Abstract
Oncolytic adenoviruses show promise as a cancer treatment. However, they generate acute inflammatory responses with production of cytokines, including tumor necrosis factor-α (TNF-α). We investigated whether inhibition of TNF-α augments efficacy of the E1A CR2-deleted adenovirus dl922-947 in ovarian cancer. dl922-947 induced transcription of TNF-α and its downstream signaling targets interleukin-6 and -8 (IL-6 and IL-8) in ovarian cancer cells. In vitro, RNAi-mediated knockdown of TNF-α reduced production of multiple inflammatory cytokines after infection and increased ovarian cancer cell sensitivity to virus cytotoxicity, as did treatment with the anti-TNF-α antibody infliximab. In vivo, stable knockdown of TNF-α in IGROV-1 xenografts increased the anticancer activity of dl922-947. In addition, inhibition of TNF-α using monoclonal antibodies also improved dl922-947 efficacy. This increased efficacy resulted from suppression of cellular inhibitor of apoptosis-1 and -2 (cIAP1 and cIAP2) transcription in malignant cells and a consequent increase in caspase-mediated apoptosis. These findings suggest that TNF-α acts as a survival factor in adenovirus-infected cells. Combining TNF-α inhibition with oncolytic adenoviruses could improve antitumor activity in clinical trials.
Introduction
Oncolytic viruses show promise as novel treatments for cancer. These viruses multiply selectively within cancer cells and cause death with release of mature viral particles. We have shown that the E1A CR2-deleted adenovirus dl922-947 can replicate specifically in human ovarian cancer cells but not in ovarian surface epithelial cells with an intact retinoblastoma pathway and has greater efficacy than either E1A wild-type adenoviruses or the E1B-55K deletion mutant dl1520 (Onyx-015).1,2 We have also shown that E1A CR2-deleted adenoviruses induce death via a novel mechanism in which apoptosis is largely absent.3 Phase 1 trials of E1A CR2-deleted adenoviruses in ovarian cancer have commenced4 and further clinical development is planned.
Marked inflammatory responses have been reported in previous gene-therapy trials involving both replicating5 and nonreplicating6 adenoviral vectors and we previously found inflammatory hepatotoxicity in nude mice following intraperitoneal (IP) delivery of dl922-947.2 This inflammation is characterized by the induction of several cytokines and chemokines, especially tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6),7 as well as IL-1α and β.8,9 Multiple signaling pathways are activated by adenoviruses upon binding to Coxsackie Adenovirus Receptor (CAR) and interactions with αvβ3/5 integrins. These include NF-κB and MAP kinases ERK and p38,7,10,11 which promote cytokine and chemokine induction, and inhibition of NF-κB may increase the oncolytic efficacy.12 The adenoviral genome contains elements designed to protect infected cells from inflammatory cytokines, some of which are located in E3. E3 proteins are dispensable for viral growth in cell culture and the majority of the adenoviruses in clinical trials (including dl922-947 and dl1520) have E3B deletions.13 It remains unclear whether acute inflammatory responses augment oncolytic activity, by promoting death of infected cells, or inhibit oncolysis, by promoting elimination of infected cells prior to productive replication.
TNF-α is a powerful cytokine with critical roles in innate and adaptive immunity.14 Bacterial pathogens and other noxious stimuli induce its expression and also enhance its translation. Early production of TNF-α is prominent in the initiation of a highly complex biological cascade involving cytokines, chemokines and endothelial adhesions, which results in the recruitment and activation of neutrophils, macrophages and lymphocytes at sites of damage and infection. When TNF-α-induced NF-κB signaling is not sustained (in cells metabolically compromised by virus infection, for example), interaction of the cytokine with its p55 type I receptor can induce apoptosis via caspase 8.15
There is now powerful evidence of a close link between inflammation and cancer (reviewed in ref. 16). We have previously reported a role for TNF-α in tumor promotion.17,18 In addition, constitutive expression of TNF-α is seen in malignant ovarian surface epithelium and we have shown that TNF-α and its receptors are expressed in many ovarian cancers.19 Autocrine production of TNF-α by ovarian carcinoma cells stimulates a network of other cytokines, angiogenic factors, and chemokines that may act in an autocrine/paracrine manner to promote peritoneal growth and spread.20 Recent clinical trials have suggested that inhibition of TNF-α may have therapeutic potential in ovarian cancer.21,22
We investigated whether suppression of TNF-α could augment the therapeutic potency of adenoviruses in ovarian cancer. We studied the efficacy of oncolytic adenoviruses in ovarian carcinoma cells with stable knockdown of TNF-α production, both in vitro and in vivo, as well as parental ovarian carcinoma cells treated with the anti-TNF-α monoclonal antibody infliximab (Remicade). We show that TNF-α expression is induced in ovarian carcinoma cells following adenoviral infection and that its suppression does indeed augment cytotoxicity. This results from a decrease in expression of cellular inhibitor of apoptosis-1 (cIAP1) and cellular inhibitor of apoptosis-2 (cIAP2) with a consequent increase in apoptosis.
Results
Adenoviruses induce TNF-α expression in ovarian cancer cells
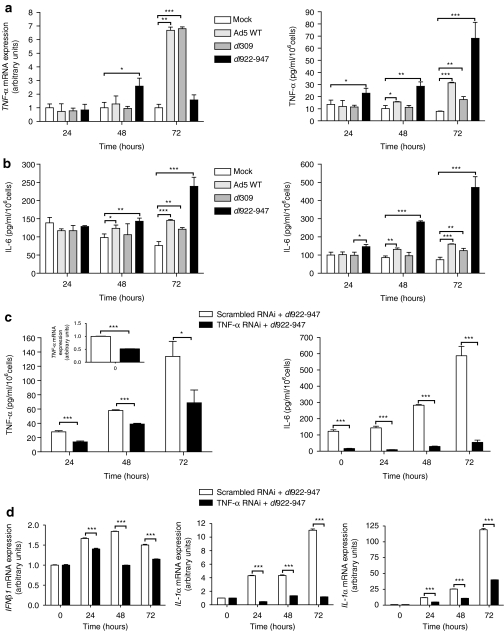
To assess adenovirus-induced TNF-α production in ovarian carcinoma cells, IGROV-1 cells were infected with three adenoviruses: dl922-947, which contains a 24 base pairs deletion in E1A CR2 and is deleted in E3B, Ad5 WT and dl309. dl309 is identical to dl922-947 except for the presence of a fully intact E1 region. All three viruses induced upregulation of TNF-α mRNA and protein (Figure 1a), with the greatest induction by dl922-947. The presence of an intact E3B region in Ad5 WT did not reduce the induction of TNF-α compared with dl309. TNF-α mRNA levels fell 72 hours following infection with dl922-947, which may have resulted from the onset of cell death–survival 72 hours following infection with dl922-947 multiplicity of infection 10 is significantly reduced compared to both Ad5 WT and dl309 (Supplementary Figure S1a). In addition, all three viruses induced expression of IL-6 and IL-8, downstream targets of TNF-α activation and, as with TNF-α, dl922-947 induced the greatest IL-6 and IL-8 production (Figure 1b). We have previously generated two clones of IGROV-1 cells with stable TNF-α knockdown [TNF-α (I) and (II) RNAi20]. Infection of TNF-α (II) RNAi cells with dl922-947 produced a significant attenuation of inflammatory responses compared to scrambled RNAi controls. This included TNF-α and IL-6 (Figure 1b) as well as interferon-β, IL-1α, and IL-1β (Figure 1d).
Figure 1.
Adenovirus infection induces cytokine expression in IGROV-1 cells. (a) IGROV-1 cells were infected with Ad5 WT, dl 309, or dl 922-947 [multiplicity of infection (MOI) 10 pfu/cell] or mock infected. TNF-α expression was assessed up to 72 hours postinfection (pi) by quantitative reverse transcription-PCR, (left) and secretion assessed by Mesoscale analysis (right). *P < 0.05; **P < 0.01; ***P < 0.001. (b) Release of IL-6 (left) and IL-8 (right) was also assessed by Mesoscale analysis. *P < 0.05; **P <0.01; ***P < 0.001. (c) IGROV-1 scrambled and tumor necrosis factor-α (TNF-α) RNAi cells were infected with dl922-947. Release of TNF-α (left) and IL-6 (right) was assessed by Mesoscale analysis up to 72 hours pi; inset, left qRT-PCR of TNF-α expression in uninfected cells. *P < 0.05; ***P < 0.001. (d) IGROV-1 scrambled and TNF-α RNAi cells were infected with dl922-947. Expression of interferon-β1, interleukin-1α and -1β was assessed up to 72 hours pi by qRT-PCR. ***P < 0.001.
Reduction of TNF-α in ovarian carcinoma cells promotes sensitization to oncolytic adenoviruses
To investigate whether TNF-α influences the efficacy of the oncolytic adenovirus dl922-947, we infected both IGROV-1 TNF-α RNAi clones and assessed cytotoxicity 144 hours later. Both TNF-α knockdown cells were significantly more sensitive to the effects of dl922-947 than either IGROV-1 parental or scrambled RNAi control cells (Figure 2a). In addition, there was significantly more cell death in both TNF-α RNAi cell lines following infection with Ad5 WT and dl309 compared to both controls (Figure 2b). TNF-α RNAi clone (II), which has greater knockdown than clone (I),20 was more sensitive to all three viruses.
Figure 2.
TNF-α knockdown and inhibition increases adenoviral cytotoxicity. (a,b) 104 IGROV-1 parental, IGROV-1 scrambled control and tumor necrosis factor-α (TNF-α) RNAi cells were infected with dl922-947 [multiplicity of infection (MOI) 0.01–1,000]. Cell survival was assessed 144 hours later (left). Mean IC50 values (± SD) from four separate experiments are plotted (right). **P < 0.01, ***P < 0.001. (b) IGROV-1 parental, IGROV-1 scrambled control and TNF-α RNAi cells were also infected with Ad5 WT and dl309 for 144 hours. Statistical comparison of survival between TNF-α RNAi cells and both parental and scrambled RNAi cells: *P < 0.05, **P < 0.01. (c) IGROV-1 cells were treated with infliximab (10 or 50 µg/ml) for 96 hours and then infected with dl922-947 (MOI 0.001–100). Cell survival was assessed 144 hours later. TNF-α release in preinfected cells was assessed by Mesoscale analysis (left).
To confirm that this increase in sensitivity resulted from TNF-α knockdown, IGROV-1 cells were treated with the antihuman TNF-α monoclonal antibody infliximab before and after infection with dl922-947. Infliximab treatment alone did not reduce cell survival (Supplementary Figure 1b) but significantly reduced the levels of TNF-α and increased sensitivity to the virus (Figure 2c).
Recombinant TNF-α reduces the efficacy of dl922-947 in ovarian carcinoma cells
To confirm that TNF-α reduces dl922-947 efficacy, we investigated the human ovarian carcinoma cell line SKOV3 and its subline SKOV3-ip1.23 SKOV3 produces markedly less TNF-α than SKOV3-ip1 (Figure 3a). As before, reduced TNF-α expression correlated with increased sensitivity to dl922-947, with the IC50 for SKOV3 cells being two logs less than for SKOV3-ip1 (Figure 3b). To investigate this further, SKOV3 cells were treated with recombinant TNF-α 2 hours following infection with dl922-947. Addition of recombinant TNF-α decreased dl922-947 efficacy, with some evidence of dose dependence, with saturation being observed above 10 ng/ml recombinant TNF-α (Figure 3c), whereas exposure of SKOV3-ip1 cells to infliximab before and after infection sensitizes them to dl922-947 (Figure 3d), as was seen with IGROV-1 cells in Figure 2c. These results together suggest that TNF-α acts as a survival factor following adenovirus infection and reduces viral efficacy in ovarian cancer cells.
Figure 3.
Recombinant TNF-α reduces dl922-947 cytotoxicity in TNF-α-low SKOV3 cells. (a) Tumor necrosis factor-α (TNF-α) expression and secretion in SKOV3 and SKOV3-ip1 cells was assessed by quantitative reverse transcription-PCR (left) and Mesoscale analysis 48 hours after plating (right). (b) 104 SKOV3 and SKOV3-ip1 cells were infected with dl922-947 [multiplicity of infection (MOI) 0.01–1,000 pfu/cell] in triplicate. Cell survival was assessed 120 hours later. (c) SKOV3 cells were infected with dl922-947 (MOI 0.01–1,000) and re-fed 2 hours later with medium containing recombinant TNF-α (0.3–30 ng/ml). Cell survival was assessed 120 hours later. (d) SKOV3-ip1 cells were treated with infliximab (10 or 50 µg/ml) for 96 hours and then infected with dl922-947 (MOI 0.03–3,000). Cell survival was assessed 120 hours later.
dl922-947 significantly prolongs median survival of mice-bearing IGROV-1 TNF-α RNAi xenografts
In two identical experiments, luciferase-expressing IGROV-1 scrambled RNAi or TNF-α RNAi (II) cells were injected IP into female nude mice. dl922-947 or nonreplicating control virus was administered on days 19–23 inclusive. A combined survival analysis is presented in Figure 4a. In this aggressive model of ovarian cancer, median survival of scrambled RNAi-bearing animals treated with control virus was only 28 days. Treatment with dl922-947 commencing on day 19 was able to prolong median survival by 9 days, although this did reach statistical significance (P = 0.001). Survival of mice bearing TNF-α RNAi cells treated with control virus was 84 days, confirming our previous findings.20 However, in mice bearing TNF-α RNAi cells, treatment with dl922-947 extended median survival significantly from 84 to 116 days (P = 0.003). Bioluminescence imaging results demonstrate that TNF-α RNAi bearing mice treated with dl922-947 had less tumor burden compared with control-treated mice (Figure 4b,c).
Figure 4.
Effect of dl922-947 in IGROV-1 TNF-α ovarian cancer xenografts. (a) 3 × 106 IGROV-1 scrambled RNAi or tumor necrosis factor-α (TNF-α) RNAi luciferase cells were injected IP into Balb C nu/nu female mice on day 1. On days 19–23 inclusive, mice were treated with Ad LM-X or dl922-947 (5 × 109 particles/injection/day) in groups of 5. Two identical experiments were performed sequentially and survival curves are presented from joint analysis. (b) Change in relative bioluminescence over time. Mice were injected with 125 mg/kg -luciferin IP, anaesthetized, then imaged for 10 seconds on a Xenogen IVIS bioluminescence system. Luminescence of TNF-α RNAi mice treated with dl922-947 was significantly (P ≤ 0.01) less than all other groups at each imaging point after virus injection. (c) Bioluminescence images from day 33.
Treatment of mice bearing ovarian carcinoma cells with dl922-947 in combination with infliximab prolongs survival
To assess the combination of dl922-947 and infliximab in vivo, two experiments were performed. In the first, mice injected IP with IGROV-1 luciferase cells were randomly allocated to receive weekly infliximab and anti-murine TNF-α antibody (2.5 mg/kg each) or control IgG (5 mg/kg) from day 2 onward. On days 8–12, mice were treated with dl922-947. Control mice received anti-TNF-α antibodies and a nonreplicating control adenovirus (Ad CMV-GFP). Tumor burden was assessed regularly using bioluminescence imaging (Figure 5a left). In the control group, as before, there was a progressive increase in luminescence, and median survival was only 34 days, indicating that anti-TNF-α antibodies had no impact upon tumor growth at these doses. In both groups treated with dl922-947, tumor burden was significantly lower than in control animals. However, from day 49 onwards, there was a significant reduction in luminescence of mice treated with anti-TNF-α antibodies compared with mice that received control IgG (P < 0.05).
Figure 5.
Effect of dl922-947 in IGROV-1 xenografts in combination with infliximab. (a) 3 × 106 IGROV-1 luciferase cells were injected IP into Balb C nu/nu female mice on day 1. On day 2 and weekly thereafter, mice were injected with anti-TNF-α antibodies (2.5 mg/kg each antihuman and anti-murine TNF-α) or control IgG (5 mg/kg). In the first experiment (left), mice in groups of 10 were treated with either dl922-947 or control Ad CMV-GFP (5 × 109 particles/injection/day) on days 8–12 inclusive. In the second experiment (right), mice were treated with either dl922-947 (5 × 109 particles/injection/day) or vehicle control on days 8–12 inclusive. Bioluminescence imaging results are presented as mean radiance per group. (b) A combined Kaplan–Meier survival analysis of the mice treated with dl922-947 + anti-TNF-α Abs and dl922-947 + control IgG is presented. (c) 4 µm liver sections were stained with haematoxylin and eosin. All images are ×100.
In the second experiment, mice were again inoculated with IGROV-1 luciferase cells on day 1 and randomly allocated to receive control or anti-TNF-α antibodies as before in groups of 20. On days 8–12, mice were randomly assigned to receive dl922-947 or vehicle in groups of 10. Again, there was persistent growth in the two control groups (Figure 5a right): median survival was 36 days in the control IgG group and 35.5 days in the anti-TNF-α antibody group (data not shown). Treatment with dl922-947 caused a reduction in luminescence; however, in the animals that received dl922-947 and control IgG, tumor burden reached a plateau following treatment, whereas there was continued reduction in bioluminescence in the anti-TNF-α antibody group until the end of the experiment (day 74).
A combined survival analysis was performed for the dl922-947-treated mice in these two experiments. Median survival for mice that received dl922-947 and control IgG was 60 days, but was statistically longer in the anti-TNF-α antibody group (P = 0.02), such that median survival was not reached (Figure 5b). Pathological examination of livers from mice treated with dl922-947 and control IgG demonstrated evidence of necrosis and thickening of the capsule (Figure 5c), as previously described.2 However, there was no increase in liver necrosis in mice treated with anti-TNF-α Ab and dl922-947.
Increased cytotoxicity in TNF-α RNAi cells does not result from increased infectivity or virus replication
To understand how TNF-α downregulation augmented cell killing by dl922-947, we first assessed infectivity. Following infection with Ad CMV-GFP, there was a small increase in GFP positivity in both IGROV-1 TNF-α RNAi lines compared to scrambled RNAi control (Supplementary Figure 1c). However, this difference was small and unlikely to account for the large increase in cytotoxicity seen previously (Figure 2a). Expression of the primary adenovirus receptor CAR was slightly increased in one TNF-α RNAi clone, but not the other, and there was no consistent change in expression of secondary integrin receptors (Supplementary Figure S1d). Viral replication was assessed using dlCR2-dsRed, which contains the same E1A CR2 deletion as dl922-947,24 but also contains a dsRed expression cassette fused to the minor capsid protein pIX. This allows noninvasive measurement of virus production using a fluorometric assay.25 There was no significant difference between scrambled and TNF-α (II) RNAi cells in either intracellular virus or virus released into the surrounding medium (Supplementary Figure S1e). Taken together these data suggest that the increased efficacy of dl922-947 and other adenoviruses in TNF-α RNAi cells is not due to increased rates of virus infection, replication, or release.
Knockdown of TNF-α increases apoptosis via downregulation of cIAP1 and 2
In keeping with the finding of similar virus replication in TNF-α knockdown cells, we found no difference in the expression of early (E1A) and late (hexon, fiber, penton) viral proteins following dl922-947 infection (Figure 6a). However, there was greater activation of caspase-3 48 and 72 hours postinfection in TNF-α RNAi cells compared to controls, accompanied by earlier poly (ADP-ribose) polymerase cleavage and greater activation of pro-caspase-8 (Figure 6b). We also observed that expression of the caspase-8 antagonist c-FLIPL was reduced in both cell lines, but to a greater degree in TNF-α RNAi cells. In addition, cellular inhibitor of apoptosis-1 (cIAP1) and cIAP2 expression was markedly downregulated in TNF-α RNAi cells compared to the scrambled RNAi cells. The downregulation was specific to cIAP1 and cIAP2, as X-linked inhibitor of apoptosis protein (XIAP) expression reduced to the same degree in both cell lines (Figure 6b).
Figure 6.
Mechanism of enhanced adenoviral cytotoxicity following TNF-α knockdown. (a) and (b) IGROV-1 scrambled and TNF-α RNAi cells were infected with dl922-947 [multiplicity of infection (MOI) 10] and harvested up to 72 hours later. Protein (20 µg) was separated on SDS-PAGE gels and analyzed by immunoblot. Figures below the caspase-8 and cFLIP blots in (b) represent expression relative to 0 hours normalised to β-actin, as quantified using ImageJ software. (c) IGROV-1 scrambled and tumor necrosis factor-α (TNF-α) RNAi cells were infected with dl922-947 (MOI 10) in the presence or absence of 12.5 µmol/l lactacystin. Protein was harvested 48 hours postinfection and blotted for cIAP1 (cellular inhibitor of apoptosis-1) expression. (d) IGROV-1 scrambled and TNF-α RNAi cells were infected with dl922-947 (MOI 10). RNA was harvested up to 48 hours pi. Expression of cIAP1 and cIAP2 was assessed by quantitative reverse transcription-PCR. ***P < 0.001. (e) IGROV-1 parental cells were transfected with scrambled (60 nmol/l), cIAP1 (30 nmol/l), cIAP2 (30 nmol/l) and cIAP1 and cIAP2 (30 nmol/l each combined) siRNA and harvested 48 hours later. Protein (20 µg) was separated on SDS-PAGE gels and analyzed by immunoblot. (f) 1 × 104 IGROV-1 parental cells were transfected with siRNA (as in e) for 24 hours before infection with dl922-947 (MOI 1 or 3 pfu/cell) in triplicate. Cell survival was assessed 120 hours later. *P < 0.05.
Since cIAP1 and cIAP2 contain a RING domain, capable of inducing autoubiquitination,26,27,28 dl922-947 infected cells were treated with the proteasome inhibitor lactacystin. However, lactacystin did not reverse cIAP1 or cIAP2 degradation in TNF-α RNAi cells, indicating that the downregulation was not due to proteasomal activity (Figure 6c and data not shown). We also confirmed, by addition of NH4Cl, that lysosomal degradation29 made little contribution to cIAP1 or cIAP2 loss (Supplementary Figure S2a and data not shown). We then assessed cIAP1/2 transcription in TNF-α RNAi and scrambled control cells following dl922-947 infection. In both cells, there was a reduction in transcription of both IAPs: however, this was significantly greater in the TNF-α RNAi cells at both 24 and 48 hours postinfection, suggesting that TNF-α knockdown reduces expression of cIAP1/2 primarily via altered gene transcription (Figure 6d).
To evaluate whether the increased dl922-947 cytotoxicity in TNF-α RNAi cells resulted from greater apoptosis, we treated cells with the pan-caspase inhibitor Z-VAD-fluoromethylketone immediately following infection with dl922-947. There was a significant reduction in cell death in the TNF-α RNAi cells at two multiplicity of infections in the presence of Z-VAD-fluoromethylketone, which was not seen in the scrambled control cells (Supplementary Figure S2b).
Finally, we used siRNA-mediated knockdown to examine the roles of cIAP1 and cIAP2 loss on oncolytic adenoviral efficacy. Knockdown of either IAP individually in uninfected cells induced an upregulation in expression of the other, both at protein (Figure 6e) and mRNA level (data not shown), as reported previously.30 Loss of either cIAP1 or cIAP2 was found to sensitize IGROV-1 cells to dl922-947, with a significant increase in cell death being observed at low multiplicity of infections when both proteins were knocked down (Figure 6f). By contrast, these effects were not seen with XIAP knockdown (data not shown). However, knockdown of cIAP1 and 2 was not able to blunt the production of TNF-α and other inflammatory cytokines in response to dl922-947 infection in these cells (Supplementary Figure S2c and data not shown). This indicates that cIAP1 and 2 are not required for the induction of the inflammatory response to adenovirus infection, but are required for inflammation-induced prosurvival signaling.
Taken together, our results suggest that the acute inflammatory response to adenovirus infection acts to reduce cell death. Inhibition of TNF-α signaling increases viral cytotoxicity, due to an augmentation of caspase-mediated apoptosis, resulting from transcriptional loss of cIAP1 and cIAP2.
Discussion
We have demonstrated that adenoviruses induce expression of TNF-α in ovarian cancer cells and that TNF-α acts as a survival factor in infected cells to decrease viral efficacy. We also found induction of IL-6 and IL-8, which are known downstream targets of TNF-α signaling, confirming our previous finding that TNF-α generates and sustains a network of other inflammatory mediators.20 Suppression of TNF-α using RNA interference or inhibitory antibodies augmented oncolytic activity both in vitro and in vivo.
TNF-α induction was greater with dl922-947 than with two E1A-wild-type viruses at both transcript and protein level. This may have resulted partially from more rapid viral protein expression and genome replication and partially from greater cell death with subsequent release of TNF-α. We have previously demonstrated that dl922-947 induces more rapid viral protein expression and viral genome replication and greater death in ovarian cancer cells than either Ad5 WT or dl309.2 The results with dl309 indicated that the E3B deletion in dl922-947 was not a determining factor, as TNF-α induction was similar with dl309 (E3B deleted) and Ad5 WT (E3B intact). We also noted an increase in TNF-α protein levels prior to any increase in mRNA levels. This may result from translation of pre-existing mRNA or a transient increase in transcription (<24 hour postinfection) resulting from signaling induced by adenovirus binding to CAR31 or αvβ3/5 integrins.8,9
Although both experiments in which TNF-α was targeted with monoclonal antibodies indicated an increase in efficacy of dl922-947, the difference in survival was not as great as that observed for mice-bearing TNF-α knockdown cells. This disparity may be due to the difference in the degree of TNF-α suppression. This may also explain why treatment with anti-TNF-α antibodies had little effect on tumor growth in mice compared with growth of TNF-α RNAi cells. Attempts were made to measure TNF-α levels at defined time points during the antibody experiments. However, we found that enzyme-linked immunosorbent assay-based systems detected both free and Ab-bound TNF-α in mouse blood, rendering results unreliable. Similar observations have been reported by others when assaying IL-6 levels following treatment with the inhibitory antibody tocilizumab.32
We previously reported hepatotoxicity following IP administration of dl922-947 in nude mice.2 There are few other data on hepatic damage following IP adenovirus administration, but hepatotoxicity is well described following four administrations.33 The etiology of this toxicity appears multifactorial, with TNF-α playing a role.34,35 We observed little hepatotoxicity in mice treated with a control adenovirus and anti-TNF-α antibodies. However, there was evidence of thickening of the liver capsule and necrosis in all dl922-947-treated mice both in the presence or absence of these antibodies. This suggests that hepatotoxicity is caused by factors other than just TNF-α and/or that the level of TNF-α inhibition with monoclonal antibodies is insufficient to block hepatic damage. Our data were obtained from nude mice, and the immune status of mice is a known determinant of toxicity following four administrations.35
We recently reported that adenoviral mutants induce a novel mode of programmed cell death in ovarian cancer cells3 with evidence of significant apoptosis only at late time points. Here, we have observed earlier and greater activation of caspase-3, with poly (ADP-ribose) polymerase cleavage, in IGROV-1 TNF-α RNAi cells treated with dl922-947. Furthermore, there was a concomitant decrease in pro-caspase-8, which coincided with a significant downregulation in expression of cIAP1 and cIAP2. By contrast, XIAP expression reduced to the same extent in TNF-α expressing and knockdown cells. XIAP can inhibit activated downstream caspases36 and the processed form of caspase-9.37 cIAP1 and cIAP2 have also been reported to block the proteolytic processing of effector caspases,38 although others have suggested that they cannot inhibit caspase activity at physiological concentrations.39 It was recently reported that cIAP1 and cIAP2 exert their anti-apoptotic effect by promoting constitutive receptor interacting protein-1 ubiquitination in cancer cells, which allows receptor interacting protein-1 to bind to the prosurvival kinase, TGF-β activated kinase 1.40 Furthermore, the authors also found that a Smac-mimetic induced the autoubiquitination and destruction of both IAPs, which prevented receptor interacting protein-1 from binding to TGF-β activated kinase 1, but permitted it to bind to caspase-8 and promote apoptosis. A similar role for cIAP1 and cIAP2 has also been reported by others.27,28 Moreover, in these reports XIAP levels were unaffected. In our system, loss of cIAP1 and cIAP2 was not a consequence of either proteasomal or lysosomal degradation.29 However, we found a significant reduction in transcription. In addition, treatment of TNF-α RNAi but not scrambled RNAi cells with the pan-caspase inhibitor Z-VAD-fluoromethylketone following dl922-947 infection was able to reduce cell death, suggesting that TNF-α knockdown was able to enhance virus-induced apoptosis. Furthermore, it was also recently shown that cIAP1 can suppress caspase-mediated apoptosis by binding to and ubiquitinating caspases-3 and 7, thereby targeting them for proteasome-dependent degradation.41 Thus, it is also possible that loss of cIAP-1 and -2 following TNF-α knockdown drives greater apoptosis by preventing degradation of executioner caspases. We also noted that knockdown of cIAP1 or 2 alone was able to sensitize cells to dl922-947. In uninfected cells, single IAP knockdown leads to a compensatory increase in the other IAP as others have seen before. However, this effect was abolished after virus infection, suggesting a complex link between IAP transcription control and adenovirus infection that will require further investigation.
There are several potential consequences of blocking the acute inflammatory response to adenovirus infection. On one hand, some of the inflammatory responses in gene-therapy clinical trials have been severe (and indeed fatal6), so inhibition of TNF-α could reduce systemic toxicity and allow greater doses to be delivered. Conversely, evidence is emerging from immunocompetent models that immune responses to some oncolytic viruses are of great importance in their overall efficacy.42 Reducing acute inflammation could risk blunting the adaptive immune response to virus-infected cells, thus compromising clinical effectiveness. Although more sophisticated preclinical models will help to answer these questions, full evaluation will ultimately require further clinical trials in patients with cancer.
In summary, we show that adenovirus infection leads to an upregulation of TNF-α and other NF-κB target genes and that suppression of TNF-α in ovarian carcinoma cells augments the oncolytic efficacy of the E1A CR2 mutant dl922-947 both in vitro and in vivo. This effect resulted from an increase in apoptosis mediated via loss of cIAP1 and cIAP2. The anti-TNF-α antibody infliximab has activity in advanced ovarian and renal cell cancers22,43 and our data suggest that treatment of women with ovarian cancer with dl922-947 in combination with infliximab may have real therapeutic potential.
Materials and Methods
Adenoviral construction and viral replication assays. dl922-947 is an Ad5 vector deleted in E1A CR2 amino acids 122–129, and in E3B.1 dl309 is an Ad5 vector with wild-type E1A, but contains the same E3B deletion as dl922-947. The construction of E1-deleted control adenovirus Ad LM-X is described elsewhere.44 Ad CMV-GFP is an E1-deleted Ad5 encoding green fluorescent protein (GFP) under cytomegalovirus (CMV) immediate early promoter/enhancer control. For virus replication assays, 3 × 105 cells were infected in phenol red-free medium with dlCR2-pIX-dsRed, a derivative of dl922-947 in which the minor capsid protein pIX is tagged with dsRed. The generation of this virus is described elsewhere.25 Fluorescence was measured up to 72 hours postinfection using a Victor3 1420 multilabel counter (Perkin-Elmer, Bucks, UK) with 544/15 nm excitation and 620/8 nm emission filters with measurement time of 1 second. The fluorescence of mock-infected cells was subtracted from that of dlCR2-pIX-dsRed-infected cells to yield net mean fluorescence. After each scan, medium was collected and cells refed with 1 ml; phenol red-free medium. The collected medium was centrifuged (5 minutes, 4,000 r.p.m.) and supernatant titered on JH293 cells by TCID50 assay.
Cell culture and cell viability assays. The source of human ovarian cancer cells was as follows: IGROV-1, Dr M. Ford (Glaxo-Wellcome, Stevenage, UK), 1999; SKOV3, Cancer Research UK Cell Services, 2001; SKOV3-ip1 cells, Dr J. Price (UT MD Anderson Cancer Center, Houston, TX) 1999. IGROV-1 and all derivatives were cultured in endotoxin-free RPMI 1640 medium plus 3.7 g/l NaHCO3 and 10% fetal calf serum. IGROV-1 scrambled RNAi and TNF-α RNAi, IGROV-1 Luc, IGROV-1 scrambled RNAi Luc and TNF-α RNAi Luc cells are all described elsewhere.20 All RNAi cells were additionally cultured in 2 µg/ml puromycin. SKOV3 and its derivative SKOV3-ip123 were cultured in Dulbecco's modified Eagle medium + 10% fetal calf serum. Cells were authenticated by single-nucleotide polymorphisms array and short tandem repeat analyses. For cell viability assays, 104 cells were infected with adenovirus in serum-free medium. After 2 hours, cells were refed with medium + 10% fetal calf serum. Cell viability was assayed by MTT assay.45 All cell viability assays were performed in triplicate on at least two occasions. Representative results are shown unless stated.
Reagents. Primary immunoblotting antibodies were: anti-Ad2 E1A (Santa Cruz Biotechnology, Santa Cruz, CA); anti-adenovirus (Abcam, Cambridge, UK); anti-caspase-8 and XIAP (BD PharMingen, Oxford, UK); anti-cIAP1 and cIAP2 (R&D Systems, Oxford, UK); anti-cleaved caspase-3 and cleaved poly (ADP-ribose) polymerase (Cell Signaling Technology, Ipswich, UK); anti-cFLIP (Alexis Biochemicals, Nottingham, UK); anti-β-actin (Sigma, Poole, UK). Z-VAD-fluoromethylketone was purchased from Merck, Nottingham, UK. Flow cytometry antibodies were: anti-CAR RmcB (a kind gift from Yaohe Wang); anti-αvβ3 LM609 (Chemicon, Hertfordshire, UK); anti-αvβ5 P1F6 (Chemicon). Mouse IgG1 (Dako, Cambridgeshire, UK) was used as isotype control. D-Luciferin was supplied by Calliper Life Sciences, Runcorn, UK. siRNAs were supplied by Dharmacon, Epsom, UK.
RNA extraction and real-time quantitative reverse transcription-PCR analysis. RNA was extracted from cells using RNeasy Mini Kit (Qiagen, Crawley, UK). DNase-treated RNA (2 µg) was reverse-transcribed with MMLV reverse transcriptase (Promega, Southampton, UK). Multiplex-real-time reverse transcription-PCR analysis was performed using ABI PRISM 7700 Sequence Detection System (PE Applied Biosystems, Warrington, UK). Expression values were normalized (ΔCt) to 18s rRNA by subtracting the cycle threshold (Ct) value of 18s rRNA from the Ct value of the experimental value. The fold differences compared with controls were calculated.
Cytokine ELISA. Cell culture supernatants were removed and cytokine concentrations measured using the Mesoscale diagnostic platform, according to manufacturer's instructions. The lower limit of sensitivity of TNF-α, IL-6, and IL-8 assays were 0.37, 0.26, and 0.10 pg/ml, respectively.
Flow cytometry. To assess infectivity by Ad5 vectors, 3 × 105 cells were infected with Ad CMV-GFP at multiplicity of infection 5 pfu/cell. Twenty-four hours later, cells were trypsinized, washed, and analyzed by flow cytometry. To assess expression of CAR and αvβ3/5 integrins, resuspended log-growth phase cells were incubated with primary antibody (4 °C, 90 minutes). Following three washes (2% fetal calf serum, 0.5 mol/l EDTA pH 8.0 in phosphate-buffered saline), they were incubated with flourescein isothiocyanate-conjugated secondary Ab (1:30; DakoCytomation, Cambridge, UK; 4 °C, 30 minutes), washed three times, counterstained with propidium iodide, then analyzed by flow cytometry.
Western blotting. Cells were scraped into 200 µl lysis buffer (150 mmol/l NaCl, 50 mmol/l Tris pH 7.5, 0.05% SDS, 1% Triton X100) and sonicated on ice. Twenty microgram protein was electrophoresed on sodium dodecyl sulfate-polyacrylamide gels and transferred onto polyvinylidene fluoride membranes. Antibody binding was visualized using electrochemiluminescence (GE Healthcare, Amersham, UK).
In vivo experiments. All experiments were done under UK Home Office authority, using 6-week-old female BALB/c nu/nu mice (Harlan, Huntington, UK). For assessment of the effect of dl922-947 on mice-bearing ovarian carcinoma cells with TNF-α knockdown, 3 × 106 IGROV-1 scrambled RNAi Luc or IGROV-1 TNF-α RNAi Luc cells were injected IP on day 1. Virus injections (5 × 109 particles/injection IP in 400 µl 20% icodextrin) were administered on days 19–23 inclusive. For experiments involving anti-TNF-α antibodies, IGROV-1-Luc cells were injected IP on day 1. On day 2 and weekly thereafter, mice were injected with either 5 mg/kg IgG control or a combination of 2.5 mg/kg each of CNTO5048 (anti-murine TNF-α monoclonal antibody) and antihuman TNF-α (infliximab). Virus (doses as above) was injected on days 8–12 inclusive. Mice were assessed daily for weight, general health and accumulation of ascites and were killed according to UK Home Office guidelines. At postmortem, livers were harvested and fixed in 10% formaldehyde. Four micrometer sections were cut and processed.
Bioluminescence imaging. Mice were injected IP with 125 mg/kg -luciferin (Calliper Life Sciences) and then anaesthetised (2% isofluorane by inhalation). While still under anesthetic, they were placed in a light-tight chamber on a warmed stage (37 °C) and imaged on a Xenogen IVIS 100 Imaging System (Xenogen, Alameda, CA). Images were obtained with a 20 cm field of view (FOV), binning (resolution) factor of 8, 1/f stop, with an imaging time of 10 seconds. Data were analyzed using Living Image software (Xenogen) and are presented as normalized mean radiance (photons/s/cm2/sr).
Statistics. All dose–response curves, Kaplan–Meier survival curves and statistical analyses were generated using GraphPad Prism version 5 (GraphPad Software, San Diego, CA). All experiments were performed in triplicate at least twice. All results are presented as mean ± SD and the unpaired, two-tailed Student's t-test was used for all statistical analyses, except for analysis of bioluminescence radiance, where comparisons were one-sided. P < 0.05 is considered statistically significant throughout.
SUPPLEMENTARY MATERIAL Figure S1. Cell sensitivity to wild-type adenovirus, infectivity and replication. 1A: IGROV1 cells were infected with d 922-947, Ad5 WT and dl 309. Cell survival was assessed 72 hours later by MTT. At MOI 10, survival following dl 922-947 infection is significantly less than with either Ad5 WT or dl 309 infection (p<0.0001 for both comparisons). 1B: Survival of cells treated with infliximab or IgG alone in figure 2C compared to mock-infected cells. 1C: IGROV-1 Scrambled RNAi and TNF-α RNAi (I) and (II) cells were infected with Ad CMV GFP (MOI 5 pfu/cell). Twenty-four hours later, cells were trypsinised, washed and analysed for GFP positivity by flow cytometry. 1D: Cell surface expression of Coxsackie Adenovirus Receptor (CAR), αvβ3 and αvβ5 integrins on IGROV-1 Scrambled RNAi and TNF-α RNAi (I) and (II) cells was assessed by flow cytometry. 1E: IGROV-1 Scrambled RNAi and TNF-α RNAi (II) cells were infected with dl CR2-dsRed (MOI 0.3 and 1 pfu/cell) as detailed in Materials and Methods. Red fluorescence was detected up to 72 h pi using a Victor3 1420 multilabel counter (left). Supernatant was collected from cells infected at MOI 1 and titred on JH293 cells by TCID50 assay (right). Figure S2. Effect of pan-caspase inhibitor on virus efficacy; TNF-α expression following cIAP1/2 knockdown. 2A: IGROV-1 Scrambled and TNF-α RNAi cells were infected with dl 922-947 (MOI 10) in the presence or absence of 100mM NH4Cl. Protein was harvested 48 hours pi and blotted for cIAP1 expression. 2B: IGROV-1 Scrambled and TNF-α RNAi cells were infected with dl 922-947 (MOI 0.01-0.1) and refed 2 hours pi with medium with or without 25μM zVAD.fmk. Cell survival was assessed up to 120 hours later. Survival is plotted as percentage survival compared to mock-infected cells. *, p<0.05. 2C: IGROV-1 parental cells were transfected with Scrambled (60 nM), cIAP1 (30 nM), cIAP2 (30 nM) and cIAP1 and cIAP2 (30 nM each) siRNA for 24 hours before being infected with dl 922-947 (MOI 10) or mock infected for 48 hours. Secretion of TNF-α was assessed by Mesoscale analysis. **, p<0.01; ***, p<0.001.
Acknowledgments
This work was largely supported by Ovarian Cancer Action. We are grateful to Dr David Shealy (Centocor) for supplying anti-TNF-α antibodies and IgG controls. We would like to thank both Keyur Trivedi and Mohammed Ikram for help with histopathology. The authors have declared no conflict of interest.
Supplementary Material
Cell sensitivity to wild-type adenovirus, infectivity and replication. 1A: IGROV1 cells were infected with d 922-947, Ad5 WT and dl 309. Cell survival was assessed 72 hours later by MTT. At MOI 10, survival following dl 922-947 infection is significantly less than with either Ad5 WT or dl 309 infection (p<0.0001 for both comparisons). 1B: Survival of cells treated with infliximab or IgG alone in figure 2C compared to mock-infected cells. 1C: IGROV-1 Scrambled RNAi and TNF-α RNAi (I) and (II) cells were infected with Ad CMV GFP (MOI 5 pfu/cell). Twenty-four hours later, cells were trypsinised, washed and analysed for GFP positivity by flow cytometry. 1D: Cell surface expression of Coxsackie Adenovirus Receptor (CAR), αvβ3 and αvβ5 integrins on IGROV-1 Scrambled RNAi and TNF-α RNAi (I) and (II) cells was assessed by flow cytometry. 1E: IGROV-1 Scrambled RNAi and TNF-α RNAi (II) cells were infected with dl CR2-dsRed (MOI 0.3 and 1 pfu/cell) as detailed in Materials and Methods. Red fluorescence was detected up to 72 h pi using a Victor3 1420 multilabel counter (left). Supernatant was collected from cells infected at MOI 1 and titred on JH293 cells by TCID50 assay (right).
Effect of pan-caspase inhibitor on virus efficacy; TNF-α expression following cIAP1/2 knockdown. 2A: IGROV-1 Scrambled and TNF-α RNAi cells were infected with dl 922-947 (MOI 10) in the presence or absence of 100mM NH4Cl. Protein was harvested 48 hours pi and blotted for cIAP1 expression. 2B: IGROV-1 Scrambled and TNF-α RNAi cells were infected with dl 922-947 (MOI 0.01-0.1) and refed 2 hours pi with medium with or without 25μM zVAD.fmk. Cell survival was assessed up to 120 hours later. Survival is plotted as percentage survival compared to mock-infected cells. *, p<0.05. 2C: IGROV-1 parental cells were transfected with Scrambled (60 nM), cIAP1 (30 nM), cIAP2 (30 nM) and cIAP1 and cIAP2 (30 nM each) siRNA for 24 hours before being infected with dl 922-947 (MOI 10) or mock infected for 48 hours. Secretion of TNF-α was assessed by Mesoscale analysis. **, p<0.01; ***, p<0.001.
REFERENCES
- Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- Lockley M, Fernandez M, Wang Y, Li NF, Conroy S, Lemoine N, et al. Activity of the adenoviral E1A deletion mutant dl922-947 in ovarian cancer: comparison with E1A wild-type viruses, bioluminescence monitoring, and intraperitoneal delivery in icodextrin. Cancer Res. 2006;66:989–998. doi: 10.1158/0008-5472.CAN-05-2691. [DOI] [PubMed] [Google Scholar]
- Baird SK, Aerts JL, Eddaoudi A, Lockley M, Lemoine NR., and, McNeish IA. Oncolytic adenoviral mutants induce a novel mode of programmed cell death in ovarian cancer. Oncogene. 2008;27:3081–3090. doi: 10.1038/sj.onc.1210977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel DT, Preuss M, Wang M, Kimball KJ, Barnes MN, Wan W.et al. (2010A phase I study of the infectivity enhanced CRAd Ad5-Δ24RGD for recurrent gynecologic cancer J Clin Oncol 28Abstr 5107]. [Google Scholar]
- Reid T, Galanis E, Abbruzzese J, Sze D, Wein LM, Andrews J, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62:6070–6079. [PubMed] [Google Scholar]
- Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zaiss AK, Colarusso P, Patel K, Haljan G, Wickham TJ, et al. The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors. Hum Gene Ther. 2003;14:627–643. doi: 10.1089/104303403321618146. [DOI] [PubMed] [Google Scholar]
- Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, Flavell RA, et al. Virus binding to a plasma membrane receptor triggers interleukin-1 α-mediated proinflammatory macrophage response in vivo. Immunity. 2009;31:110–121. doi: 10.1016/j.immuni.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve DA, Pétrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- Bowen GP, Borgland SL, Lam M, Libermann TA, Wong NC., and, Muruve DA. Adenovirus vector-induced inflammation: capsid-dependent induction of the C-C chemokine RANTES requires NF-κB. Hum Gene Ther. 2002;13:367–379. doi: 10.1089/10430340252792503. [DOI] [PubMed] [Google Scholar]
- Tibbles LA, Spurrell JC, Bowen GP, Liu Q, Lam M, Zaiss AK, et al. Activation of p38 and ERK signaling during adenovirus vector cell entry lead to expression of the C-X-C chemokine IP-10. J Virol. 2002;76:1559–1568. doi: 10.1128/JVI.76.4.1559-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DH, Chen MJ, Searle PF, Kerr DJ., and, Young LS. Inhibition of NF-κB enhances the cytotoxicity of virus-directed enzyme prodrug therapy and oncolytic adenovirus cancer gene therapy. Gene Ther. 2005;12:1187–1197. doi: 10.1038/sj.gt.3302510. [DOI] [PubMed] [Google Scholar]
- Kirn D. Replication-selective oncolytic adenoviruses: virotherapy aimed at genetic targets in cancer. Oncogene. 2000;19:6660–6669. doi: 10.1038/sj.onc.1204094. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Killeen N., and, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Micheau O., and, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, et al. Mice deficient in tumor necrosis factor-α are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- Arnott CH, Scott KA, Moore RJ, Hewer A, Phillips DH, Parker P, et al. Tumour necrosis factor-α mediates tumour promotion via a PKC α- and AP-1-dependent pathway. Oncogene. 2002;21:4728–4738. doi: 10.1038/sj.onc.1205588. [DOI] [PubMed] [Google Scholar]
- Naylor MS, Stamp GW, Foulkes WD, Eccles D., and, Balkwill FR. Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. J Clin Invest. 1993;91:2194–2206. doi: 10.1172/JCI116446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulbe H, Thompson R, Wilson JL, Robinson S, Hagemann T, Fatah R, et al. The inflammatory cytokine tumor necrosis factor-α generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 2007;67:585–592. doi: 10.1158/0008-5472.CAN-06-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhusudan S, Muthuramalingam SR, Braybrooke JP, Wilner S, Kaur K, Han C, et al. Study of etanercept, a tumor necrosis factor-α inhibitor, in recurrent ovarian cancer. J Clin Oncol. 2005;23:5950–5959. doi: 10.1200/JCO.2005.04.127. [DOI] [PubMed] [Google Scholar]
- Brown ER, Charles KA, Hoare SA, Rye RL, Jodrell DI, Aird RE, et al. A clinical study assessing the tolerability and biological effects of infliximab, a TNF-α inhibitor, in patients with advanced cancer. Ann Oncol. 2008;19:1340–1346. doi: 10.1093/annonc/mdn054. [DOI] [PubMed] [Google Scholar]
- Yu D, Wolf JK, Scanlon M, Price JE., and, Hung MC. Enhanced c-erbB-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Res. 1993;53:891–898. [PubMed] [Google Scholar]
- Leyton J, Lockley M, Aerts JL, Baird SK, Aboagye EO, Lemoine NR, et al. Quantifying the activity of adenoviral E1A CR2 deletion mutants using renilla luciferase bioluminescence and 3'-deoxy-3'-[18F]fluorothymidine positron emission tomography imaging. Cancer Res. 2006;66:9178–9185. doi: 10.1158/0008-5472.CAN-06-1539. [DOI] [PubMed] [Google Scholar]
- Ingemarsdotter CK, Baird SK, Connell CM, Oberg D, Hallden G., and, McNeish IA.2010Low-dose paclitaxel synergizes with oncolytic adenoviruses via mitotic slippage and apoptosis in ovarian cancer Oncogeneepub ahead of print). [DOI] [PMC free article] [PubMed]
- Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor α (TNFα)-induced NF-κB activation. J Biol Chem. 2008;283:24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, et al. IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Vince JE, Chau D, Callus B, Wong WW, Hawkins CJ, Schneider P, et al. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFα. J Cell Biol. 2008;182:171–184. doi: 10.1083/jcb.200801010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conze DB, Albert L, Ferrick DA, Goeddel DV, Yeh WC, Mak T, et al. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol Cell Biol. 2005;25:3348–3356. doi: 10.1128/MCB.25.8.3348-3356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanini A, Nicolis E, Bonizzato A, Bezzerri V, Melotti P, Assael BM, et al. Interaction of adenovirus type 5 fiber with the coxsackievirus and adenovirus receptor activates inflammatory response in human respiratory cells. J Virol. 2006;80:11241–11254. doi: 10.1128/JVI.00721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N., and, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959–3964. doi: 10.1182/blood-2008-05-155846. [DOI] [PubMed] [Google Scholar]
- Shayakhmetov DM, Li ZY, Ni S., and, Lieber A. Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J Virol. 2004;78:5368–5381. doi: 10.1128/JVI.78.10.5368-5381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov DM, Gaggar A, Ni S, Li ZY., and, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Machemer T, Philopena J, Wen SF, Quijano E, Ramachandra M, et al. Acute hepatotoxicity of oncolytic adenoviruses in mouse models is associated with expression of wild-type E1a and induction of TNF-α. Virology. 2004;328:52–61. doi: 10.1016/j.virol.2004.06.043. [DOI] [PubMed] [Google Scholar]
- Scott FL, Denault JB, Riedl SJ, Shin H, Renatus M., and, Salvesen GS. XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs. EMBO J. 2005;24:645–655. doi: 10.1038/sj.emboj.7600544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, et al. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–116. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenev T, Zachariou A, Wilson R, Ditzel M., and, Meier P. IAPs are functionally non-equivalent and regulate effector caspases through distinct mechanisms. Nat Cell Biol. 2005;7:70–77. doi: 10.1038/ncb1204. [DOI] [PubMed] [Google Scholar]
- Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Choi YE, Butterworth M, Malladi S, Duckett CS, Cohen GM., and, Bratton SB. The E3 ubiquitin ligase cIAP1 binds and ubiquitinates caspase-3 and -7 via unique mechanisms at distinct steps in their processing. J Biol Chem. 2009;284:12772–12782. doi: 10.1074/jbc.M807550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res. 2009;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison ML, Obermueller E, Maisey NR, Hoare S, Edmonds K, Li NF, et al. Tumor necrosis factor α as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. J Clin Oncol. 2007;25:4542–4549. doi: 10.1200/JCO.2007.11.2136. [DOI] [PubMed] [Google Scholar]
- McNeish IA, Tenev T, Bell S, Marani M, Vassaux G., and, Lemoine N. Herpes simplex virus thymidine kinase/ganciclovir-induced cell death is enhanced by co-expression of caspase-3 in ovarian carcinoma cells. Cancer Gene Ther. 2001;8:308–319. doi: 10.1038/sj.cgt.7700305. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cell sensitivity to wild-type adenovirus, infectivity and replication. 1A: IGROV1 cells were infected with d 922-947, Ad5 WT and dl 309. Cell survival was assessed 72 hours later by MTT. At MOI 10, survival following dl 922-947 infection is significantly less than with either Ad5 WT or dl 309 infection (p<0.0001 for both comparisons). 1B: Survival of cells treated with infliximab or IgG alone in figure 2C compared to mock-infected cells. 1C: IGROV-1 Scrambled RNAi and TNF-α RNAi (I) and (II) cells were infected with Ad CMV GFP (MOI 5 pfu/cell). Twenty-four hours later, cells were trypsinised, washed and analysed for GFP positivity by flow cytometry. 1D: Cell surface expression of Coxsackie Adenovirus Receptor (CAR), αvβ3 and αvβ5 integrins on IGROV-1 Scrambled RNAi and TNF-α RNAi (I) and (II) cells was assessed by flow cytometry. 1E: IGROV-1 Scrambled RNAi and TNF-α RNAi (II) cells were infected with dl CR2-dsRed (MOI 0.3 and 1 pfu/cell) as detailed in Materials and Methods. Red fluorescence was detected up to 72 h pi using a Victor3 1420 multilabel counter (left). Supernatant was collected from cells infected at MOI 1 and titred on JH293 cells by TCID50 assay (right).
Effect of pan-caspase inhibitor on virus efficacy; TNF-α expression following cIAP1/2 knockdown. 2A: IGROV-1 Scrambled and TNF-α RNAi cells were infected with dl 922-947 (MOI 10) in the presence or absence of 100mM NH4Cl. Protein was harvested 48 hours pi and blotted for cIAP1 expression. 2B: IGROV-1 Scrambled and TNF-α RNAi cells were infected with dl 922-947 (MOI 0.01-0.1) and refed 2 hours pi with medium with or without 25μM zVAD.fmk. Cell survival was assessed up to 120 hours later. Survival is plotted as percentage survival compared to mock-infected cells. *, p<0.05. 2C: IGROV-1 parental cells were transfected with Scrambled (60 nM), cIAP1 (30 nM), cIAP2 (30 nM) and cIAP1 and cIAP2 (30 nM each) siRNA for 24 hours before being infected with dl 922-947 (MOI 10) or mock infected for 48 hours. Secretion of TNF-α was assessed by Mesoscale analysis. **, p<0.01; ***, p<0.001.