Abstract
The induction of potent virus-specific immune responses at mucosal surfaces where virus transmission occurs is a major challenge for vaccination strategies. In the case of influenza vaccination, this has been achieved only by intranasal delivery of live-attenuated vaccines that otherwise pose safety problems. Here, we demonstrate that potent mucosal and systemic immune responses, both cellular and humoral, are induced by intranasal immunization using formulated DNA. We show that formulation with the DNA carrier polyethylenimine (PEI) improved by a 1,000-fold the efficiency of gene transfer in the respiratory track following intranasal administration of luciferase-coding DNA. Using PEI formulation, intranasal vaccination with DNA-encoding hemagglutinin (HA) from influenza A H5N1 or (H1N1)2009 viruses induced high levels of HA-specific immunoglobulin A (IgA) antibodies that were detected in bronchoalveolar lavages (BALs) and the serum. No mucosal responses could be detected after parenteral or intranasal immunization with naked-DNA. Furthermore, intranasal DNA vaccination with HA from a given H5N1 virus elicited full protection against the parental strain and partial cross-protection against a distinct highly pathogenic H5N1 strain that could be improved by adding neuraminidase (NA) DNA plasmids. Our observations warrant further investigation of intranasal DNA as an effective vaccination route.
Introduction
Recent outbreaks of avian influenza A (H5N1) and the new influenza A (H1N1) 2009 viruses emphasize the urgent need for efficient and safe vaccines that can be used for mass vaccination within a short time. Although these pathogens are transmitted through mucosal routes, including the respiratory route, currently available vaccines are mostly administrated parenterally, usually by intramuscular (i.m.) or subcutaneous (s.c.) injection. Although parenteral immunization is effective in inducing systemic immune responses, it is often suboptimal or nonefficacious for inducing local immunity at mucosal sites. In contrast, potent mucosal vaccines can induce both local and systemic immunity.1 Thus, novel mucosal vaccines that are fully efficient against drifting influenza viruses could potentially play an important role in preventing the spread of highly pathogenic strains.2
Mucosal immunity is mainly mediated by secretory immunoglobulin A (IgA) that, unlike other antibody isotypes, are resistant to degradation in the protease-rich external environment of mucosal surfaces. Secretory IgA have multiple roles in mucosal defense, promoting the capture of antigens or microorganisms in the mucus and preventing infection by epithelial attachment inhibition.3 Local immunoglobulin G (IgG) synthesis is also observed after mucosal infection or vaccination4 and contributes to neutralization of pathogens, but IgG concentration is generally 30–100-fold1 lower than that of IgA due to the susceptibility of IgG to degradation. Notably, cytotoxic T lymphocytes (CTLs) may also play a crucial role in the local clearance or containment of mucosal viral infections.
To initiate mucosal immune responses, antigens must first access the mucosal-associated lymphoid tissue. In this regard, the new spray formulation of cold-adapted live attenuated influenza vaccines (LAIV) seems to offer a more effective way to induce CTLs and mucosal immunity than parenteral vaccines.5,6 Nevertheless, LAIV is approved only for healthy people in the 5–49 age range and thus cannot be used in high-risk groups such as infants and elderly individuals, highlighting the need for alternative vaccine strategies targeting the mucosal pathway. Indeed, effective mucosal vaccination by nonliving vaccines is difficult to achieve due to their poor immunogenicity and usually requires mucosal adjuvants7,8 and/or various delivery systems including cationic liposomes, virosomes, immunostimulating complexes (ISCOMS) or nanodiscs.9,10,11,12
In the present study, we investigated the immunogenicity and protective capacity of intranasal (i.n.) vaccination with H5N1- or influenza A H1N1(2009)-specific hemagglutinin (HA) DNA vaccines. DNA vaccine technology provides an attractive alternative for the rapid manufacturing of influenza vaccines that could offer a faster response time in the event of a pandemic threat.13 The ability of plasmid DNA-encoding HA to induce immune protection after parenteral injection has been shown in influenza virus challenges in animals.14,15,16 Here, we evaluated the effectiveness of i.n. influenza DNA vaccine administration and its ability to provide protective and cross-protective immunity against a mouse-adapted H5N1 influenza virus. To improve antigen expression after i.n. immunization, plasmid DNA was delivered after formulation with polyethylenimine (PEI). The cationic polymer PEI is known to be an efficient carrier of DNA that promotes uptake by and transfection of cells.17 PEI is highly effective as a gene transfer agent both in vitro and in vivo, and is used in lung gene therapies.18 In this study, we investigated the capacity of PEI-formulated DNA nasal vaccines to induce systemic and mucosal T-cell and B-cell immune responses compared with nonformulated plasmid DNA administrated by i.n. or intradermal (i.d.) injection. Additionally, their ability to induce immune protection against a H5N1 virus challenge was tested and we demonstrated that i.n. PEI/DNA vaccines induced protective immunity and cross-protection that can be enhanced by adding neuraminidase (NA)-encoding plasmids.
Results
Gene expression after intranasal DNA injection
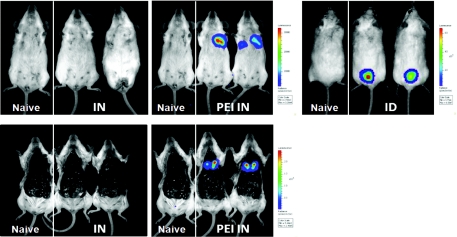
We sought to evaluate the level and distribution of gene expression after i.n. DNA administration. In vivo bioimaging of luciferase was performed in mice 48 hours after i.n. injection of 10 µg of unformulated or PEI-formulated luciferase-expressing plasmids, and compared to mice injected by i.d. DNA electroporation. Luciferase expression in mice receiving i.n. unformulated plasmid DNA was below the detection level (about 102 photons/second) while mice receiving i.n. PEI/DNA complexes expressed significant level of luciferase in the thorax (2.4 ± 0.8 × 105 photons/second; Figure 1). Imaging of the surgically exposed thoracic cavity confirmed the undetectable gene expression after i.n. injection of naked plasmid DNA and revealed that light emission originated from the lungs in the PEI/DNA group. This highly specific luciferase expression after i.n. PEI/DNA injection reached 1.2 ± 0.2 × 106 photons/second locally in the lungs. Alternative methods of plasmid DNA formulation (Lipofectamine-2000, DOTAP, ESCORT) were investigated but significant level of gene expression was only observed with PEI (data not shown). However, this level is much lower than the expression measured in the skin after i.d. DNA administration in combination with electroporation (8.4 ± 9.6 × 108 photons/second; Figure 1), a method known to induce high levels of antigen expression.19
Figure 1.
In vivo expression of plasmid DNA after intranasal or intradermal injection. BALB/c mice (n = 2) were injected with 10 µg of a plasmid (pLuc) encoding the luciferase reporter enzyme, either by i.n. injection or by i.d. injection followed by skin electroporation (ID). For i.n. injection, pLuc was injected either unformulated (IN) or PEI-formulated (PEI IN). As negative control, naive mice received 50 µl of control buffer by i.n. injection. Forty-eight hours after injection, luciferase activity was measured after an intraperitoneal injection of luciferin. Bioluminescence imaging was acquired for 120 seconds with the IVIS Spectrum imaging system on animals either alive (top panels) or euthanized and with the thoracic cavity surgically exposed (bottom panels). Same animals are shown in top and bottom panels.
Systemic and mucosal humoral immune responses
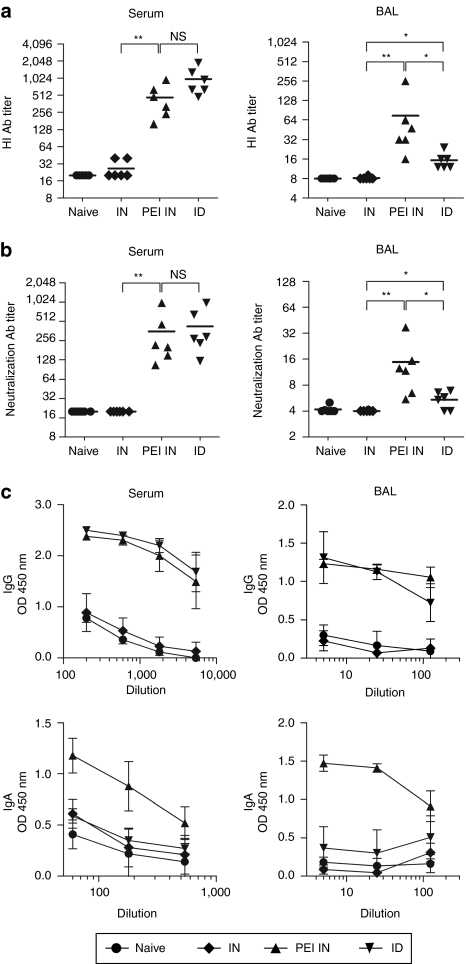
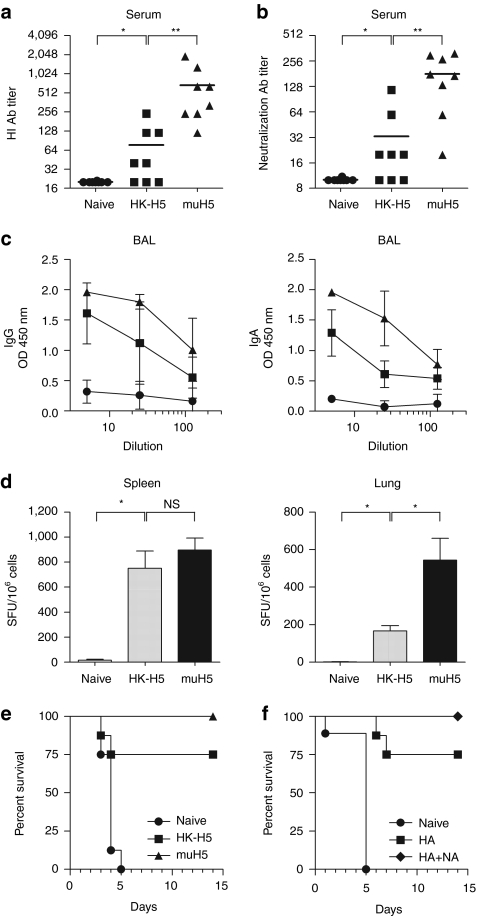
We next compared mucosal versus parenteral DNA vaccination for their ability to induce mucosal immune responses against the H5N1 virus. We used a virus obtained from the Influenza A/crested eagle/Belgium/01/2004 that has been adapted to mice by serial lung-to-lung passages (muH5N1) and is highly pathogenic.20 Mice were immunized twice with 10 µg of HA-encoding DNA either i.n. or i.d. We measured HA-specific antibodies in sera and bronchoalveolar lavages (BALs) of mice by indirect enzyme-linked immunosorbent assay (ELISA) and we evaluated antibody titers by hemagglutination inhibition (HI) or microneutralization assays using retrovirus-derived particles pseudotyped with muH5N1 glycoproteins. In agreement with the luciferase expression data (Figure 1), no antibody response could be detected after i.n. DNA immunization in the absence of formulation (Figure 2), regardless of the assay used. As shown in Figure 2, similar levels of HI (Figure 2a) or virus neutralization (Figure 2b) were measured using sera of mice immunized with i.n. PEI/DNA or i.d. DNA vaccines (NS, P = 0.06, Figure 2a; P = 0.4, Figure 2b; Mann–Whitney). In contrast, weak HI and low neutralizing antibody titers were detected in the BAL of i.d. immunized mice, while significantly higher titers were measured in BAL from mice i.n. immunized with HA-specific PEI/DNA vaccines (Figure 2a,b).
Figure 2.
Humoral immune responses in muH5N1 HA vaccinated mice. BALB/c mice (n = 6 per group) were immunized twice at 4 weeks of interval, either by i.n. injection with 10 µg of unformulated (IN; diamonds) or PEI-formulated (PEI IN; triangles) pH5mu DNA, or by i.d. injection followed by skin electroporation with 10 µg of unformulated pH5mu (ID; inverted triangles). As negative control, naive mice received 50 µl of control buffer by i.n. injection (circles). Sera (left) and BAL (right) were collected, respectively, 3 and 4 weeks after the last immunization and tested individually for the presence of muH5N1 specific antibodies. (a) Hemagglutination inhibition (HI) antibody responses. Samples were tested for specific antibodies by HI assay using purified muH5N1-pseudotyped retrovirus particles. HI antibody titers are expressed as the reciprocal of the highest dilution of samples inhibiting agglutination. (b) Neutralization antibody responses. Samples were tested for specific antibodies by neutralization assay using purified muH5N1-pseudotyped retrovirus particles. Neutralizing antibody titers are expressed as the reciprocal of the dilution of samples allowing 50% neutralization. (a,b) Symbols represent individual mice and horizontal lines represent the geometric mean of each group. **P < 0.01; *P < 0.05; NS, P > 0.05 (Mann–Whitney test). (c) IgG- and IgA-specific antibodies responses. Samples were serially diluted and muH5-specific IgG (top panels) and IgA (bottom panels) were detected by enzyme-linked immunosorbent assay (ELISA). Results are expressed as the geometric mean of optical densities (OD450) in each group, and error bars indicate the standard deviation. Results are representative of three independent experiments.
We next analyzed the Ig isotype of virus-specific antibodies in the serum and BAL of immunized mice. High and similar levels of IgG were detected in serum and BAL of mice immunized with i.n. PEI/DNA or i.d. DNA administration (Figure 2c). In contrast, high levels of H5N1-specific IgA antibodies could only be observed in serum and BAL of mice vaccinated i.n. with PEI/DNA complexes. Therefore, both mucosal and systemic anti-HA IgA antibodies are induced only after i.n. immunization with PEI/DNA complexes.
Systemic and mucosal cellular immune responses
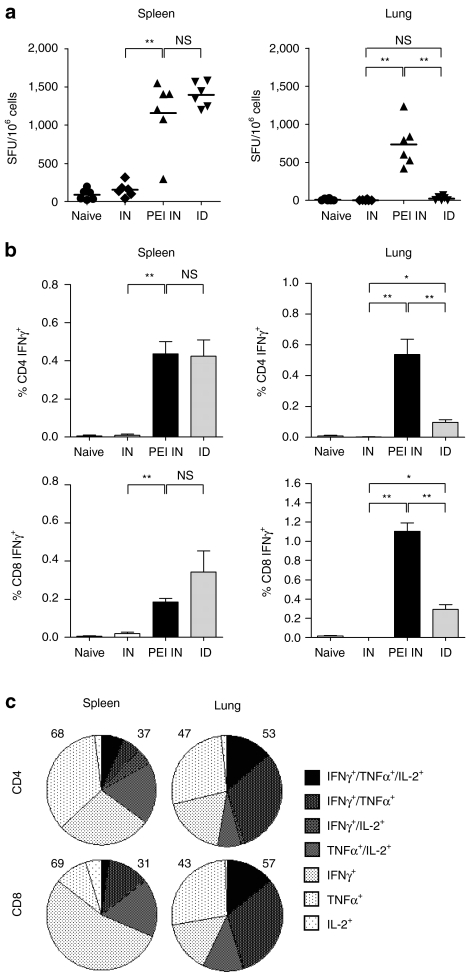
We next evaluated whether the i.n. route is able to efficiently induce T cell immune responses. We first measured the frequency of interferon-γ (IFNγ) secreting T cells in spleen or lungs from immunized or control mice by ELISPOT, 4 weeks after the last immunization with muH5N1 HA-encoding DNA. As shown in Figure 3a, both i.n. PEI/DNA and i.d. DNA immunizations induced high and similar numbers of HA-specific memory T cells in the spleen as detected by ELISPOT (P = 0.3; Mann–Whitney). In contrast, IFNγ secreting T cells in the lungs were only detected in mice i.n. immunized with PEI/DNA complexes. Detection of IFNγ-producing cells by flow cytometry confirmed these results and revealed that both memory CD8+ and CD4+ T cells were induced in the lungs specifically after i.n. immunization with PEI/DNA (Figure 3b). Specific memory T cells were also detected in the cervical draining lymph nodes but the frequency was low in comparison with lungs (data not shown). Additionally, we demonstrated that a high proportion of memory CD4+ and CD8+ T cells generated in i.n. PEI/DNA-vaccinated mice are multifunctional, as defined by the simultaneous secretion of at least two cytokines among IFNγ, tumor necrosis factor-α, and interleukin-2. The highest percentages of multifunctional T cells after i.n. immunization were observed in lungs corresponding to 57 ± 13% and 53 ± 9% of CD8+ and CD4+ memory T cells, respectively (Figure 3c). These percentages were comparable to those measured in spleen after i.d. immunization (51 ± 2 % and 39 ± 6% of CD8+ and CD4+ memory T cells respectively; data not shown). Altogether, these data show that although both mucosal and parenteral DNA immunizations could induce a systemic cellular immunity, only i.n. PEI/DNA vaccines generated muH5N1-specific memory T cells in mucosal tissues.
Figure 3.
Cellular immune responses in muH5N1 HA vaccinated mice. BALB/c mice (n = 6 per group) were immunized as described in Figure 2. Mice were euthanized 4 weeks after the last injection, and spleens and lungs were harvested to analyze muH5N1-specific cellular responses. (a,b) Specific IFNγ immune responses. muH5N1-specific IFNγ-secreting cells were quantified in spleen (left) or lung (right) with ELISPOT assays (a) and intracellular staining assays (ICS; b). (a) Symbols represent individual mice and horizontal lines represent the geometric mean of each group. (b) Average frequencies of IFNγ-producing CD4+ (top panels) and CD8+ (bottom panels) T cells were analyzed by flow cytometry, and results are expressed as the mean ± SD (n = 6). (a,b) **P < 0.01; *P < 0.05; NS, P > 0.05 (Mann–Whitney test). (c) Multifunctional specific cytokine-producing CD4+ and CD8+ T cells. In the PEI IN group, CD4+ and CD8+ T cells from spleen (left) or lung (right) were analyzed for specific production of IFNγ, tumor necrosis factor-α (TNF-α), and interleukin-2 (IL-2) production by intracellular staining. The pie charts show the cell fraction of the total specific CD4+ (top) or CD8+ (bottom) responses, expressing each of the seven possible combinations of IFNγ, TNF-α, and IL-2.The total percentages of monofunctional and multifunctional T cells are done on the left and the right of each graph respectively. Results are representative of three independent experiments.
Protection from a lethal H5N1 virus challenge
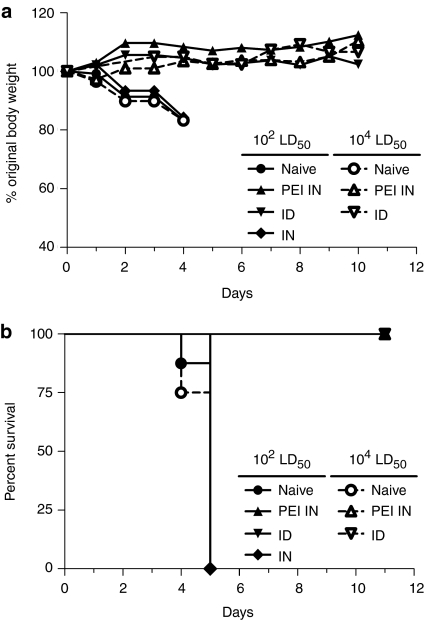
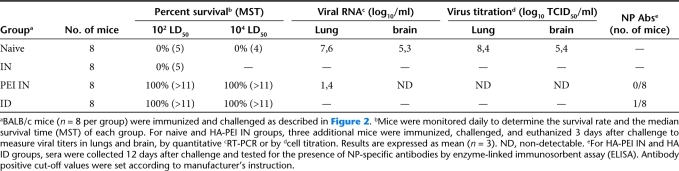
We compared mucosal versus parenteral DNA vaccination for their ability to induce immune protection against i.n. H5N1 virus challenge. Mice were immunized twice with 10 µg of muH5N1 HA-encoding DNA either i.n. or i.d. and subsequently challenged 4 weeks later with 102 LD50 of muH5N1 virus. Mice immunized with i.n. PEI/DNA or i.d. DNA exhibited no significant weight loss over time in contrast to mice i.n. immunized with unformulated DNA vaccines (Figure 4a). Mice in the latter group succumbed to the lethal muH5N1 virus infection between days 4 and 5, similarly to naive control mice (Figure 4b and Table 1). Mice i.n. immunized with PEI/DNA complexes survived at least 12 days and developed efficient immune responses capable of suppressing virus replication, as demonstrated by the absence of detectable virus in lungs or brain at day 3 after challenge (Table 1). The absence of virus replication and spreading in immunized mice was confirmed by undetectable levels of muH5N1 nucleoprotein-specific antibodies in sera, 12 days after challenge (Table 1). Additionally, the efficacy of i.n. PEI/DNA vaccination was highlighted by the complete protection of immunized mice after challenge with a higher dose of lethal virus (104 LD50 of muH5N1 virus; Table 1 and Figure 4). In this condition, all mice i.n. immunized with PEI/DNA complexes survived without morbidity as evidenced by a stable bodyweight and no difference with i.d. immunized mice was observed, highlighting the i.n. vaccination efficacy.
Figure 4.
Protection against lethal H5N1 challenge. BALB/c mice (n = 8 per group) were immunized as described in Figure 2. Four weeks after the second immunization, mice were i.n. challenged with two different lethal doses of a mouse-adapted strain of the H5N1 virus: 102 LD50 (closed symbols) or 104 LD50 (open symbols). Mice were then monitored daily to determine (a) body weight changes as an indicator of morbidity and (b) survival. (a) Results are expressed as the average percentage of initial weight recorded on the day of challenge. (b) Kaplan–Meier survival curves are shown for each group.
Table 1. Protection against lethal muH5N1 virus challenge in muH5N1 HA vaccinated mice.
H5N1 HA-specific cross-protective immune responses
Ideally, influenza vaccines should protect not only against the virus strain used to generate the vaccine but also against viruses that have undergone antigenic drift. Thus, we investigated whether i.n. PEI/DNA vaccines could induce neutralizing antibodies, T-cell responses and protection against a heterologous virus strain. Mice were immunized i.n. with PEI/DNA complexes encoding HA from the heterologous A/HongKong/156/97 (HK-H5N1, clade 3) or homologous muH5N1 virus strains and immune reactivity against muH5N1 virus (clade 1) was evaluated. We observed that i.n. PEI/DNA vaccination with HK-H5N1 induced antibodies in the serum that were capable of inhibiting hemagglutination and infectious properties of muH5N1 HA-pseudotyped retroviral particles (Figure 5a,b), even though the crossreactive antibody titers were lower than those measured in mice immunized with homologous vaccines. ELISA assays revealed that both IgG and IgA antibodies present in the BAL of HK-H5N1 i.n. immunized mice were crossreactive with muH5N1 (Figure 5c). Moreover, crossreactive T cell responses were efficiently induced in spleen and lungs of immunized mice (Figure 5d). Notably, the frequency of muH5N1-reactive splenic T cells was similar in HK-H5N1-immunized mice and muH5N1-immunized controls.
Figure 5.
Cross-reactive immune responses and cross-protection in mice i.n. immunized with DNA vaccines encoding HA from heterologous influenza A H5N1 virus. BALB/c mice (n = 4–8 per group) were immunized twice at 4 weeks interval by i.n. injection with pH5HK (HK-H5; squares) or pH5mu (muH5; triangles) PEI/DNA complexes (a–e). As negative control, naive mice received i.n. 50 µl of control buffer (circles). Sera, BAL, splenocytes and pulmonary lymphocytes were collected as described in Figures 2 and 3. (a) HI antibody responses. Sera were tested for HI antibodies against purified muH5N1-pseudotyped retrovirus particles and HI antibody titers are shown. (b) Neutralization antibody responses. Sera were tested for neutralizing antibodies against purified muH5N1-pseudotyped retrovectors and titers calculated as in Figure 2 are shown. (a,b) Symbols represent individual mice and horizontal lines represent the geometric mean of each group. (c) IgG- and IgA-specific antibodies responses. BAL were serially diluted and muH5 specific IgG (left) and IgA (right) were detected by enzyme-linked immunosorbent assay (ELISA). Results are expressed as the geometric mean of OD450 in each group (n = 4) and error bars indicate the SD (d) Specific IFNγ responses. Secretion of IFNγ by splenocytes (left) and pulmonary lymphocytes (right) were measured by ELISPOT assay. Results are expressed as the mean ± SD (n = 4). (a–d) **P < 0.01; *P < 0.05; NS, P > 0.05 (Mann–Whitney test). (e,f) Cross-protection against muH5N1 virus challenge. Four weeks after the last immunization, mice were i.n. challenged with a lethal dose (102 LD50) of the muH5N1 strain and monitored daily for survival. Kaplan–Meier survival curves are shown for each group. (f) Mice were immunized with pH5HK (HA; squares) or pH5HK + pN1HK (HA+NA; diamonds) PEI/DNA complexes. Results are representative of three (e) and two (f) independent experiments.
Finally, we examined whether an i.n. PEI/DNA vaccine could induce a cross-protective immunity against heterologous virus strains. Mice i.n. immunized with HK-H5N1 or muH5N1 HA-specific formulated DNA were challenged i.n. with muH5N1 virus and survival rates were determined. As expected, all unimmunized mice died within 5 days upon infection while 100% of mice immunized with muH5NI DNA were protected (Figure 5e). Importantly, mice immunized with the HK-H5N1 DNA vaccine were partially protected against muH5N1 viral infection and had a survival rate of 75% (Figure 5e). Of note, surviving mice of this group did not significantly lose bodyweight (data not shown), further indicating cross-protection upon infection.
Because HA and NA are the most highly antigenic proteins in influenza, and both are included in standard influenza vaccines, we examined whether addition of NA-encoding plasmid DNA into the HK-H5N1 PEI/DNA vaccine could enhance the cross-protection from muH5N1 infection. Thus, mice were i.n. immunized twice with either HK-H5N1 HA or HA+NA plasmid DNA and challenged i.n. with muH5N1 virus. Bodyweight loss and survival rate were determined. We confirmed that mice i.n. immunized with HK-H5N1 HA DNA were partially protected against muH5N1 virus challenge (75% survival; Figure 5f). In contrast, all mice immunized with HK-H5N1 HA+NA DNA survived a subsequent infection with muH5N1 (100% survival; Figure 5f) without morbidity as evidenced by a stable bodyweight (data not shown). We thus sought to evaluate the level of protection afforded by the NA DNA vaccine. Mice were i.n. immunized with HK-H5N1 or muH5N1 NA plasmid DNA and challenged i.n. with muH5N1 virus. Both vaccines led to a significant prolongation of survival of the mice, superior with homologous vaccine, but all mice ultimately succumbed (Supplementary Figure S1). These results highlight that NA antigens are not sufficient to induce robust protective immunity but contribute to stimulate broader immune responses and cross-protection in i.n. DNA influenza vaccines.
Vaccination for influenza A (H1N1) 2009 virus
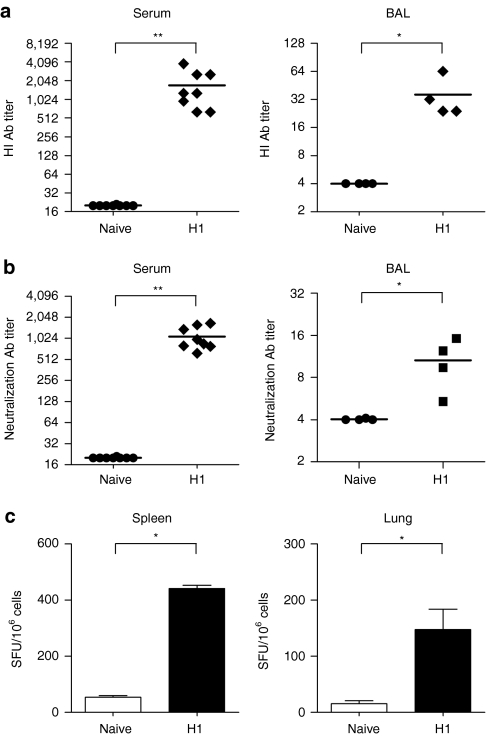
We determined whether the i.n. DNA vaccination protocol could induce responses against the pandemic influenza A (H1N1)2009 virus. Mice were i.n. immunized twice, at a 4-week interval, with HA DNA derived from the swine-originating influenza A (H1N1)2009 virus strain. The results demonstrate that i.n. PEI/DNA vaccination induced (H1N1)2009-specific functional antibody activity as evidenced by HI and neutralization assays (Figure 6a,b). Specific antibodies were detected both in serum and BAL and titers measured in mice immunized with (H1N1)2009-specific PEI/DNA vaccines were similar to those observed previously using muH5N1 vaccines (Figure 2). Additionally, ELISPOT results showed that PEI/DNA vaccines elicited high frequencies of memory T cells secreting IFNγ in spleen and lungs (Figure 6c). These data confirm in the influenza A (H1N1)2009 HA vaccination model that i.n. PEI/DNA vaccines induce potent mucosal and systemic immune responses.
Figure 6.
Immune responses in mice i.n. immunized with DNA vaccines encoding HA from influenza A H1N1(2009). BALB/c mice (n = 4–8 per group) were immunized twice at 4 weeks interval by i.n. injection with 10 µg of pH12009PEI/DNA complexes (H1; diamonds). As negative control, naive mice received i.n. 50 µl of control buffer (circles). Sera, BAL, splenocytes and pulmonary lymphocytes were collected as described in Figures 2 and 3. (a) HI and (b) neutralization antibody responses. Sera (left) or BAL (right) were tested for specific antibodies using purified H1N1-pseudotyped retrovirus particles. (a,b) HI and neutralizing antibody titers were calculated as in Figure 2. Symbols represent individual mice and horizontal lines represent the geometric mean of each group. (c) Specific IFNγ responses. Secretion of IFNγ by splenocytes (left) and pulmonary lymphocytes (right) were measured by ELISPOT assay, after a 48-h restimulation with recombinant H1N1 lentiviral vectors. Results are expressed as the mean ± SD (n = 4). **P < 0.01; *P < 0.05 (Mann–Whitney test).
Discussion
Development of i.n. delivered vaccines may offer superior protection against pathogens that infect via mucosal surfaces by stimulating the local immunity. In this regard, the new spray formulation of cold adapted LAIV may offer a more effective way to induce local immunity. However, the use of live virus inevitably raises safety concerns, and likewise the use of LAIV vaccine is approved only for limited target populations. Intranasal administration of inactivated vaccines represents a potential solution to overcome these problems. In clinical trials, conventional inactivated influenza virus vaccines provided protection against influenza when administered intranasally.21,22,23 Nevertheless, manufacturing logistics of conventional inactivated influenza virus vaccines place some limitations on their availability and use.24 DNA vaccine technology provides an attractive alternative that would allow a rapid manufacturing of influenza vaccines and offer a faster response time to a pandemic threat.13 DNA vaccines are produced in bacteria and therefore bypass the need for egg or mammalian cell culture methods, allowing the manufacture of vaccines against a new strain to start as soon as the relevant HA gene sequence is available. DNA vaccines have been proposed as an attractive alternative to protein-based approaches because of their simplicity of manufacture, and ability to generate serological and cell-mediated immune responses. However, despite exciting results in preclinical models and the approved use of DNA vaccines for veterinary purposes,25 an effective DNA vaccine in humans has remained elusive. One particular aspect of DNA vaccination that has been the subject of intensive study is the optimal mode of delivery. Here, we have explored a new strategy of nasal priming with PEI/DNA complexes to induce mucosal immunity and determined its potential in the context of influenza vaccination. PEI, a readily available synthetic polycation, was previously shown to enhance the efficacy of gene delivery both in vitro and in vivo18,26 and was thus an ideal candidate for our study. PEI is capable of binding and compacting DNA and can participate in achieving efficient transfection. In contrast to unformulated DNA, we observed that i.n. administration of PEI/DNA was able to induce efficient gene expression locally as proven using a luciferase reporter model. Bioluminescence images analysis revealed that i.n. PEI/DNA complexes poorly transfected the nose but efficiently the pulmonary tissue, resulting in high-level transgene expression in lungs, in line with previous reports.26 The level of gene expression after i.n. PEI/DNA administration was nevertheless 2 logs lower than after i.d. DNA injection with electroporation, one of the most efficient DNA delivery methods available to date.19,27 Remarkably, despite this difference in gene expression, systemic immune responses induced by i.n. PEI/DNA and i.d. immunization were similar, and i.n. PEI/DNA vaccines were even far superior to i.d. vaccines in their ability to stimulate mucosal immunity. Despite the lower efficacy of gene transfer per se, PEI/DNA complexes may contribute to the immunogenicity of this approach. Indeed, mucosal administration of PEI could function as a potent immunostimulator, as previously suggested by gene expression analysis28 that revealed the upregulation of genes indicative of a mixed Th1/Th2 responses, the activation of both CD8+ and CD4+ T cells and B cell differentiation upon immunization with PEI formulated DNA. Taken together, our data demonstrate that PEI is effective in delivering DNA to mucosal surfaces and improving the immunogenicity of DNA vaccines.
The ability of i.n. PEI/DNA vaccines to induce humoral and cellular immune responses against HA from influenza A (H5N1) and (H1N1)2009 viruses, both systemically and in the mucosal tissue, reveals that expression of plasmid DNA in the peripheral lung tissue can elicit strong local and systemic immune responses. Systemic responses were similar in mice immunized with H5N1-specific i.n. PEI/DNA or i.d. DNA vaccines, with no detectable differences in serum antibody titers and frequency of IFNγ-secreting splenocytes (Figures 2 and 3). Conversely, intranasal PEI/DNA vaccines uniquely induced high-magnitude CD4+ and CD8+ memory T cell responses in spleen and lungs, a high frequency of which further demonstrated multifunctional properties characterized by the concomitant production of IFNγ, tumor necrosis factor-α, and interleukin-2. It is important to note that the induction of multifunctional T-cell populations coexpressing several cytokines has been previously associated with superior antiviral protection and vaccine efficacy in mouse and man.29,30,31,32 When mucosal immunity was investigated, virus-specific IgA antibodies were only found in BAL (Figure 2c) and vaginal secretions (data not shown) of mice immunized i.n. with PEI/DNA complexes. The presence of IgA in vaginal secretions after i.n. vaccination has been observed previously after i.n. DNA immunization in different models33,34 and was expected because of overlapping tissue sites in the common mucosal immune system.35 Production of IgA in the lungs could be important to establish protective immunity against influenza. Indeed, it has been shown that IgA inhibit initial pathogen colonization by performing immune exclusion both on the mucosal surface and within infected secretory epithelial cells without causing tissue damage.36 The local role of IgA is here highlighted by the correlation between the HI/neutralizing activity and the presence of IgA antibodies in BAL of immunized mice. Nevertheless, it is difficult to determine unequivocally the relative importance of IgA versus serum antibodies for protection, because we did not observe IgA in i.d. immunized mice who survived H5N1 infection. Other experiments of IgA transfer in naive mice support the notion that IgA exert a decisive role in cross-protection against influenza.37
In our study, i.n. PEI/DNA vaccines induced cellular and humoral immune responses capable of providing protective immunity against a divergent virus of H5N1 subtype. The broad protection profile of this DNA vaccine strategy thus compares favorably with the extent of protection observed in novel consensus DNA immunogen approaches.38,39 Remarkably, as shown previously,40,41 addition of NA antigens in vaccine formulation clearly enhanced the protective efficacy of i.n. PEI/DNA immunization as shown in our cross-protection model, and thus should be included in i.n. vaccine formulations to induce broad immunity.
Overall, our results show that the delivery vehicle and route of immunization are critical factors governing the efficiency with which plasmid DNA can elicit specific mucosal immune responses and immune protection in different influenza models. We believe that the vaccine strategy described herein warrants further evaluation as a potentially fast, affordable, and stable prophylactic approach for combating pandemic influenza viruses, and should further be evaluated in settings where mucosal immunity is desired such as for the development of HIV vaccines. Despite the extensive use of PEI as a nonviral gene delivery vector and the promising results obtained for the treatment of a wide range of pulmonary and nonpulmonary disorders, numerous practical issues would need to be worked out in order to apply the delivery of genes by inhalation methods for clinical vaccines.
Materials and Methods
Cell lines. 293T human embryo kidney cells (CRL-1573, ATCC) were cultured at 37 °C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 mmol/l of -glutamin, 100 U/ml of penicillin and streptomycin (all from Invitrogen, Cergy Pontoise, France) and 10% of heat-inactivated fetal calf serum (FCS; PAA, Les Mureaux, France).
Virus. A mouse-adapted variant strain (muH5N1) was obtained from an avian H5N1 virus (A/crested eagle/Belgium/1/2004) by lung-to-lung passaging as described elsewhere.20 After five passages, the mouse-adapted virus (muH5N1) rapidly caused the death of naive mice and began to propagate stably in lungs.
Antigens and plasmids. The cDNA sequences encoding the HA and NA from the mouse-adapted muH5N1 Influenza A/crested eagle/Belgium/01/2004 (H5N1) virus (ABA70758.1 and ABA70757.1 GenBank sequences, respectively), from A/Hong Kong/156/97 (H5N1) virus (AF028709, AF046089) and from the pandemic Influenza A/New Mexico/04/2009 (H1N1) virus (ACR08530, ACR08568) were codon-optimized, synthesized and cloned in pUC57 plasmid (GenScript, Piscataway, NJ). The following sequences 5′-CTCGAGAGCGCTGAATTCCGCCACCATG-3′ and 5′-TGAGAATTCGTTAACGGATCC-3′ were added at the 5′- and 3′-end of the optimized sequence, respectively, introducing a start and stop codon (in bold) and unique restriction sites for subsequent cloning (XhoI-BamHI) in phCMV expression plasmid. The corresponding DNA plasmids (pH5mu, pN1mu, pH5HK, pN1HK, pH12009, pN12009) were produced by amplification in Escherichia coli competent bacteria (DH5α Invitrogen, Cergy Pontoise, France) and purification with endotoxin-free preparation kits (Nucleobond PC 2000 EF; Macherey-Nagel, Hoerd, France). All plasmids were verified after amplification by enzymatic digestion.
Production of retroviral particles. HIV-Gag-based pseudoparticles: To generate influenza or control VSV-G pseudotyped lentiviral particles, 293T cells were transfected with expression vectors encoding the viral components (i.e., HA and NA glycoproteins or VSV-G and lentiviral core proteins), as previously described.42 An HIV p24-specific ELISA assay (Kit RETRO-TEK HIV-1 p24 Antigen ELISA; ZeptoMetrix, New York) was used to determine the p24 concentrations in the lentiviral vector samples, according to the manufacturer's instructions. MLV-Gag-based pseudoparticles: Influenza-pseudotyped MLV-retroviral particles were produced by Epixis (Lyon, France) using recombinant baculovirus vectors encoding the viral components (HA and NA glycoproteins and Moloney MLV Gag proteins) in insect cells. MLV-Gag-based vectors: Infectious particles used in neutralization assays were generated as previously described43 by transfection of HEK-293T cells (ATCC CRL-11268) with plasmids encoding MLV-derived core and packaging (Gag-Pol) components, HA and NA proteins from influenza virus, and a retroviral transfer vector harboring a marker gene encoding the luciferase firefly (F-Luc).
Mice. Female BALB/c mice were purchased from Elevage Janvier (Le Genest Saint Isle, France) and were 8 weeks old when the experiments were initiated. Mice were maintained under specific pathogen-free conditions, and manipulations were performed according to the European Economic Community guidelines and approved by the local ethics committee.
Immunizations. Mice were anesthetized by intraperitoneal (i.p.) injection of xylazin (10 mg/kg; Rompun 2%, Bayer Pharma, France) and ketamin (150 mg/kg; Imalgène 1000, Merial, France) and vaccinated twice at days 0 and 28. Intranasal immunization: 50 µl of a 5% glucose solution with 10 µg of unformulated or PEI-formulated HA-plasmid DNA was injected slowly into one nostril where the fluid was inhaled. In HA and NA combination vaccine, 10 µg and 2.5 µg of HA and NA-specific plasmid DNAs were mixed respectively before formulation. As a negative control, naive mice were i.n. injected with 50 µl of a 5% glucose solution without DNA plasmid. PEI-DNA formulation was performed using in vivo jet-PEI (Ozyme, Saint-Quentin-en-Yvelines, France) according to the manufacturer's recommendations with a N/P ratio of 8. Intradermic immunization: Mice were shaved on the lower back and injected with 40 µl of a 5% glucose solution containing 10 µg of HA plasmid DNA in two sites separated by a distance of 0.5 cm. The skin was immediately electroporated with tweezer type electrodes (CUY650 P3 electrodes; Sonidel Limited, Dublin, Ireland) using a BTX ECM830 generator (Harvard Apparatus, Les Ulis, France). Eight pulses of 60 V were given for 20 ms duration with a 200 ms interval.
Samples. Mice were bled for serum collection prior to the first immunization and 3 weeks after the last immunization. Sera were heat-inactivated at 56 °C for 30 minutes. Mice were euthanized 4 weeks after the last immunization and the spleen was harvested. BAL were collected through the trachea by two series of injection-aspiration with 1 ml phosphate-buffered saline (PBS) + protease inhibitor (Complete Mini Protease Inhibitor Cocktail; Roche Diagnostics, Meylan, France). Lungs were removed after perfusion with 2 ml of PBS via the heart left ventricle and cell suspensions were prepared by digestion with collagenase IV (4 mg/ml; Sigma-Aldrich, Saint-Quentin Fallavier, France) and DNAse I (250 µg/ml; Roche Diagnostics, Meylan, France) in RPMI medium. A 40/60% Percoll gradient (Sigma-Aldrich) was used to isolate pulmonary lymphocytes.
Bioluminescence imaging. Anesthetized mice were i.n. or i.d. injected with 10 µg of a plasmid (pLuc) encoding the luciferase reporter enzyme, either unformulated or PEI-formulated. Forty-eight hours later, luciferase activity was measured by i.p. administration of -luciferin (150 mg/kg; Promega, Charbonnières-les-Bains, France) and bioluminescence images were acquired during 120 seconds with the IVIS Spectrum imaging system (Caliper Life Sciences, Tremblay, France). Luciferase expression was analyzed with the Living Image 3.2 software (Caliper Life Sciences) and shown in photons per second (p/second).
Virus challenges. Four weeks after the last immunization, mice were anesthetized and i.n. challenged with 50 µl of a suspension containing 102 or 104 50% lethal dose (LD50) of mouse-adapted variant strain muH5N1 virus. Mice were monitored daily for weight loss and survival during 2 weeks.
Virus titration. Lungs and brain were removed, weighed, crushed and homogenized in cold PBS with antibiotics to obtain an ~10% homogenates. Tenfold serial dilutions of tissue homogenates in L15/McCoy's Medium (Sigma-Aldrich) supplemented with 4% FCS were used to infect chick embryo fibroblast cells (6.105 cells/ml). Seven days after infection, cell supernatants were tested for HA by hemagglutination assay using chicken erythrocytes. Infectious titers from each organ were calculated by the Reed and Muench method and expressed as mean log of 50% tissue culture infectious dose per ml (TCID50/ml).
Real-time RT-PCR assays. Viral RNA from lung and brain suspensions were extracted using the MagMax AI/ND 96 Viral RNA Kit (Applied Biosystems, Courtaboeuf, France) adapted for semiautomated extraction using a Kingfisher magnetic particle processor. RT-PCR was used for H5N1 HPAI detection.44 Cycle threshold (Ct) values higher than 40 were considered as negative. RT-PCR targeting β-actin was used as reaction control.45
HI assay. For determination of HI titers, sera or BAL were first incubated overnight at 37 °C with receptor-destroying enzyme (Cholera filtrate; Sigma-Aldrich) and then incubated 30 minutes at 56 °C to inactivate the enzyme. Samples were serially diluted twofold starting from 1:10 or 1:2 dilutions, respectively, in 25 µl of PBS in V-bottom 96-well plates and incubated 45 minutes at 37 °C with 4 HA units of muH5N1- or H1N1-pseudotyped MLV-Gag based particles in 25 µl PBS. Finally, 50 µl of a 0.5% chicken erythrocyte (Charles River, L'Arbresle, France) suspension in PBS was added to each well. The highest sample dilution able to prevent hemagglutination was scored as the HI titer.
Neutralization assay. Neutralization assays were performed by Epixis by incubating serially diluted sera with muH5N1- or H1N1- pseudotyped MLV-Gag-based particles harboring the F-Lucmarker gene. Luciferase assay was performed using a Bright-Glo Luciferase assay system (Promega) and a CentroXS luminometer (Berthold Technologies, Thoiry, France). Data were expressed in RLU (relative luminescence units). The percentage of neutralization was calculated as 100 × [1 − (RLU in infected target cells in the presence of serum/RLU in infected control cells)].
ELISA. 96-well microtiter plates (Nunc Life Technologies, Rochester, NY) were precoated with Galanthus nivalis lectin (0.5 µg in 100 µl of 50 mmol/l carbonate buffer pH 9.6 per well; Sigma-Aldrich) at 4 °C for 24 hours, and then coated with H5N1mu or H1N1-pseudotyped MLV-Gag pseudoparticles (0.5 µg in 100 µl of PBS per well) at 4 °C overnight. Wells were blocked with 200 µl of Superblock Blocking Buffer (Thermo Fisher Scientific, Brebières, France) for 1 hour at room temperature. Serial dilutions of sera or BAL in PBS 2% (w/v) bovine serum albumin (Sigma-Aldrich) 0.1% Tween-20 (Bio-Rad Laboratories, Marnes-la-Coquette, France) were added and incubated for 2 hours at room temperature, revealed for 1 hour at room temperature with biotin-labeled goat anti-mouse IgG or anti-mouse IgA (both from CliniSciences, Montrouge, France), and for 1 hour at room temperature with an ultrasensitive streptavidin-peroxidase polymer (Sigma-Aldrich). Plates were thoroughly washed between each step by multiple washes with PBS 0.1% Tween-20. Peroxidase activity was measured using TMB substrate (Sigma-Aldrich) and optical densities were read at 450 nm (OD450) after blocking the reaction by adding HCl 1 mol/l.
NP competitive ELISA. Virus challenged mouse sera reacting with the nucleoprotein of avian influenza viruses were detected using the ID Screen Influenza A antibody Competition kit (ID.Vet, Montpellier, France), according to the manufacturer's recommendations. Values >50% were considered as negative.
ELISPOT assay. HA-specific IFNγ production was determined by a standard ELISPOT assay (Mabtech, Sophia Antipolis, France). Splenocytes (5 × 105 cells/well) or pulmonary lymphocytes (2 × 105 cells/well) were stimulated for 48 hours at 37 °C in 5% CO2 with 20 ng of HIV-Gag-based particles pseudotyped with HA and NA. Medium alone or concanavalin A (2 µl/ml; concanavalin A; Sigma-Aldrich) were used as negative and positive controls, respectively. After revelation, spots were counted using the AID ELISPOT reader (ELR03, AID Autoimmun Diagnostika, Strassberg, Germany).
Immunostaining and flow cytometry. Spleen cells and pulmonary lymphocytes were incubated (2 × 106 cells/well) overnight at 37 °C in 5% CO2 with 100 ng of lentiviral particles pseudotyped with HA and NA in complete RPMI medium supplemented with Brefeldin A (10 µg/ml, BD Biosciences, Le Pont de Claix, France). As negative control, cells were incubated in complete RPMI medium without antigen. Cells were stained with the following antibodies for cell surface analysis: PerCP labeled anti-CD3, Alexa700 labeled anti-CD8, APC-Alexa750 labeled anti-CD4 (all from BD Biosciences). Cells were then permeabilized with the BD Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions and stained with antibodies for intracellular cytokine staining: FITC labeled anti-interleukin-2, PE-labeled anti–tumor necrosis factor-α, APC-labeled anti-IFNγ (all from BD Biosciences). Acquisition was done with a BD LSRII flow cytometer and results were analyzed with FlowJo v7.5.3 software (TreeStar, Ashland, OR).
SUPPLEMENTARY MATERIAL Figure S1. Protection against lethal H5N1 challenge in mice i.n. immunized with DNA vaccines encoding NA from homologous or heterologous influenza A H5N1 virus.
Acknowledgments
The authors thank Xavier Dervillez, Béatrice Baut, Isabelle Ragon from UPMC UMR7211 and Pierre Garrone and Anne-Catherine Fluckiger from Epixis for technical assistance. This research was funded by grants from EuroTransBio (DV3) and ANR (PseudoFlu).
Supplementary Material
Protection against lethal H5N1 challenge in mice i.n. immunized with DNA vaccines encoding NA from homologous or heterologous influenza A H5N1 virus.
REFERENCES
- Neutra MR., and, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Ichinohe T, Tamura S., and, Kurata T. Development of a mucosal vaccine for influenza viruses: preparation for a potential influenza pandemic. Expert Rev Vaccines. 2007;6:193–201. doi: 10.1586/14760584.6.2.193. [DOI] [PubMed] [Google Scholar]
- Hutchings AB, Helander A, Silvey KJ, Chandran K, Lucas WT, Nibert ML, et al. Secretory immunoglobulin A antibodies against the sigma1 outer capsid protein of reovirus type 1 Lang prevent infection of mouse Peyer's patches. J Virol. 2004;78:947–957. doi: 10.1128/JVI.78.2.947-957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu Z, Clements ML, Prince SJ, Murphy BR., and, Mestecky J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine. 1995;13:1006–1012. doi: 10.1016/0264-410x(95)00016-t. [DOI] [PubMed] [Google Scholar]
- Belshe RB, Nichol KL, Black SB, Shinefield H, Cordova J, Walker R, et al. Safety, efficacy, and effectiveness of live, attenuated, cold-adapted influenza vaccine in an indicated population aged 5-49 years. Clin Infect Dis. 2004;39:920–927. doi: 10.1086/423001. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Mendelman PM, Mallon KP, Jackson LA, Gorse GJ, Belshe RB, et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA. 1999;282:137–144. doi: 10.1001/jama.282.2.137. [DOI] [PubMed] [Google Scholar]
- Ichinohe T, Ainai A, Tashiro M, Sata T., and, Hasegawa H. PolyI:polyC12U adjuvant-combined intranasal vaccine protects mice against highly pathogenic H5N1 influenza virus variants. Vaccine. 2009;27:6276–6279. doi: 10.1016/j.vaccine.2009.04.074. [DOI] [PubMed] [Google Scholar]
- Xiaowen Z, Qinghua Y, Xiaofei Z., and, Qian Y. Co-administration of inactivated avian influenza virus with CpG or rIL-2 strongly enhances the local immune response after intranasal immunization in chicken. Vaccine. 2009;27:5628–5632. doi: 10.1016/j.vaccine.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Durrer P, Glück U, Spyr C, Lang AB, Zurbriggen R, Herzog C, et al. Mucosal antibody response induced with a nasal virosome-based influenza vaccine. Vaccine. 2003;21:4328–4334. doi: 10.1016/s0264-410x(03)00457-2. [DOI] [PubMed] [Google Scholar]
- Coulter A, Harris R, Davis R, Drane D, Cox J, Ryan D, et al. Intranasal vaccination with ISCOMATRIX adjuvanted influenza vaccine. Vaccine. 2003;21:946–949. doi: 10.1016/s0264-410x(02)00545-5. [DOI] [PubMed] [Google Scholar]
- Sanders MT, Deliyannis G, Pearse MJ, McNamara MK., and, Brown LE. Single dose intranasal immunization with ISCOMATRIX vaccines to elicit antibody-mediated clearance of influenza virus requires delivery to the lower respiratory tract. Vaccine. 2009;27:2475–2482. doi: 10.1016/j.vaccine.2009.02.054. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, Grimme S, Ganesh B, Gopisetty A, Sheng JR, Martinez O, et al. Nanodisc-incorporated hemagglutinin provides protective immunity against influenza virus infection. J Virol. 2010;84:361–371. doi: 10.1128/JVI.01355-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG. Potential advantages of DNA immunization for influenza epidemic and pandemic planning. Clin Infect Dis. 1999;28:225–229. doi: 10.1086/515123. [DOI] [PubMed] [Google Scholar]
- Zheng L, Wang F, Yang Z, Chen J, Chang H., and, Chen Z. A single immunization with HA DNA vaccine by electroporation induces early protection against H5N1 avian influenza virus challenge in mice. BMC Infect Dis. 2009;9:17. doi: 10.1186/1471-2334-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Kuang H, Wang H, Fang F, Yang Z, Zhang Z, et al. Comparing the ability of a series of viral protein-expressing plasmid DNAs to protect against H5N1 influenza virus. Virus Genes. 2009;38:30–38. doi: 10.1007/s11262-008-0305-2. [DOI] [PubMed] [Google Scholar]
- Rao S, Kong WP, Wei CJ, Yang ZY, Nason M, Styles D, et al. Multivalent HA DNA vaccination protects against highly pathogenic H5N1 avian influenza infection in chickens and mice. PLoS ONE. 2008;3:e2432. doi: 10.1371/journal.pone.0002432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LA, McLachlan G, Sumner-Jones SG, Ferguson D, Baker A, Tennant P, et al. Enhanced lung gene expression after aerosol delivery of concentrated pDNA/PEI complexes. Mol Ther. 2008;16:1283–1290. doi: 10.1038/mt.2008.96. [DOI] [PubMed] [Google Scholar]
- Roos AK, Eriksson F, Timmons JA, Gerhardt J, Nyman U, Gudmundsdotter L, et al. Skin electroporation: effects on transgene expression, DNA persistence and local tissue environment. PLoS ONE. 2009;4:e7226. doi: 10.1371/journal.pone.0007226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garigliany MM, Habyarimana A, Lambrecht B, Van de Paar E, Cornet A, van den Berg T, et al. Influenza A strain-dependent pathogenesis in fatal H1N1 and H5N1 subtype infections of mice. Emerg Infect Dis. 2010;16:595–603. doi: 10.3201/eid1604.091061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum E, Engelhard D, Levy R, Schlezinger M, Morag A., and, Zakay-Rones Z. Mucosal (SIgA) and serum (IgG) immunologic responses in young adults following intranasal administration of one or two doses of inactivated, trivalent anti-influenza vaccine. Vaccine. 2004;22:2566–2577. doi: 10.1016/j.vaccine.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Muszkat M, Greenbaum E, Ben-Yehuda A, Oster M, Yeu'l E, Heimann S, et al. Local and systemic immune response in nursing-home elderly following intranasal or intramuscular immunization with inactivated influenza vaccine. Vaccine. 2003;21:1180–1186. doi: 10.1016/s0264-410x(02)00481-4. [DOI] [PubMed] [Google Scholar]
- Hagenaars N, Mastrobattista E, Glansbeek H, Heldens J, van den Bosch H, Schijns V, et al. Head-to-head comparison of four nonadjuvanted inactivated cell culture-derived influenza vaccines: effect of composition, spatial organization and immunization route on the immunogenicity in a murine challenge model. Vaccine. 2008;26:6555–6563. doi: 10.1016/j.vaccine.2008.09.057. [DOI] [PubMed] [Google Scholar]
- Forde GM. Rapid-response vaccines–does DNA offer a solution. Nat Biotechnol. 2005;23:1059–1062. doi: 10.1038/nbt0905-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonius K, Simard N, Harland R., and, Ulmer JB. The road to licensure of a DNA vaccine. Curr Opin Investig Drugs. 2007;8:635–641. [PubMed] [Google Scholar]
- Densmore CL, Orson FM, Xu B, Kinsey BM, Waldrep JC, Hua P, et al. Aerosol delivery of robust polyethyleneimine-DNA complexes for gene therapy and genetic immunization. Mol Ther. 2000;1:180–188. doi: 10.1006/mthe.1999.0021. [DOI] [PubMed] [Google Scholar]
- Luxembourg A, Evans CF., and, Hannaman D. Electroporation-based DNA immunisation: translation to the clinic. Expert Opin Biol Ther. 2007;7:1647–1664. doi: 10.1517/14712598.7.11.1647. [DOI] [PubMed] [Google Scholar]
- Regnström K, Ragnarsson EG, Köping-Höggård M, Torstensson E, Nyblom H., and, Artursson P. PEI - a potent, but not harmless, mucosal immuno-stimulator of mixed T-helper cell response and FasL-mediated cell death in mice. Gene Ther. 2003;10:1575–1583. doi: 10.1038/sj.gt.3302054. [DOI] [PubMed] [Google Scholar]
- Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda D, Comte D, Cavassini M, Giostra E, Bühler L, Perruchoud M, et al. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur J Immunol. 2008;38:2665–2677. doi: 10.1002/eji.200838336. [DOI] [PubMed] [Google Scholar]
- Klavinskis LS, Barnfield C, Gao L., and, Parker S. Intranasal immunization with plasmid DNA-lipid complexes elicits mucosal immunity in the female genital and rectal tracts. J Immunol. 1999;162:254–262. [PubMed] [Google Scholar]
- Huang X, Xu J, Qiu C, Ren L, Liu L, Wan Y, et al. Mucosal priming with PEI/DNA complex and systemic boosting with recombinant TianTan vaccinia stimulate vigorous mucosal and systemic immune responses. Vaccine. 2007;25:2620–2629. doi: 10.1016/j.vaccine.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–5484. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Tamura S, Funato H, Hirabayashi Y, Kikuta K, Suzuki Y, Nagamine T, et al. Functional role of respiratory tract haemagglutinin-specific IgA antibodies in protection against influenza. Vaccine. 1990;8:479–485. doi: 10.1016/0264-410x(90)90250-p. [DOI] [PubMed] [Google Scholar]
- Chen MW, Cheng TJ, Huang Y, Jan JT, Ma SH, Yu AL, et al. A consensus-hemagglutinin-based DNA vaccine that protects mice against divergent H5N1 influenza viruses. Proc Natl Acad Sci USA. 2008;105:13538–13543. doi: 10.1073/pnas.0806901105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddy DJ, Yan J, Corbitt N, Kobasa D, Kobinger GP., and, Weiner DB. Immunogenicity of novel consensus-based DNA vaccines against avian influenza. Vaccine. 2007;25:2984–2989. doi: 10.1016/j.vaccine.2007.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Kadowaki S, Hagiwara Y, Yoshikawa T, Matsuo K, Kurata T, et al. Cross-protection against a lethal influenza virus infection by DNA vaccine to neuraminidase. Vaccine. 2000;18:3214–3222. doi: 10.1016/s0264-410x(00)00149-3. [DOI] [PubMed] [Google Scholar]
- Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ., and, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 2007;4:e59. doi: 10.1371/journal.pmed.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellier B, Huret C, Miyalou M, Desjardins D, Frenkiel MP, Despres P, et al. DNA vaccines expressing retrovirus-like particles are efficient immunogens to induce neutralizing antibodies. Vaccine. 2009;27:5772–5780. doi: 10.1016/j.vaccine.2009.07.059. [DOI] [PubMed] [Google Scholar]
- Bartosch B, Bukh J, Meunier JC, Granier C, Engle RE, Blackwelder WC, et al. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc Natl Acad Sci USA. 2003;100:14199–14204. doi: 10.1073/pnas.2335981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensels M, Bublot M, Van Borm S, De Vriese J, Lambrecht B, Richard-Mazet A, et al. Prime-boost vaccination with a fowlpox vector and an inactivated avian influenza vaccine is highly immunogenic in Pekin ducks challenged with Asian H5N1 HPAI. Vaccine. 2009;27:646–654. doi: 10.1016/j.vaccine.2008.11.044. [DOI] [PubMed] [Google Scholar]
- Van Borm S, Steensels M, Ferreira HL, Boschmans M, De Vriese J, Lambrecht B, et al. A universal avian endogenous real-time reverse transcriptase-polymerase chain reaction control and its application to avian influenza diagnosis and quantification. Avian Dis. 2007;51 1 Suppl:213–220. doi: 10.1637/7552-033106R.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protection against lethal H5N1 challenge in mice i.n. immunized with DNA vaccines encoding NA from homologous or heterologous influenza A H5N1 virus.