Abstract
Efficient production of insulin in response to changes in glucose levels has been a major issue for insulin gene therapy to treat diabetes. To express target genes in response to glucose specifically in hepatocytes, we generated a synthetic promoter library containing hepatocyte nuclear factor-1, CAAT/enhancer-binding protein (C/EBP) response element, and glucose-response element. Combinations of these three cis-elements in 3-, 6-, or 9-element configurations were screened for transcriptional activity and then glucose responsiveness in vitro. The most effective promoter (SP23137) was selected for further study. Intravenous administration of a recombinant adenovirus expressing furin-cleavable rat insulin under control of the SP23137 promoter into streptozotocin (STZ)-induced diabetic mice resulted in normoglycemia, which was maintained for >30 days. Glucose tolerance tests showed that treated mice produced insulin in response to glucose and cleared exogenous glucose from the blood in a manner similar to nondiabetic control mice, although the clearance was somewhat delayed. Insulin expression was seen specifically in the liver and not in other organs. These observations indicate the potential of this synthetic, artificial promoter to regulate glucose-responsive insulin production and remit hyperglycemia, thus providing a new method of liver-directed insulin gene therapy for type 1 diabetes.
Introduction
Type 1 diabetes is a metabolic disorder caused by the autoimmune destruction of insulin-producing pancreatic β cells, which results in the lack of insulin.1 Exogenous insulin therapy is used to treat diabetes and delay the progression of long-term complications; however, multiple daily insulin injections or continuous subcutaneous insulin infusion by pump is cumbersome and sometimes causes hypoglycemic episodes, which can be life-threatening.2,3 Therefore, there is great interest in developing more efficient methods for providing physiological levels of insulin in a safe and cost-effective manner as replacements for conventional insulin therapy.
Because insulin-producing pancreatic β-cells are destroyed in type 1 diabetes, one strategy is to induce insulin production in an appropriate target cell by gene therapy. Early attempts to replace the function of β-cells by introducing various components of the insulin secretory machinery into non-β-cells were unsuccessful, as insulin secretion was constitutive and not responsive to extracellular glucose.4,5 In order to regulate blood glucose levels effectively, insulin must be produced and released in response to a rise in blood glucose levels and suppressed as blood glucose concentrations decrease. The liver is an important organ for glucose homeostasis, and hepatocytes are particularly attractive as target cells for insulin gene therapy as they have the ability to sense glucose. Several attempts have been made to create glucose-responsive biosynthesis of insulin in the liver using liver-specific and glucose-responsive promoters, including the promoters for the genes encoding L-type pyruvate kinase (L-PK), insulin-like growth factor-binding protein 1, phosphoenolpyruvate carboxykinase, and glucose-6-phosphatase.6,7,8,9,10,11,12 The activities of these promoters are closely coupled with the metabolic control of carbohydrates. However, available naturally occurring promoters are not always capable of regulating transcription in a desired manner. In addition, these transcriptionally regulated systems have relatively slow kinetics in both up- and downregulation of biosynthesis of insulin compared to secretory machinery of pancreatic β-cells.13 Eukaryotic gene expression is regulated by a promoter region that consists of a combination of several cis-regulatory elements. The composition and arrangement of each element determines the characteristics of the regulatory region through combinatorial interaction between transcription regulators.14,15 Moreover, efficient transcription requires the combinatorial and synergistic interaction of multiple activators bound to the DNA counterpart, and many different combinations of transcription factors are capable of transcriptional synergy. As a consequence, a limited number of activators can be arranged in numerous possible combinations, each of which is biologically distinct.
In order to develop an efficient promoter for liver-specific, glucose-regulated insulin expression, we developed synthetic promoters consisting of various combinations of several cis-elements including hepatocyte nuclear factor-1 (HNF-1), CATT/enhancer-binding protein (C/EBP), and glucose-responsive element (GlRE). One of these promoters, SP23137, expressed the target gene specifically in hepatocytes and regulated target gene expression in response to extracellular glucose changes in vitro and in vivo.
Results
Construction of synthetic promoter libraries
The strategy for the construction of the synthetic promoter libraries is shown in Figure 1a. First, we generated 3-element modules that consisted of three cis-elements (HNF-1 and C/EBP, binding sites for strong hepatocyte-specific transcriptional activity, and GlRE, a binding site for glucose responsiveness of the synthetic promoter) in all possible 27 combinations by sequential insertion of each element at the sites of KpnI/BamHI, BamHI/EcoRV, and EcoRV/EcoRI in the pcDNA3.1 plasmid wherein the original multiple cloning sites were replaced with new ones (Supplementary Figure S1). Subsequently, individual 3-element modules were transferred upstream of the L-PK basal promoter [pLPK(-96/+12)], followed by the luciferase reporter gene, to generate 3-element synthetic promoter (SP-Luc) plasmids (Figure 1a). To generate 6-element synthetic promoters, an additional 3-element module was inserted into the 3-element SP-Luc plasmids. To generate 9-element synthetic promoter libraries (9-element SP-Luc plasmids), an additional 3-element module was inserted at the XhoI/NheI site in selected 6-element SP-Luc plasmids (Figure 1a). A total of 19 6-element SP-Luc plasmids with activity >7.5% of the cytomegalovirus (CMV) promoter were chosen for further elongation of cis-elements. More than 40 clones of 9-element SP-Luc plasmids were produced from the individual selected 6-element SP-Luc plasmids to cover all possible combinations arising from the addition of 3-element modules.
Figure 1.
Generation of synthetic promoters. (a) Schematic diagram of synthetic promoters containing 3-, 6-, and 9-cis-regulatory elements. The proximal region of the L-PK promoter (-96/+12 relative to transcriptional initiation site) was used as a basal promoter for generation of synthetic promoter-reporter constructs. Each box represents one of the following elements: HNF-1, C/EBP, or GlRE. Distribution pattern of transcriptional activities of (b) 6-element SP-Luc and (c) 9-element SP-Luc in H4IIE cells. Luciferase activities of synthetic promoter constructs are indicated as a percentage of CMV promoter activity. “Percentage of total plasmids” on the y-axis indicates the proportion of the plasmids showing the indicated range of luciferase activity as a percentage of all plasmids that were screened. C/EBP, CAAT/enhancer-binding protein; CMV, cytomegalovirus; GlRE, glucose-responsive element; HNF-1, hepatocyte nuclear factor-1; L-PK, L-type pyruvate kinase.
In vitro transcriptional activity of synthetic promoters
To determine the transcriptional activities of 3-element synthetic promoters, rat hepatoma cells (H4IIE cells) were transfected with 3-element SP-Luc plasmids, and luciferase activity was measured. The CMV promoter-Luc construct and L-PK promoter-Luc construct were used as controls for a strong ubiquitous promoter activity and for a naturally occurring liver-specific glucose-responsive promoter, respectively. Most of the 3-element SP-Luc plasmids showed transcriptional activity between 2 and 5% of CMV promoter activity and were comparable to the activity of L-PK promoter (Supplementary Figure S2a).
For determination of activities of 6-element synthetic promoters, >300 clones of 6-element SP-Luc plasmids were examined. In most of the 6-element synthetic promoters, transcriptional activity was <7.5% of the CMV promoter (Figure 1b). However, some 6-element synthetic promoters showed more than twofold greater activity than that of L-PK promoter and >7.5 % of CMV promoter activity (Supplementary Figure S2b), and these synthetic promoters were used for generation of 9-element synthetic promoters. To determine whether there was any pattern in those promoters with strong transcriptional activity, we sequenced those 6-element SP-Luc plasmids that showed the highest promoter activity (n = 19). There was no similarity in the order of elements among individual synthetic promoters, and no common pattern in the composition of cis-elements could be found (Supplementary Figure S3a).
Overall transcriptional activity of the 9-element promoters was higher than the original 6-element synthetic promoters (Figure 1c), and a considerable number showed activity that was greater than any of the 6-element SP-Luc plasmids (>21% of CMV promoter activity), indicating that an increase in the number of cis-elements had a positive effect on transcriptional activity. The composition of cis-elements in these synthetic promoters was examined (n = 8); however, there was no similarity in the order of cis-elements among the synthetic promoters (Supplementary Figure S3b).
Glucose responsiveness of synthetic promoters in vitro
To determine whether selected 9-element synthetic promoters could respond to changes in glucose concentration, we transfected rat primary hepatocytes with selected 9-element SP-Luc plasmids and examined luciferase activity in the presence of low and high glucose. Since hepatoma cell lines do not retain their glucose responsiveness,16 primary hepatocytes that still had glucose-sensing activity were used for this purpose. We found that all of the selected 9-element SP-Luc plasmids showed increased luciferase activity in the presence of a high concentration of glucose compared to a low glucose concentration (Figure 2), indicating that our synthetic promoters are able to respond to glucose change. Synthetic promoters 23142 and 6117 showed relatively high luciferase activity in high glucose concentration compared to other promoters. However, they also showed relatively high basal promoter activity in response to low glucose concentration. In contrast, synthetic promoter 23137 (SP23137) showed the strongest glucose responsiveness with relatively low basal promoter activity, therefore it was selected for further investigation in vivo.
Figure 2.
Glucose responsiveness of selected synthetic promoters in vitro. Selected synthetic promoters were transfected into primary rat hepatocytes, followed by incubation in media with the indicated concentrations of glucose. Luciferase assay was performed to measure the promoter activity in response to glucose change and fold induction calculated as a percentage of L-PK promoter activity in the presence of low glucose. Four independent experiments were performed using duplicate wells, and the data from the four experiments were combined. All values are means ± SD. L-PK, L-type pyruvate kinase.
Tissue specificity of SP23137 in vitro
Because the synthetic promoters were designed for liver-specific expression of the target transgene, the tissue specificity of SP23137 needed to be tested. For this purpose, we constructed an adenoviral vector-expressing insulin under the control of SP23137 (rAd-SP23137-rINSfur). We then infected this virus into several different cell lines, namely the HeLa cell line from human cervix, L6 cell line from rat skeletal muscle, L929 cell line from mouse subcutaneous connective tissue, NRK cell line from rat kidney, and H4IIE cell line from rat liver, followed by examination of the expression of insulin gene in the cells by immunohistochemical staining. Insulin-positive cells were found only in H4IIE cells, whereas insulin-positive cells were observed in all cell lines infected with rAd-CMV-rINSfur (Figure 3a). To measure the promoter activity in different cell lines, CMV-Luc or SP23137-Luc plasmids were transfected into the cells, followed by luciferase assay 24 hours after transfection (Figure 3b). In H4IIE cells, SP23137 showed 18% ± 0.6 of CMV promoter activity, whereas it showed very low promoter activity in HeLa (0.1% ± 0.01), L929 (0.5% ± 0.055), and NRK (1% ± 0.26) cells. These results indicate that SP23137 shows hepatocyte-specific expression in vitro.
Figure 3.
Liver cell-specific insulin gene expression in rAd-SP23137-rINSfur-infected cell lines. (a) rAd-SP23137-rINSfur or rAd-CMV-rINSfur viruses were infected into several cell lines as follows: H4IIE (rat hepatoma cell line), L929 (mouse fibroblast cell line), L6 (mouse muscle cell line), HeLa (human cervical carcinoma cell line), and NRK (normal rat kidney cell line). Cells were stained using specific antibody against rat insulin. Cells without virus infection (no virus) were used as negative control. (b) CMV-Luc or SP23137-Luc plasmids were transfected into the same cell lines for reporter assay. After 24 hours of incubation, cell lysates were obtained and luciferase activity was measured and expressed as a percentage of CMV-Luc activity in each cell line. CMV, cytomegalovirus.
Remission of hyperglycemia in diabetic mice treated with rAd-SP23137-rINSfur
To evaluate the ability of synthetic promoter SP23137 to produce insulin and control glucose levels in vivo, we injected rAd-SP23137-rINSfur or rAd-CMV-rINSfur intravenously into streptozotocin (STZ)-induced diabetic NOD.scid mice. First, we examined the effects of different doses of rAd-SP23137-rINSfur (3 × 1010, 1 × 1010, or 3 × 109 viral particles) on blood glucose levels. Diabetic mice treated with the highest dose, 3 × 1010 viral particles, showed a very quick drop in blood glucose levels to a normal range within a few days, whereas mice treated with 1 × 1010 viral particles exhibited a relatively slow decrease in blood glucose concentration (Figure 4a). After blood glucose levels reached the normal range, normoglycemia was maintained for up to 1 month in mice treated with 1 × 1010 viral particles and longer than 1 month in mice treated with 3 × 1010 viral particles, after which hyperglycemia recurred. Mice treated with 3 × 109 viral particles of rAd-SP23137-rINSfur showed moderately reduced blood glucose levels compared to mice treated with higher doses of virus, indicating that the amount of insulin from this titer of virus was not enough to lower blood glucose levels to a normal range (Figure 4a). Although mild hypoglycemic episodes were observed early in mice treated with 3 × 1010 viral particles, no animals died.
Figure 4.
Activity of synthetic promoter SP23137 in vivo. (a) The indicated doses of rAd-SP23137-rINSfur were administered into STZ-induced diabetic NOD.scid mice, and blood glucose levels were monitored. As a negative control, diabetic NOD.scid mice that were not treated with virus were used. As a nondiabetic normal control, untreated NOD.scid mice were used. Blood glucose levels were measured between 2 and 4 during the day, and food and water were given ad libitum during the experiments. All values are means ± SD. (b) Presence of recombinant adenoviral genome in the liver of treated animals. Livers were obtained from the mice treated with rAd-SP23137-rINSfur viruses (1010 viral particles) at various times following virus administration. Whole DNA was extracted from the livers at the indicated times after virus injection, and PCR was performed to detect the adenoviral genome. pAd-SP23137-rINSfur plasmid DNA was used as positive control. STZ, streptozotocin; VP, viral particles.
To determine whether the recurrence of hyperglycemia was a result of the disappearance of the injected viral genome, we examined the presence of the injected viral genome in the liver of rAd-SP23137-rINSfur-treated NOD.scid mice on 10, 25, and 50 days following virus administration, using PCR with specific primers for the adenoviral genome. Clear bands were observed at 10 and 25 days, whereas a very faint band was seen at 50 days, when the hyperglycemia recurred (Figure 4b). These results indicate that recurrence of hyperglycemia after treatment most likely resulted from the reduced adenoviral genome in the liver of treated animals.
Glucose responsiveness of SP23137 in vivo
To examine the importance of regulated expression of insulin gene by the promoter, we injected rAd-CMV-rINSfur virus, which constitutively produces insulin. As expected, all mice treated with rAd-CMV-rINSfur died within 8 days after virus injection due to hypoglycemia. In contrast, all mice treated with the same dose of rAd-SP23137-rINSfur, which produces insulin under the control of SP23137, lowered blood glucose levels and survived (Supplementary Figure S4). It is notable that administration of rAd-CMV-rINSfur virus lead to death of the treated animals, even when given at titers 10 times lower than rAd-SP23137-rINSfur, indicating that an uncontrolled strong promoter is potential risk for insulin gene therapy.
Next, we checked glucose responsiveness of SP23137 in vivo, using a glucose tolerance test, at 2 weeks after virus injection. We found that blood glucose levels peaked at 30 minutes after glucose injection and gradually decreased to normal levels at 120 minutes after injection in rAd-SP23137-rINSfur-treated mice, whereas blood glucose levels in diabetic control mice did not decrease. Glucose clearance in rAd-SP23137-rINSfur-treated mice was somewhat delayed as compared with normal control mice, evidenced by significantly higher blood glucose levels at 60 and 90 minutes after injection (Figure 5a). When we examined serum insulin levels at 30 and 120 minutes after glucose injection, we found no significant increase in rAd-SP23137-rINSfur-treated mice compared to basal levels, whereas there was about a fourfold increase in normal control mice at 30 minutes after glucose injection (Figure 5b). To determine whether the expression of insulin mRNA in the liver was increased in response to glucose, we analyzed insulin mRNA by real-time quantitative PCR before and 90 minutes after glucose injection. We found that insulin mRNA expression was increased about fourfold after glucose challenge in rAd-SP23137-rINSfur-treated mice. Insulin mRNA was not detected in the liver of normal control mice (Figure 5c). Next, we investigated the effect of fasting on blood glucose levels, at 3 weeks after virus injection. When animals were fasted for 9 or 24 hours, blood glucose levels were low, but not hypoglycemic, in both normal and rAd-SP23137-rINSfur-treated animals. In contrast, STZ-induced diabetic mice showed significantly higher blood glucose levels even after 24 hours of fasting (Figure 5d).
Figure 5.
Glucose responsiveness of SP23137 in vivo. (a) Glucose tolerance test. At 2 weeks after virus injection, animals were fasted for 16 hours, glucose was administered by intraperitoneal injection, and blood glucose levels were measured. All values are means ± SD. (b) Serum insulin levels after glucose challenge. Serum was obtained before (basal) and after glucose loading (glucose-stimulated) from normal or normoglycemic, virus-treated animals. All values are means ± SD. *P < 0.001 as compared with basal level. (c) Insulin mRNA induction after glucose challenge. The liver samples were prepared before (basal) and 90 minutes after the glucose loading (glucose-stimulated), total RNA was prepared, and quantitative real-time PCR was performed. Fold induction of insulin mRNA was calculated as a percentage of the basal level. ND, not detected. All values are means ± SD. *P < 0.001 as compared with basal level. (d) Fasting glucose levels. At 3 weeks after viral treatment, animals were fasted for 9 or 24 hours and blood glucose levels were measured.
Liver-specific insulin gene expression by SP23137 in vivo
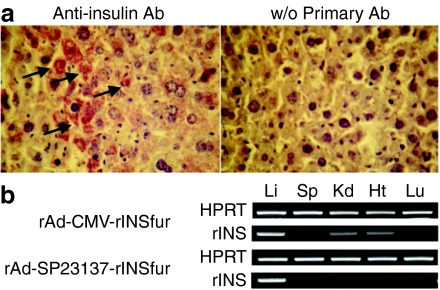
To determine the liver-specific promoter activity of SP23137 in vivo, liver samples from rAd-SP23137-rINSfur- or rAd-CMV-rINSfur-treated mice were obtained for immunohistochemistry. Insulin protein was present in samples from both rAd-SP23137-rINSfur-treated (Figure 6a) and rAd-CMV-rINSfur-treated mice, indicating that SP23137 had promoter activity for the expression of insulin in the liver in vivo. Next, we checked the insulin gene expression in other tissues by reverse transcriptase-PCR. We detected transgenic rat insulin mRNA expression only in the liver from rAd-SP23137-rINSfur-treated mice (Figure 6b), which was consistent with the in vitro results. In contrast, transgenic rat insulin mRNA was detected in all tested tissues (kidney, spleen, liver, lung, and heart) from rAd-CMV-rINSfur-treated mice (Figure 6b).
Figure 6.
Liver-specific insulin gene expression in rAd-SP23137-rINSfur-treated NOD.scid mice. rAd-SP23137-rINSfur (1010 VP) viruses were administered into STZ-induced diabetic NOD.scid mice. After the treated animals showed normal glucose levels at 2 weeks postinfection, they were sacrificed and the various organs were removed. (a) Immunohistochemical staining for insulin in the liver. The stained cells are indicated as arrows. (b) RT-PCR. Total RNAs were isolated and RT-PCR was performed using primers for rat insulin or HPRT. Ht, heart; Kd, kidney; Li, liver; Lu, lung; RT-PCR, reverse transcriptase-PCR; Sp, spleen; STZ, streptozotocin; VP, viral particles.
Discussion
Type 1 diabetes results from insulin deficiency caused by autoimmune destruction of insulin-producing pancreatic β cells,1 requiring life-long administration of insulin for type 1 diabetic patients. However, daily insulin injections are cumbersome and not always successful in maintaining a tight control of blood glucose levels. Insulin gene therapy has been studied as a possible therapeutic approach; however, simple replacement of insulin gene expression by genetic engineering would not be useful unless there is an appropriate system to regulate insulin gene expression in response to glucose levels. Many attempts have been made to achieve glucose-responsive insulin production using naturally occurring glucose-regulated promoters.7,8,9,10,11,12 However, since these naturally occurring promoters show weak transcriptional activities, use of these promoters for insulin expression results in incomplete remission of hyperglycemia under nonfasting conditions. In addition, available naturally occurring promoters are not always capable of regulating transcription in a desired manner, due to the complex interaction of transcription factors that bind to the promoter region, making it more difficult to manipulate transcriptional regulation.17 Several attempts have been made to modify a naturally occurring promoter, the insulin growth factor-binding protein 1 basal promoter, by adding three copies of GlRE upstream of the promoter.8,12,18 Although this modified promoter was able to improve glycemic control in STZ-treated diabetic rats, there was a wide range of blood glucose levels in the random feeding state and elevated free fatty acid levels in the serum. This phenomenon is likely due to improper regulation of this promoter in response to changes in glucose levels.
The liver is a particularly good candidate target for insulin gene therapy. First, it is a major target organ of insulin action and plays a critical role in glucose homeostasis.19 Second, like the pancreatic β-cells, the liver has the intrinsic ability to respond to changes in blood glucose concentrations because it contains glucose transporter and glucokinase.20 Third, hepatocytes are not susceptible to pre-existing β-cell-specific autoimmune responses.21,22,23 On the basis of these features, we chose the liver for insulin gene expression. In this study, synthetic promoters were constructed from combinations HNF-1 and C/EBP binding elements, for enhanced liver-specific transcriptional activity, and GlRE, for glucose responsiveness, and the promoters were used for liver-directed insulin gene therapy. Expression of furin-cleavable insulin under the control of one of these synthetic promoters, SP23137, controlled blood glucose levels and remitted hyperglycemia in diabetic mice.
Normoglycemia was achieved after injection of rAd-SP23137-rINSfur, but hyperglycemia recurred at the time at which adenoviral genome had almost disappeared. Adenovirus elicits an innate immune response through the induction of high levels of type I interferons by both plasmacytoid dendritic cells and non-plasmacytoid dendritic cells such as conventional DCs and macrophages and mediated by Toll-like receptor 9.24 In addition, Toll-like receptor expression levels in NOD, NOD.scid, and Balb/c mice were comparable,25 even though NOD.scid mice are B and T cell deficient. Therefore, we consider that cells expressing the transgene are cleared by an innate immune response to adenovirus.
Synthetic promoters with 3 elements (combinations of HNF-1, C/EBP, and GlRE) had comparable transcriptional activities to the naturally occurring L-PK promoter, although multimerized single elements had relatively low activity, consistent with previous reports.17 However, the transcriptional activities of these 3-element synthetic promoters did not result in sufficient insulin production to correct hyperglycemia (data not shown). It was reported that the inclusion of additional transcription-factor binding elements within a construct can enhance gene expression.26,27 Therefore, we tried to enhance the activity of our synthetic promoters by introducing additional 3-element modules into the constructs. As expected, the overall activity of 6-element promoters was enhanced compared with 3-element promoters. These observations indicate that some combinations of cis-elements might have a synergistic effect on promoter activity. Sequence analysis of the 6-element promoters found no common features. Similar results were observed for 9-element synthetic promoters, which were constructed by inserting additional 3-element modules into those 6-element synthetic promoters that had activity over 7.5% that of the CMV promoter. The 9-element synthetic promoters had less, the same, or more activity than their parental constructs, with no sequence similarity in those 9-element promoters with high activity.
Although high transcriptional activity is important, glucose responsiveness is the most critical feature of insulin gene therapy. Analysis of glucose responsiveness of selected 9-element synthetic promoters in primary rat hepatocytes revealed that these promoters expressed the reporter gene in response to glucose. In addition, insulin mRNA was increased in the liver after glucose injection in diabetic mice treated with one of these 9-element promoters, rAd-SP23137, expressing insulin. However, no significant induction of serum insulin levels was observed in rAd-SP23137-rINSfur-treated diabetic mice after glucose challenge. This result is similar to a previous report that showed only a small change in serum insulin levels after glucose challenge in diabetic BioBreeding rats treated with an adenovirus containing a glucose-regulated transgenic insulin.12 The absence of an increase in serum insulin levels during glucose tolerance tests can be partly explained by the fact that the liver is the major target organ for insulin and routinely removes 50% of insulin secreted from β-cells.28 In addition, a relatively normal blood glucose clearance after glucose challenge without a significant change in serum insulin levels was found in an animal model having β-cells with compromised secretory function.29 Therefore, it is likely that SP23137 promoter produced sufficient amounts of insulin to maintain the blood glucose level in a normal range and to clear exogenous glucose, but insufficient amounts to result in a significant increase in serum insulin after glucose challenge.
It is notable that no animals died during the viral treatment, although mild hypoglycemia was observed in the early treatment period in animals treated with 3 × 1010 viral particles, which was the maximum nonlethal dose of adenoviral vector. Glucose tolerance tests showed that rAd-SP23137-rINSfur-treated animals had a delayed return to normoglycemia compared with nondiabetic control animals and were hypoglycemic between 180 and 240 minutes after glucose challenge. These results indicate that our newly developed synthetic promoter still has some limitations as a glucose regulatory system, especially in negative feedback. The negative regulation of synthetic promoter might be improved by introduction of insulin responsive sequence, which has been shown to repress promoter activity in the presence of insulin.30 In addition, it might be possible to control the half-life of transgenic insulin mRNA by incorporating specific elements into its sequence that result in rapid degradation,31 as the potential risk of insulin-induced hypoglycemia depends largely on the relative stability of insulin mRNA.32
Our newly developed synthetic promoter, SP23137, has several advantages. First, it showed comparable promoter activity in response to 11 mmol/l glucose but stronger promoter activity in response to high glucose (27 mmol/l) compared with the naturally occurring L-PK promoter in vitro. This stronger promoter activity will shorten the exposure time to high glucose after nutrient uptake and subsequently will reduce the level of hemoglobin A1c. Second, our synthetic promoter induced liver-specific expression of the insulin gene. Using promoters to drive insulin expression in nonhepatic tissues that are not capable of glucose sensing may result in the insulin gene being constitutively expressed, which would result in hypoglycemia. This phenomenon might be avoided with our liver-specific promoter. Third, although our newly developed synthetic promoter did not show optimal regulation of insulin gene expression in response to glucose level change especially in negative feedback, it has great potential to be manipulated for improved control by adding other responsive elements such as the insulin responsive sequence. Taken together, our liver-specific synthetic promoter may have therapeutic potential for the possible cure of diabetes in humans through insulin gene therapy or engineered hepatocyte therapy.
Materials and Methods
Animals. NOD.scid mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Sprague–Dawley rats were purchased from Charles River Laboratory (Wilmington, MA). Animals were maintained under specific pathogen-free conditions and provided with sterile food and water ad libitum at the Animal Resource Center, Faculty of Medicine, University of Calgary, Calgary, Alberta, Canada. The use and care of the animals used in this study were approved by the Animal Care Committee, Faculty of Medicine, University of Calgary.
Construction of plasmids. pLPK-TA plasmid was constructed using direct insertion of PCR amplified L-PK promoter into the TA cloning vector (Invitrogen, Carlsbad, CA). A 2.5 kbp SacI/HindIII fragment of the L-PK promoter was removed from plasmid pLPK-TA, and then cloned into the SacI/HindIII sites of pGL3E (Promega, Madison, WI) which has an enhancer region at the end of luciferase gene, generating pLPK-Luc plasmid that was used as a positive control in the promoter assay. For construction of CMV-Luc plasmids, a Mlu1/HindIII fragment of pcDNA3.1 containing the CMV promoter region was inserted into same enzyme site of pGL3E (Promega). A 108-bp NheI/HindIII fragment of the L-PK promoter was removed from the pLPK-TA, and this fragment was inserted into the same enzyme sites of pGL3E (Promega), generating pLPK(-96/±12)-Luc plasmid for use in the generation of synthetic promoter-reporter constructs.
Generation of synthetic promoter library. Two complementary oligonucleotides were synthesized for each control element, phosphorylated, and annealed to yield short DNA fragments. The oligonucleotides sequences were as follows: HNF-1 (sense), 5′-CTA GCT GGT TAA TGA TTA ACC AGG ACT-3′ HNF-1 (antisense), 5′-AGT CCT GGT TAA TCA TTA ACC AGC TAG-3′ C/EBP (sense), 5′-TCG CAA ATT GCG CAA TAT CGC G-3′ C/EBP (antisense), 5′-C GCG TAT TTG CGC AAT TTG CGA -3′ G1RE (sense), 5′-GGG CGC ACG GGG CAC TCC CGT GGT TCC TGG ACT CTG GCC CCC AGT GTA -3′, GlRE (antisense), 5′-TAC ACT GGG GGC CAG AGT CCA GGA ACC ACG GGA GTG CCC CGT GCG CCC-3′. HNF-1 and C/EBP sequence were obtained from the TRANFAC database, and GlRE sequence was obtained from the GlREs of L-PK promoter. Each synthetic fragment was synthesized with respective restriction enzyme cohesive ends at both ends of the oligonucleotide for the generation of the synthetic promoter library. The introduced restriction enzyme sites were KpnI/BamHI, BamHI/EcoRV, and EcoRV/EcoRI. Each complementary oligonucleotide (100 pmol each) was annealed and phosphorylated using T4 polynucleotide kinase (New England BioLabs, Ipswich, MA), followed by phenol/chloroform/isoamyl alcohol extraction. Each cis-element containing the KpnI/BamHI, BamHI/EcoRV, and EcoRV/EcoRI enzyme sites was sequentially inserted into the same enzyme sites of the modified pcDNA3.1 vector (pcDNA3-NewMCS) (Supplementary Figure S1) (Invitrogen), generating 27 different 3-element SP-D3 plasmids containing different combinations of the 3 cis-elements (3-element module). To generate synthetic promoter-reporter plasmids, the 3-element modules from 3-element SP-D3 plasmid were subcloned upstream of the pLPK (-96/+12)-Luc plasmid, generating 3-element SP-Luc plasmids. For generation of 6-element SP-Luc plasmids, an additional 3-element module was inserted into 3-element SP-Luc plasmids (Figure 1a). After screening for high transcriptional activity of the synthetic promoters, another 3-element module was inserted into selected 6-element SP-Luc plasmids to generate 9-element SP-Luc plasmids (Figure 1a).
Screening of synthetic promoters in vitro. All plasmids used in the transfection experiment for initial screening of synthetic promoter were prepared using miniprep kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. H4IIE cells were cultured as described below. One day before transfection, cells were seeded into 96-well plates at a number of 5,000 cells/well. Cells were transfected with 150 ng plasmid/well using lipofectamine plus reagent (Invitrogen) according to the manufacturer's instructions and collected 24 hours after transfection. Cells were lysed with Glo Lysis Buffer (Promega, Madison, WI), and luciferase activity was measured using Steady-Glo Luciferase Assay System (Promega). The assay was performed in quadruplicate in at least two different rounds of transfection.
Primary rat hepatocyte isolation. Male Sprague–Dawley rats (180–300 g) were anesthetized by intraperitoneal injection of nembutal sodium solution (0.10 ml/100 g body weight; Ovation Pharmaceutical, Deerfield, IL). Primary hepatocytes were isolated by the collagenase perfusion method and plated in 6-well Primaria plates (BD) at a cell density of 1.2 × 106 cells/well as described previously.33 After a 16-hour attachment period, transfection was performed using Fugene 6 (Roche, Indianapolis, IN) in modified William E medium (Invitrogen) with 3.3 mmol/l glucose for 12–14 hours. Subsequently, cells were cultured for 24 hours in medium containing either 3.3, 11, or 27 mmol/l glucose and harvested for luciferase activity.
Immunohistochemical staining of cells. All cells used in the present study were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen), penicillin (200 IU/ml), streptomycin (100 µg/ml), and -glutamine (2 mmol/l) at 37 °C in a 5% CO2/95% air humidified atmosphere. The cell lines used were L6 (rat skeletal muscle cell line),34 L929 (mouse subcutaneous connective tissue cell line), H4IIE (rat hepatoma cell line), HeLa (human epithelial cell line), and NRK (rat kidney cell line) cells. Cells were seeded onto specifically designed slides (Labtek; Nalge Nucc, Naperville, IL) at 2 × 104 cells/well, incubated for 1 day, and infected with virus at 10 multiplicity of infection. After 24 hours of infection, cells were fixed using 4% paraformaldehyde in phosphate-buffered saline for 15 minutes at room temperature, then permeablized with 1% Triton X-100 in phosphate-buffered saline. Nonspecific antibody binding was blocked using blocking buffer [1% (wt/vol) bovine serum albumin, 0.2% (vol/vol) Tween-20 in phosphate-buffered saline] for 1 hour. Cells were incubated with guinea-pig anti-rat insulin antibody for 1 hour (1/200 in blocking buffer; DAKO, Carpiteria, CA), washed, and incubated in biotinylated anti-guinea-pig antibody (1/300) for 1 hour. After washing, horseradish peroxidase-conjugated streptavidin was diluted in blocking buffer (1/300) and added to cells for 1 hour, followed by color development using Vector VIP (Vector Laboratories, Burlingham, CA) according to the manufacturer's instructions.
Luciferase reporter assay. One day before transfection, cells were seeded into 24-well plates. Cells were transfected with 200 ng plasmid/well using lipofectamine plus reagent (Invitrogen). After 24-hour incubation, cells were lysed and luciferase activity was measured as described above. The assay was performed in quadruplicate in at least two different rounds of transfection.
Generation of recombinant adenovirus. Transpose-Ad Adenoviral Vector System (Qbiogene, Carlsbad, CA) was utilized and all the procedures were carried out according to the manufacturer's instruction. Furin-cleavable rat insulin cDNA (rINSfur) was obtained from VR3503 plasmid that contained rat insulin gene with furin cleavage sites at the B-chain and C-peptide junction.35 rINSfur DNA fragment was digested at XbaI site and cloned into PCR276 adenoviral transfer plasmids, generating pCR276-rINSfur that was used as backbone for further construction of adenoviral vectors containing synthetic promoter. pCMV-rINSfur was generated by insertion of the rINSfur region from pCR276-rINSfur into pCR259 adenoviral transfer vector that had CMV promoter to drive transgene expression.
NotI/SalI-digested fragments of SP-rINSfur containing synthetic promoter, furin-cleavable rat insulin cDNA, poly-A region, and SV40 enhancer, were cloned into the PCR276 transfer vector at the same sites, generating PCR276-SP-rINSfur plasmids. The resulting plasmids were transformed into High-Q Transpose-AdTM 294 chemically competent cells, where the transposition of transgene from the recombinant adenovirus transfer vector to the Transpose-AdTM 294 plasmid, generating pAd-SP-rINSfur plasmid. pAd-CMV-rINSfur plasmid was generated from pCMV-rINSfur in the same way. DNA extracted from transposition-occurred white colony was retransformed into chemically competent HighA-1TM cells in order to segregate and amplify the pAd-SP-rINSfur plasmid from adenovirus transfer vector. The segregated pAd-SP-rINSfur plasmids were then transfected into human embryonic kidney 293 cells to generate recombinant adenovirus expressing the insulin gene (rAd-SP-rINSfur) under the control of the synthetic promoters. rAd-CMV-rINSfur virus was generated in the same way. Recombinant viruses were amplified on a large scale in human embryonic kidney 293 cells, purified by double CsCl density-gradient centrifugation, and dialyzed against adenovirus dilution buffer (10 mmol/l Tris–Cl, pH 8.0, 2 mmol/l MgCl2, 4% sucrose). The dialyzed virus was kept at -80 °C for storage. Viral titer was determined by the measurement of optical density at 260 nm.
Blood glucose monitoring and glucose tolerance tests. STZ was administered to NOD.scid mice via an intraperitoneal injection at a dose of 140 mg/kg body weight in citrate buffer pH 4.0. After blood glucose was increased to 400 mg/dl and continued for three consecutive days, recombinant adenoviruses were administered intravenously through tail vein. All mice were given ad libitum access to food and water during the experiments, and blood glucose was measured between 2 and 4 during the day via the glucose oxidase method, using tail-blood and a One-Touch Profile portable blood glucose monitor (Lifescan, Milpitas, CA). For glucose tolerance tests, animals were fasted for 16 hours and then a 1 mol/l glucose solution was administered via an intraperitoneal injection at a dose of 2 g/kg body weight. Blood samples were collected from a small cut at the tip of the mice tail before glucose injection, at 0, 15, 30, 60, 90, 120, and 240 minutes after the glucose load.
Insulin ELISA. Animals were fasted for 6 hours and then a 1 mol/l glucose solution was administered via an intraperitoneal injection at a dose of 2 g/kg body weight. Serum was obtained before and 30 and 120 minutes after glucose loading. Serum insulin levels were measured with rat insulin ELISA kit (Crystal Chem, Downers Grove, IL), which shows 100% crossreactivity with rat and mouse insulin, according to manufacturer's instructions.
Reverse transcriptase-PCR. Several organs (liver, spleen, lung, heart, and kidney) of animals were placed in 10 volumes of RNA later RNA stabilization Reagent (Qiagen), stored at -80 °C. Total RNA was isolated using RNeasy Mini Kit (Qiagen) according to the manufacturer's instruction, and stored at -80 °C. Five microgram of total RNA was used to synthesize cDNA using Superscript II reverse transcriptase (Invitrogen) and oligo(dT)12–18 (Invitrogen). PCR was performed using specific primers for rat insulin. Mouse hypoxanthine phosphoribosyl transferase was used as an internal standard. The upstream and downstream primers used were as follows: rat insulin (sense) GTGGATGCGCTTCCTG; rat insulin (antisense) ACAATGCCACGCTTCTG; mouse hypoxanthine phosphoribosyl transferase (sense) GTAATGATCAGTCAACGGGGGAC; mouse hypoxanthine phosphoribosyl transferase (antisense) CCAGCAAGCTTGCAACCTTAACCA. The PCR condition was optimized for each set of primers. The PCR mixture36 contained 0.2 mmol/l of each deoxynucleotide triphosphate, 1 µmol/l of each specific primer, 1.5 mmol/l or 2 mmol/l MgCl2, 50 mmol/l KCl, 10 mmol/l Tris–Cl, pH 9.0, and 2.5 U of Taq polymerase (New England BioLabs). After amplification, the products were subjected to electrophoresis on a 1% agarose gel and visualized by ethidium bromide staining.
Histology. The liver, kidney, spleen, and pancreas were removed from virus-treated NOD.scid mice when blood glucose level fell <150 mg/dl, which occurred at 2 weeks postinfection. Glucose (2 g/kg body weight) was administered intraperitoneally to boost the insulin expression 2 hours before tissue removal. The samples were fixed with 10% buffered formalin, embedded in paraffin, sectioned at 4.5 µm, and mounted on glass slides. Slides were treated with xylene, dehydrated in ethanol, and washed with tap water. Slides were immunohistochemically stained using anti-insulin antibody as described for the cell lines. After washing with tap water, samples were counterstained with Meyer's hematoxylin solution.
DNA extraction from tissues and PCR. Genomic DNA was purified from the liver of rAd-SP23137-rINSfur-treated NOD.scid mice with DNeasy Tissue kit (Qiagen) according to the manufacturer's instructions at 5, 10, 25, and 50 days after viral administration. As positive control, DNA from the liver of rAd-CMV-rINSfur-treated animals was used. The upstream and downstream primers were as follows: sense, GTGGATGCGCTTCCTG; antisense, ACAATGCCACGCTTCTG. After amplification, the products were subjected to electrophoresis on a 1% agarose gel and visualized by ethidium bromide staining.
Quantitative real-time reverse transcriptase-PCR analysis. Total RNA was isolated from the liver, and cDNA was synthesized using a Superscript III Reverse Transcriptase Kit (Invitrogen). PCR was carried out in a LightCycler (Roche Applied Science, Indianapolis, IN) at 95 °C for 15 min, followed by 45 cycles of 95 °C for 15 seconds, 55 °C for 20 seconds, and 72 °C for 35 seconds. The rat insulin primer sequences used were as above. As an internal control, GAPDH mRNA was amplified. Relative copy number was calculated using the threshold crossing point (Ct) as calculated by the LightCycler software combined with the ΔΔCt calculations.
SUPPLEMENTARY MATERIAL Figure S1. Generation of synthetic promoter. (a) Schematic diagram of new multiple-cloning sites in the pcDNA3.1 plasmid. Two complementary oligonucleotides containing restriction enzymes sites for Kpn I-BamH I-EcoR V-EcoR I were synthesized and subcloned into the Xho I/Nhe I sites to replace the original multiple-cloning sites containing EcoR V-EcoR I-Kpn I-Hind III. (b) Nucleotide sequences for the cis -elements used for generation of synthetic promoters. Figure S2. Luciferase activities of synthetic promoters in rat hepatoma cells. (a) Activities of 3-element synthetic promoters. All 3-element modules were transferred to a luciferase reporter vector generating 3-element SP-Luc, followed by luciferase assay. (b) 6-element synthetic promoters that had more than 7.5% of CMV promoter activity. Luciferase activities of synthetic promoter constructs were calculated as percentage of CMV promoter activity. All values are means ± S.D. L-PK, pLPK-Luc plasmid; h, HNF-1 binding element; c, C/EBP binding element; g, glucose-responsive element. Figure S3. The arrangement of cis-elements. (a) 6-element SP-Luc plasmids that showed over 7.5% of CMV promoter activity and (b) 9-element SP-Luc plasmid that showed over 21% of CMV promoter activity. Figure S4. Survival rate after virus administration. rAd-CMV-rINSfur or rAd-SP23137-rINSfur viruses were injected at the indicated dosage into STZ-treated NOD.scid mice.
Acknowledgments
The work presented in this paper was supported by a grant of the Korea Healthcare technology R&D project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A062260) and National Institutes of Health grant 1R21DK60192. We thank Dr Ann Kyle for editorial assistance.
Supplementary Material
Generation of synthetic promoter. (a) Schematic diagram of new multiple-cloning sites in the pcDNA3.1 plasmid. Two complementary oligonucleotides containing restriction enzymes sites for Kpn I-BamH I-EcoR V-EcoR I were synthesized and subcloned into the Xho I/Nhe I sites to replace the original multiple-cloning sites containing EcoR V-EcoR I-Kpn I-Hind III. (b) Nucleotide sequences for the cis -elements used for generation of synthetic promoters.
Luciferase activities of synthetic promoters in rat hepatoma cells. (a) Activities of 3-element synthetic promoters. All 3-element modules were transferred to a luciferase reporter vector generating 3-element SP-Luc, followed by luciferase assay. (b) 6-element synthetic promoters that had more than 7.5% of CMV promoter activity. Luciferase activities of synthetic promoter constructs were calculated as percentage of CMV promoter activity. All values are means ± S.D. L-PK, pLPK-Luc plasmid; h, HNF-1 binding element; c, C/EBP binding element; g, glucose-responsive element.
The arrangement of cis-elements. (a) 6-element SP-Luc plasmids that showed over 7.5% of CMV promoter activity and (b) 9-element SP-Luc plasmid that showed over 21% of CMV promoter activity.
Survival rate after virus administration. rAd-CMV-rINSfur or rAd-SP23137-rINSfur viruses were injected at the indicated dosage into STZ-treated NOD.scid mice.
REFERENCES
- Yoon JW., and, Jun HS.1998. Insulin-dependent diabetes mellitus. In:Roitt IM., and, Delves PJ.eds). Encyclopedia of Immunology. Academic Press, London. pp; 1390–1398. [Google Scholar]
- The DCCT Research Group Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am. J. Med. 1991;90:450–459. [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Levine F., and, Leibowitz G. Towards gene therapy of diabetes mellitus. Mol Med Today. 1999;5:165–171. doi: 10.1016/s1357-4310(98)01425-7. [DOI] [PubMed] [Google Scholar]
- Yoon JW., and, Jun HS. Recent advances in insulin gene therapy for type 1 diabetes. Trends Mol Med. 2002;8:62–68. doi: 10.1016/s1471-4914(02)02279-7. [DOI] [PubMed] [Google Scholar]
- Won JC, Rhee BD., and, Ko KS. Glucose-responsive gene expression system for gene therapy. Adv Drug Deliv Rev. 2009;61:633–640. doi: 10.1016/j.addr.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Gros L, Montoliu L, Riu E, Lebrigand L., and, Bosch F. Regulated production of mature insulin by non-beta-cells. Hum Gene Ther. 1997;8:2249–2259. doi: 10.1089/hum.1997.8.18-2249. [DOI] [PubMed] [Google Scholar]
- Thulé PM., and, Liu JM. Regulated hepatic insulin gene therapy of STZ-diabetic rats. Gene Ther. 2000;7:1744–1752. doi: 10.1038/sj.gt.3301297. [DOI] [PubMed] [Google Scholar]
- Chen R, Meseck ML., and, Woo SL. Auto-regulated hepatic insulin gene expression in type 1 diabetic rats. Mol Ther. 2001;3:584–590. doi: 10.1006/mthe.2001.0299. [DOI] [PubMed] [Google Scholar]
- Kozlowski M, Olson DE, Rubin J, Lyszkowicz D, Campbell A., and, Thulé PM. Adeno-associated viral delivery of a metabolically regulated insulin transgene to hepatocytes. Mol Cell Endocrinol. 2007;273:6–15. doi: 10.1016/j.mce.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Alam T., and, Sollinger HW. Glucose-regulated insulin production in hepatocytes. Transplantation. 2002;74:1781–1787. doi: 10.1097/00007890-200212270-00024. [DOI] [PubMed] [Google Scholar]
- Olson DE, Paveglio SA, Huey PU, Porter MH., and, Thulé PM. Glucose-responsive hepatic insulin gene therapy of spontaneously diabetic BB/Wor rats. Hum Gene Ther. 2003;14:1401–1413. doi: 10.1089/104303403769211628. [DOI] [PubMed] [Google Scholar]
- Halban PA, Kahn SE, Lernmark A., and, Rhodes CJ. Gene and cell-replacement therapy in the treatment of type 1 diabetes: how high must the standards be set. Diabetes. 2001;50:2181–2191. doi: 10.2337/diabetes.50.10.2181. [DOI] [PubMed] [Google Scholar]
- Chen L. Combinatorial gene regulation by eukaryotic transcription factors. Curr Opin Struct Biol. 1999;9:48–55. doi: 10.1016/s0959-440x(99)80007-4. [DOI] [PubMed] [Google Scholar]
- Reményi A, Schöler HR., and, Wilmanns M. Combinatorial control of gene expression. Nat Struct Mol Biol. 2004;11:812–815. doi: 10.1038/nsmb820. [DOI] [PubMed] [Google Scholar]
- Meienhofer MC, De Medicis E, Cognet M., and, Kahn A. Regulation of genes for glycolytic enzymes in cultured rat hepatoma cell lines. Eur J Biochem. 1987;169:237–243. doi: 10.1111/j.1432-1033.1987.tb13603.x. [DOI] [PubMed] [Google Scholar]
- Li X, Eastman EM, Schwartz RJ., and, Draghia-Akli R. Synthetic muscle promoters: activities exceeding naturally occurring regulatory sequences. Nat Biotechnol. 1999;17:241–245. doi: 10.1038/6981. [DOI] [PubMed] [Google Scholar]
- Olson DE, Campbell AG, Porter MH, Freeman KG, Kelso E, Flatt WP, et al. Hepatic insulin gene therapy normalizes diurnal fluctuation of oxidative metabolism in diabetic BB/Wor rats. Mol Ther. 2008;16:1235–1242. doi: 10.1038/mt.2008.97. [DOI] [PubMed] [Google Scholar]
- Taylor R., and, Agius L. The biochemistry of diabetes. Biochem J. 1988;250:625–640. doi: 10.1042/bj2500625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rencurel F, Waeber G, Antoine B, Rocchiccioli F, Maulard P, Girard J, et al. Requirement of glucose metabolism for regulation of glucose transporter type 2 (GLUT2) gene expression in liver. Biochem J. 1996;314 (Pt 3):903–909. doi: 10.1042/bj3140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipes MA, Cooper EM, Skelly R, Rhodes CJ, Boschetti E, Weir GC, et al. Insulin-secreting non-islet cells are resistant to autoimmune destruction. Proc Natl Acad Sci USA. 1996;93:8595–8600. doi: 10.1073/pnas.93.16.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabiin MT, Tuch BE, Bai L, Han XG., and, Simpson AM. Susceptibility of insulin-secreting hepatocytes to the toxicity of pro-inflammatory cytokines. J Autoimmun. 2001;17:229–242. doi: 10.1006/jaut.2001.0539. [DOI] [PubMed] [Google Scholar]
- Tabiin MT, White CP, Morahan G., and, Tuch BE. Insulin expressing hepatocytes not destroyed in transgenic NOD mice. J Autoimmune Dis. 2004;1:3. doi: 10.1186/1740-2557-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Huang X., and, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giarratana N, Penna G, Amuchastegui S, Mariani R, Daniel KC., and, Adorini L. A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. J Immunol. 2004;173:2280–2287. doi: 10.4049/jimmunol.173.4.2280. [DOI] [PubMed] [Google Scholar]
- Walters MC, Fiering S, Eidemiller J, Magis W, Groudine M., and, Martin DI. Enhancers increase the probability but not the level of gene expression. Proc Natl Acad Sci USA. 1995;92:7125–7129. doi: 10.1073/pnas.92.15.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland HG, Martin DI., and, Whitelaw E. A globin enhancer acts by increasing the proportion of erythrocytes expressing a linked transgene. Mol Cell Biol. 1997;17:1607–1614. doi: 10.1128/mcb.17.3.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth WC, Bennett RG., and, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. 1998;19:608–624. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, et al. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 2000;408:994–997. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- O'Brien RM, Streeper RS, Ayala JE, Stadelmaier BT., and, Hornbuckle LA. Insulin-regulated gene expression. Biochem Soc Trans. 2001;29 Pt 4:552–558. doi: 10.1042/bst0290552. [DOI] [PubMed] [Google Scholar]
- Wilusz CJ., and, Wilusz J. Bringing the role of mRNA decay in the control of gene expression into focus. Trends Genet. 2004;20:491–497. doi: 10.1016/j.tig.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Dong H., and, Woo SL. Hepatic insulin production for type 1 diabetes. Trends Endocrinol Metab. 2001;12:441–446. doi: 10.1016/s1043-2760(01)00491-x. [DOI] [PubMed] [Google Scholar]
- Shih HM, Liu Z., and, Towle HC. Two CACGTG motifs with proper spacing dictate the carbohydrate regulation of hepatic gene transcription. J Biol Chem. 1995;270:21991–21997. doi: 10.1074/jbc.270.37.21991. [DOI] [PubMed] [Google Scholar]
- Dutheil N, Shi F, Dupressoir T., and, Linden RM. Adeno-associated virus site-specifically integrates into a muscle-specific DNA region. Proc Natl Acad Sci USA. 2000;97:4862–4866. doi: 10.1073/pnas.080079397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abai AM, Hobart PM., and, Barnhart KM. Insulin delivery with plasmid DNA. Hum Gene Ther. 1999;10:2637–2649. doi: 10.1089/10430349950016672. [DOI] [PubMed] [Google Scholar]
- Chalkley GE., and, Verrijzer CP. DNA binding site selection by RNA polymerase II TAFs: a TAF(II)250-TAF(II)150 complex recognizes the initiator. EMBO J. 1999;18:4835–4845. doi: 10.1093/emboj/18.17.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generation of synthetic promoter. (a) Schematic diagram of new multiple-cloning sites in the pcDNA3.1 plasmid. Two complementary oligonucleotides containing restriction enzymes sites for Kpn I-BamH I-EcoR V-EcoR I were synthesized and subcloned into the Xho I/Nhe I sites to replace the original multiple-cloning sites containing EcoR V-EcoR I-Kpn I-Hind III. (b) Nucleotide sequences for the cis -elements used for generation of synthetic promoters.
Luciferase activities of synthetic promoters in rat hepatoma cells. (a) Activities of 3-element synthetic promoters. All 3-element modules were transferred to a luciferase reporter vector generating 3-element SP-Luc, followed by luciferase assay. (b) 6-element synthetic promoters that had more than 7.5% of CMV promoter activity. Luciferase activities of synthetic promoter constructs were calculated as percentage of CMV promoter activity. All values are means ± S.D. L-PK, pLPK-Luc plasmid; h, HNF-1 binding element; c, C/EBP binding element; g, glucose-responsive element.
The arrangement of cis-elements. (a) 6-element SP-Luc plasmids that showed over 7.5% of CMV promoter activity and (b) 9-element SP-Luc plasmid that showed over 21% of CMV promoter activity.
Survival rate after virus administration. rAd-CMV-rINSfur or rAd-SP23137-rINSfur viruses were injected at the indicated dosage into STZ-treated NOD.scid mice.