K+ channels 27. There are also many potential synaptic mechanisms of desensitization that are independent of amacrine cell inhibition, such as the depletion of the readily releasable pool of synaptic vesicles, inactivation of calcium currents do to run down of the concentration gradient (Tsukamoto et al., 2001), or the desensitization of postsynaptic receptors (e.g. AMPA/kainate, NMDA channels), glutamate transporters, or metabotropic receptors in bipolar cell terminals 28. Further study will be needed to discern the precise mechanisms of desensitization.
Implications for Brightness Perception in Human Subjects
The data reported here are consistent with preliminary reports from clinical trials with retinal prostheses 6. The retina of human subjects was electrically stimulated with pulse trains from a chronically implanted, epi-retinal prosthesis. Each subject reported the perceived brightness as a function of time throughout the duration of the stimulus. Subjects reported a brief, “flash-like” increase in brightness that quickly reduced over hundreds of milliseconds. The timing of this fading is consistent with the timing of the rapid mechanism of desensitization reported here. Also, in response to stimulation lasting 60sec, several subjects reported a gradual reduction in brightness that decreased for the duration of the stimulus. Both the rapid and slow reduction in brightness seen in human subjects was present at all stimulation rates tested (5Hz, 20Hz, 60Hz), consistent with our observation that ganglion cell desensitization occurred for all pulse rates ≥2Hz that we tested. The fact that the brightness fading in human subjects occurred with two different time constants that closely matched the two time constants in our study raises the possibility that the desensitization we observed in retinal ganglion cells could be contributing to brightness fading. Our results do not preclude the possibility that one or more alternative mechanisms may also contribute to brightness fading, such as the involvement of saccadic eye movements 29,30, or adaptation of downstream neurons in thalamus31 or primary visual cortex 32.
There was a surprising amount of cell-to-cell variability in the level of desensitization. Even for ganglion cells of the same class, some cells responded robustly to repetitive stimulation, while others showed significant desensitization (Figure 1). This finding also has implications for interpreting data from human trials. A typical retinal prosthesis uses disc electrodes of 260-520μm diameter 5. The density of ganglion cells in the mid-periphery of human retina is ~103cells/mm2 33, resulting in approximately 53-212 ganglion cells within the area of each electrode. Therefore, hundreds of ganglion cells could potentially be activated by a given electrode. If we considered all ganglion cells to behave identically, then stimulation with a given electrode at 4Hz would presumably elicit a robust response in all cells for the first pulse, but by the tenth pulse the response of each cell would be reduced by approximately half (Figure 2). However, if we consider the cell-to-cell variability that we observed here, then some cells will respond robustly to all pulses, showing little desensitization, while the response of other cells will be largely abolished by the tenth pulse. This raises the possibility that the fading in brightness over time seen in human subjects may result from desensitization of a subset of ganglion cells, causing reduced spiking in some but not all ganglion cells.
Relationship to Deep Brain Stimulation In-Vitro Studies
There are several in-vitro studies that have examined the effects of repetitive stimulation on neurons in the subthalamic nucleus (STN), a common target for deep brain stimulation in the treatment of Parkinson's disease 34. In response to high-frequency pulse trains (>100Hz), the spontaneous activity of STN neurons was completely suppressed. This effect persisted in the presence of synaptic blockers 35,36, and therefore was attributed to inactivation of intrinsic voltage gated channels. This differs from the rapid desensitization reported here because the spike generator showed little desensitization even when the synaptic response was completely abolished (Figure 7). It is possible, however, that such inactivation of voltage-gated channels may underlie the slow desensitization we observed (Figure 8). Consistent with this notion, the suppression of spiking in STN neurons often required several seconds of stimulation 36, indicating the desensitization of STN neurons also occurs with a slow time course.
Interestingly, stimulating STN neurons at a relatively low rate (10Hz) had little or no effect on their spontaneous activity even though each pulse was found to elicit EPSPs 37,38. In contrast, modulating the synaptic input to retinal ganglion cells often elicits robust bursts of spiking (Figure 1). The difference between data from STN and from retina could be a result of pulse width; in the retina, longer pulse widths are associated with stronger synaptic responses 16, while studies in the STN often use short-duration pulses (<500μsec). Alternatively, the differences in desensitization between STN neurons and retinal ganglion cells could be related to the fact that ribbon synapses are present in the retina but not the STN 39. These ribbon synapses are specialized structures that allow neurotransmitter to be continuously released over long periods of time, may result in synaptically mediated responses that are more pronounced in ganglion cells than STN neurons. Likewise, the significant level of synaptic input provided by ribbon synapses could make ganglion cells more susceptible to desensitization of the synaptically mediated response.
Acknowledgements
Thank you to Angelica Perez-Fornos for helpful discussions on data from human trials, as well as Donald Eddington, Neal Desai, and Richard Masland for their contributions.
References
- 1.Weiland JD, Liu W, Humayun MS. Retinal Prothesis. Annu Rev Biomed Eng. 2004 doi: 10.1146/annurev.bioeng.7.060804.100435. [DOI] [PubMed] [Google Scholar]
- 2.Winter JO, Cogan SF, Rizzo JF., 3rd Retinal prostheses: current challenges and future outlook. J Biomater Sci Polym Ed. 2007;18:1031–1055. doi: 10.1163/156856207781494403. [DOI] [PubMed] [Google Scholar]
- 3.Zrenner E. Will retinal implants restore vision? Science. 2002;295:1022–1025. doi: 10.1126/science.1067996. [DOI] [PubMed] [Google Scholar]
- 4.Caspi A, Dorn JD, McClure KH, Humayun MS, Greenberg RJ, McMahon MJ. Feasibility study of a retinal prosthesis: spatial vision with a 16-electrode implant. Arch Ophthalmol. 2009;127:398–401. doi: 10.1001/archophthalmol.2009.20. [DOI] [PubMed] [Google Scholar]
- 5.Greenwald S. Brightness as a function of current amplitude in human retinal electrical stimulation. Invest Ophthalmol Vis Sci. 2009;50:5017–5025. doi: 10.1167/iovs.08-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez Fornos A, Sommerhalder J, Pelizzone M. Dynamics of Visual Perception Upon Electrical Stimulation of the Retina.. Annual Meeting, Association for Research in Vision and Ophthalmology; Fort Lauderdale, FL. 2010. [Google Scholar]
- 7.Fried SI, Hsueh HA, Werblin FS. A method for generating precise temporal patterns of retinal spiking using prosthetic stimulation. J Neurophysiol. 2006;95:970–978. doi: 10.1152/jn.00849.2005. [DOI] [PubMed] [Google Scholar]
- 8.Ahuja AK, Behrend MR, Kuroda M, Humayun MS, Weiland JD. An In Vitro Model of a Retinal Prosthesis. IEEE Trans Biomed Eng. 2008;55:1744–1753. doi: 10.1109/tbme.2008.919126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekirnjak C, et al. High-resolution electrical stimulation of primate retina for epiretinal implant design. J Neurosci. 2008;28:4446–4456. doi: 10.1523/JNEUROSCI.5138-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen RJ, Rizzo JF., 3rd Responses of ganglion cells to repetitive electrical stimulation of the retina. J Neural Eng. 2007;4:S1–6. doi: 10.1088/1741-2560/4/1/S01. [DOI] [PubMed] [Google Scholar]
- 11.Jensen RJ. Spatiotemporal aspects of pulsed electrical stimuli on the response of rabbit retinal ganglion cells. Exp Eye Res. 2009;89:972–979. doi: 10.1016/j.exer.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Margalit E, Thoreson WB. Inner retinal mechanisms engaged by retinal electrical stimulation. Invest Ophthalmol Vis Sci. 2006;47:2606–2612. doi: 10.1167/iovs.05-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen RJ, Ziv OR, Rizzo JF. Responses of rabbit retinal ganglion cells to electrical stimulation with an epiretinal electrode. J Neural Eng. 2005;2:S16–21. doi: 10.1088/1741-2560/2/1/003. [DOI] [PubMed] [Google Scholar]
- 14.Tsai D, Morley JW, Suaning GJ, Lovell NH. Direct activation and temporal response properties of rabbit retinal ganglion cells following subretinal stimulation. J Neurophysiol. 2009;102:2982–2993. doi: 10.1152/jn.00545.2009. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg R. Analysis of electrical stimulation of the vertebrate retina - work towards a retinal prosthesis. Johns Hopkins; 1998. [Google Scholar]
- 16.Jensen RJ, Ziv OR, Rizzo JF., 3rd Thresholds for activation of rabbit retinal ganglion cells with relatively large, extracellular microelectrodes. Invest Ophthalmol Vis Sci. 2005;46:1486–1496. doi: 10.1167/iovs.04-1018. [DOI] [PubMed] [Google Scholar]
- 17.Fried SI, Lasker AC, Desai NJ, Eddington DK, Rizzo JF., 3rd Axonal sodium-channel bands shape the response to electric stimulation in retinal ganglion cells. J Neurophysiol. 2009;101:1972–1987. doi: 10.1152/jn.91081.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen RJ, Rizzo JF, 3rd, Ziv OR, Grumet A, Wyatt J. Thresholds for activation of rabbit retinal ganglion cells with an ultrafine, extracellular microelectrode. Invest Ophthalmol Vis Sci. 2003;44:3533–3543. doi: 10.1167/iovs.02-1041. [DOI] [PubMed] [Google Scholar]
- 19.Troy JB, Lee BB. Steady discharge of macaque retinal ganglion cells. Vis Neurosci. 1994;11:111–118. doi: 10.1017/s0952523800011159. [DOI] [PubMed] [Google Scholar]
- 20.Freeman DK. The maintained discharge of rat retinal ganglion cells. Vis Neurosci. 2008;25:535–548. doi: 10.1017/S095252380808067X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troy JB, Robson JG. Steady discharge of X and Y retinal ganglion cells under cat photopic luminance. Vis Neurosci. 1992;9:535–553. doi: 10.1017/s0952523800001784. [DOI] [PubMed] [Google Scholar]
- 22.Berry MJ, Warland DK, Meister M. The structure and precision of retinal spike trains. Proc Natl Acad Sci U S A. 1997;94:5411–5416. doi: 10.1073/pnas.94.10.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margolis DJ, Detwiler PB. Different mechanisms generate maintained activity in ON and OFF retinal ganglion cells. J Neurosci. 2007;27:5994–6005. doi: 10.1523/JNEUROSCI.0130-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stasheff SF. Increased spontaneous activity and high frequency rhythmic firing among retinal ganglion cells accompanies outer retinal degeneration in the rd1 mouse. Invest. Ophthalmol. Vis. Sci. 2004;45:5070. [Google Scholar]
- 25.O'Brien BJ, Isayama T, Richardson R, Berson DM. Intrinsic physiological properties of cat retinal ganglion cells. J Physiol. 2002;538:787–802. doi: 10.1113/jphysiol.2001.013009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Vives MV, Nowak LG, McCormick DA. Cellular mechanisms of long-lasting adaptation in visual cortical neurons in vitro. J Neurosci. 2000;20:4286–4299. doi: 10.1523/JNEUROSCI.20-11-04286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barraza D, Hitoshi K, Wilson CJ. Slow spike frequency adaptation in neurons of the subthalamic nucleus. J Neurophysiol. 2009;102:3689–3697. doi: 10.1152/jn.00759.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukasiewicz PD. Synaptic mechanisms that shape visual signaling in the inner retina. Prog Brain Res. 2005;147:205–218. doi: 10.1016/S0079-6123(04)47016-2. [DOI] [PubMed] [Google Scholar]
- 29.Rucci M. Fixation eye movements, natural image statistics, and fine spatial vision. Network-Comp Neural. 2008;19:253–285. doi: 10.1080/09548980802520992. [DOI] [PubMed] [Google Scholar]
- 30.Bremmer F, Kubischik M, Hoffman KP, Krekelberg B. Neural dynamics of saccadic suppression. J Neurosci. 2009;29:12374–12383. doi: 10.1523/JNEUROSCI.2908-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mante V, Frazor RA, Bonin V, Geisler WS, Carandini M. Independence of luminance and contrast in natural scenes in the early visual system. Nat Neurosci. 2005;8:1690–1697. doi: 10.1038/nn1556. [DOI] [PubMed] [Google Scholar]
- 32.Carandini M, Ferster D. A tonic hyperpolarization underlying contrast adaptaiton in cat visual cortex. Science. 1997;276:949–952. doi: 10.1126/science.276.5314.949. [DOI] [PubMed] [Google Scholar]
- 33.Dacey DM. The mosaic of midget ganglion cells in the human retina. J Neurosci. 1993;13:5334–5355. doi: 10.1523/JNEUROSCI.13-12-05334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang JY, Shi L,H, Luo F, Zhang WM, Woodward DJ. Studies of neural mechanisms of deep brain stimulation in rodent models of Parkinson's disease. Neurosci Biobehav Rev. 2008;32:352–366. doi: 10.1016/j.neubiorev.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol. 2001;85:1351–1356. doi: 10.1152/jn.2001.85.4.1351. [DOI] [PubMed] [Google Scholar]
- 36.Margarinos-Ascone C, Pazo JH, Macadar O, Buno W. High-frequency stimulation of the subthalamic nucleus silences subthalamic neurons: a possible cellular mechanism in Parkinson's disease. Neuroscience. 2002;115:1109–1117. doi: 10.1016/s0306-4522(02)00538-9. [DOI] [PubMed] [Google Scholar]
- 37.Garcia L, Audin J, D'Alessandro G, Bioulac B, Hammond C. Dual effect of high-frequency stimualtion on subthalamic neuron activity. J Neurosci. 2003;23:8743–8751. doi: 10.1523/JNEUROSCI.23-25-08743.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia L, D'Alessandro G, Fernagut PO, Bioulac B, Hammond C. Impact of high-frequency stimulation parameters on the pattern of discharge of subthalamic nucleus. J Neurophysiol. 2005;94:3662–3669. doi: 10.1152/jn.00496.2005. [DOI] [PubMed] [Google Scholar]
- 39.Thoreson WB. Kinetics of synaptic transmission at ribbon synpases of rods and cones. Mol Neurobiol. 2007;36:205–223. doi: 10.1007/s12035-007-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]