Abstract
Mucopolysaccharidosis VI (MPS VI) is caused by deficient arylsulfatase B (ARSB) activity resulting in lysosomal storage of glycosaminoglycans (GAGs). MPS VI is characterized by dysostosis multiplex, organomegaly, corneal clouding, and heart valve thickening. Gene transfer to a factory organ like liver may provide a lifetime source of secreted ARSB. We show that intravascular administration of adeno-associated viral vectors (AAV) 2/8-TBG-felineARSB in MPS VI cats resulted in ARSB expression up to 1 year, the last time point of the study. In newborn cats, normal circulating ARSB activity was achieved following delivery of high vector doses (6 × 1013 genome copies (gc)/kg) whereas delivery of AAV2/8 vector doses as low as 2 × 1012 gc/kg resulted in higher than normal serum ARSB levels in juvenile MPS VI cats. In MPS VI cats showing high serum ARSB levels, independent of the age at treatment, we observed: (i) clearance of GAG storage, (ii) improvement of long bone length, (iii) reduction of heart valve thickness, and (iv) improvement in spontaneous mobility. Thus, AAV2/ 8-mediated liver gene transfer represents a promising therapeutic strategy for MPS VI patients.
Introduction
Mucopolysaccharidosis VI (MPS VI) or Maroteaux–Lamy syndrome, is an autosomal recessive lysosomal storage disease caused by deficient activity of arylsulfatase B (ARSB) resulting in lysosomal storage of the glycosaminoglycan (GAG) dermatan sulfate.1 The disorder is characterized by growth retardation and skeletal dysplasia, joint stiffness, organomegaly, heart valve dysfunction, and corneal clouding.1 Despite physical disabilities and the wide range of musculoskeletal problems, MPS VI patients typically have normal intelligence.1,2 MPS VI diagnosis is usually made in early childhood when clinical features become apparent.
Enzyme replacement therapy (ERT) is the current treatment for MPS VI,3,4 relying on the ability of ARSB to be taken-up by most cells via the mannose-6-phosphate receptor pathway.5 However, ERT is associated with inconvenient weekly infusions of costly recombinant enzyme.6 In addition, despite amelioration of the visceral phenotype, related to significant reduction in tissue GAG storage, ERT shows limited efficacy for several MPS VI features such as the ocular and skeletal abnormalities.4,7 To some extent the issue here is early intervention and correct dosing.8,9 Ocular pathology is unlikely to be treated with ERT. Alternative strategies with similar or better therapeutic outcomes and increased patient compliance to treatment are desirable.
Several MPS VI animal models are available to allow testing of novel therapeutic approaches. There are the naturally occurring MPS VI rat10 and cat models11 bearing spontaneous mutations in the ARSB gene and closely mimicking the human disease, and an ARSB knockout mouse has been generated.12 MPS VI rats carry the 507insC ARSB frameshift mutation which results in the generation of a premature stop codon and no ARSB protein.10,13 MPS VI cats are homozygous for the L476P ARSB missense mutation resulting in production of unstable ARSB protein.11
We have recently tested a gene therapy approach for MPS VI based on systemic delivery of adeno-associated viral (AAV) vectors13,14 serotype 8 (AAV2/8) which efficiently target liver.15,16,17 Thus, a single treatment could convert the liver in a factory for secretion of therapeutic ARSB.
We have shown that systemic delivery of AAV2/8 vectors resulted in long-term production of therapeutic ARSB levels in MPS VI animals.13,14 However, in the null MPS VI rats, development of a neutralizing humoral immune response to the ARSB transgene product resulted in reduced levels of circulating ARSB after gene transfer and limited therapeutic efficacy.13 This was overcome by the use of immunosuppressant drugs with gene transfer resulting in high ARSB expression levels and rescue of the MPS VI rat phenotype.14 Our study, as well as those of others testing gene therapy for MPS,18,19,20 points to the requirement for high and stable serum enzyme levels to obtain therapeutic efficacy in tissues refractory to ERT, such as bone. Our preliminary results in MPS VI cats, bearing a homozygous missense mutation resulting in production of inactive ARSB,11 showed that close to normal serum ARSB activity levels can be obtained after neonatal liver AAV2/8-mediated gene transfer without an immune response to the transgene product.13
Therefore, we tested the effect of various AAV2/8 vector doses and the age of treatment on ARSB circulating levels and phenotype rescue in MPS VI cats with the goal of transferring this strategy to MPS VI patients.
Results
Long-term expression of therapeutic ARSB levels in newborn and juvenile MPS VI cats systemically administered with AAV2/8 vectors
Newborn (postnatal day 5, p5) and juvenile (p50) MPS VI cats were injected with various doses of AAV2/8-TBG-fARSB vectors encoding for feline ARSB (fARSB) under the control of the liver-specific thyroxin binding globulin (TBG) promoter,21 with or without the woodchuck hepatitis virus post-transcriptional regulatory element (WPRE22). As control, cats received AAV2/8-TBG-eGFP vector either at p5 (6 × 1013 genome copies (gc)/kg) or at p50 (6 × 1012 gc/kg).
ARSB activity levels were measured in sera from injected and control animals for up to 1 year after gene delivery, when cats were sacrificed for postmortem analysis (Figure 1). In newborn cats injected with 6 × 1013 gc/kg of AAV2/8-TBG-fARSB, serum ARSB activity peaked about 10 days after injection, declined to levels similar to those measured in normal cats (NR), and remained stable for the duration of the study (Figure 1a). Animals receiving lower vector doses (ranging from 3 × 1012 to 3 × 1013 gc/kg, Figure 1a) showed enzyme activity levels <50% of NR levels (on average 4.6 ± 0.8 nmol/ml/hour in the group receiving 3 × 1013 gc/kg, P < 0.05 versus affected cats, AF) or similar to those measured in AF (in the groups receiving either 6 × 1012 or 3 × 1012 gc/kg: 2.5 and 2.4 ± 0.1 nmol/ml/hour, respectively, P < 0.05 versus AF for the group receiving 3 × 1012 gc/kg). Given their low serum ARSB activity levels, these animals were excluded from the following analysis of phenotypic rescue.
Figure 1.
Serum ARSB activity levels in MPS VI cats treated with AAV2/8-TBG-fARSB. MPS VI cats were injected either at (a) postnatal day 5 (p5) or at (b–e) p50 with various doses of AAV2/8-TBG-fARSB (as reported inside each panel). Serum ARSB activity was monitored for 12 months after vector administration. Each curve represents ARSB values over time from a single animal. In cats injected at (a) p5 ARSB activity levels similar to those measured in normal cats (NR) were obtained in animals receiving 6 × 1013 genome copies (gc/kg) of AAV2/8-TBG-fARSB vectors. In animals injected at (b–e) p50, ARSB activity levels similar or above NR were achieved more consistently in the 6 × 1013–2 × 1012 gc/kg vector dose range. AF, untreated MPS VI cats; °, animals receiving AAV vector constructs containing the WPRE element. Arrows point at the time point of AAV vectors injection. The number of animals analyzed in each group is reported inside each panel. AAV, adeno-associated viral; ARSB, arylsulfatase B; MPS VI, mucopolysaccharidosis VI; TBG, thyroxin binding globulin.
In contrast, in animals injected at p50 we measured higher than NR serum ARSB activity levels in several (but not all) cats receiving AAV vector doses between 2 × 1012 and 6 × 1013 gc/kg (Figure 1b,c). With lower AAV vector doses (2 × 1011 and 6 × 1011 gc/kg, Figure 1d,e), most injected animals showed circulating enzyme levels similar to AF, with only one cat in each group reaching ARSB activity in the NR levels range (Figure 1d, 6 × 1011 gc/kg) or slightly below it (Figure 1e, 2 × 1011 gc/kg). There was no relationship between the inclusion of WPRE in our constructs and the ARSB transduction efficiency (Figure 1). As expected, AF cats receiving AAV-eGFP vectors showed serum ARSB activity similar to untreated AF animals (data not shown). Thus, we obtained stable, long-term ARSB expression and secretion after AAV-mediated liver-directed gene transfer in MPS VI cats. High (normal or above) serum ARSB activity was measured more consistently in juvenile animals receiving vector doses in the 2 × 1012–6 × 1013 gc/kg range whereas in newborn cats normal circulating ARSB activity levels were exclusively obtained following systemic delivery of high vector doses (6 × 1013 gc/kg). Notably, the high serum ARSB activity levels obtained in some AAV vector-treated cats suggest that transduced hepatocytes can produce large amounts of active ARSB protein. However, since ARSB, like any other sulfatase, requires the post-translational activation operated by the sulfatase modifying factor 1,23 we cannot exclude that sulfatase modifying factor 1 levels in transduced hepatocytes may limit active ARSB production, thus potentially explaining the nonlinear relationship between the serum ARSB levels we observe and the AAV vector doses injected in MPS VI cats.
Importantly, as we previously reported,13 MPS VI cats injected with AAV2/8 vectors did not develop immune responses to the ARSB transgene product (Supplementary Figure S1).
To confirm long-term transgene expression, we measured ARSB enzyme activity, GAG levels, and AAV vector gc in livers from 12-month-old injected and control (NR and AF cats uninjected or receiving the AAV-eGFP vector) animals (Table 1). As expected, we observed higher than NR liver ARSB activity in several AAV-injected cats. (Table 1). This resulted in reduction of liver GAG storage in treated animals, which was more efficient in animals receiving higher vector doses (6 × 1012–6 × 1013 gc/kg), independent of the age the cat at treatment (Table 1). In addition, persistence of liver transduction was confirmed by the presence of detectable AAV vector gc at the end of the study. In general, animals receiving higher vector doses and having higher serum enzyme activity have higher liver ARSB activity and AAV vector genome copy numbers (Table 1).
Table 1. ARSB activity, GAG, and liver vector genome copies in MPS VI cats treated with AAV vectors.
Amelioration of biochemical, visceral, and skeletal anomalies in MPS VI cats treated with AAV2/8 vectors
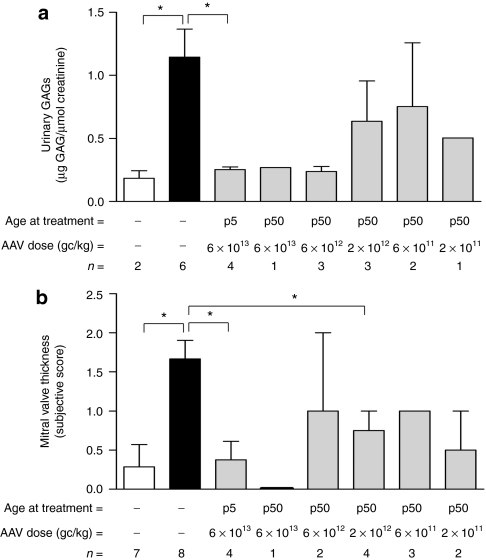
To assess the uptake of circulating ARSB protein by tissues of MPS VI cats treated with AAV vectors, we measured ARSB as well as GAG levels in spleen and kidney, and urine. Tissue ARSB activity was increased in most cats treated with AAV vectors compared to AF (Table 1). Consistent with levels of serum ARSB activity, higher ARSB levels were measured in the spleen (Table 1) and kidney (data not shown) of cats receiving AAV vector doses between 2 × 1012 and 6 × 1013 gc/kg at p50 or 6 × 1013 gc/kg at p5; reduction of GAG storage was evident in tissues from most treated cats independent of the tissue enzyme levels (Table 1 and data not shown), confirming than even minor increases in ARSB activity were sufficient to reduce GAG storage in visceral tissues of MPS VI animals, as previously reported.13,14 However, complete GAG clearance was only observed in animals receiving higher vector doses and showing serum ARSB levels in or above the NR range. Urinary GAGs were reduced in several AAV vector-treated cats, and their levels inversely correlate with serum enzyme levels (R = 0.61, P = 0.005). Complete normalization of urinary GAG excretion was obtained in cats receiving AAV vector doses between 6 × 1012 and 6 × 1013 gc/kg with statistically significant reduction observed in those treated at p5; animals receiving lower vector doses showed GAG levels lower than AF but higher than NR (Figure 2a).
Figure 2.
Reduced urinary GAG excretion and mitral valve thickness in MPS VI cats treated with AAV2/8-TBG-fARSB. (a) Urinary GAG levels were measured in 12-month-old normal (NR, white bar), affected (AF1, black bar) and MPS VI cats receiving different AAV2/8-TBG-fARSB vector doses (reported under each bar) either at p5 or at p50. Urinary GAGs were reduced in animals receiving AAV compared to AF. Stronger reduction was obtained in animals receiving AAV vector doses between 6 × 1013 and 6 × 1012 gc/kg (p50) or 6 × 1013 gc/kg (p5). (b) Heart ultrasound analysis of 10-month-old NR, AF, and MPS VI cats treated with AAV vectors. Mitral valve thickness (MVT, subjective score) was significantly increased in AF compared to NR cats. MVT reduction was obtained in cats treated with AAV2/8-TBG-fARSB, independent of the age at treatment and of the AAV vector dose injected. Results are reported as mean ± SE when n > 1; *P value ≤0.05. The number of animals analyzed in each group (n=), the vector doses used and the age at treatment are reported under each bar. AAV, adeno-associated viral; fARSB, feline arylsulfatase B; GAG, glycosaminoglycan; gc, genome copies; MPS VI, mucopolysaccharidosis VI; p5, cats injected at postnatal day 5; p50, cats injected at postnatal day 50; TBG, thyroxin binding globulin.
Heart valve dysfunction is a common feature of MPS VI patients and animal models due to extensive GAG accumulation in the valve leaflets.24,25 To assess the impact of our gene transfer therapeutic approach on heart disease in MPS VI cats, we performed echocardiographic analysis in 9–12-month-old treated and control animals. Subjective echocardiographic assessments were scored from 0 (normal or none) to 4 (severe).24 As previously reported, MPS VI cats showed significant mitral valve thickening24 which was reduced and in some cases normalized in several treated animals, independent of the AAV vector dose injected or of the age of the cat at treatment (Figure 2b); statistically significant mitral valve thickening improvements are evident in the groups receiving 6 × 1013 gc/kg at p5 and 2 × 1012 gc at p50.
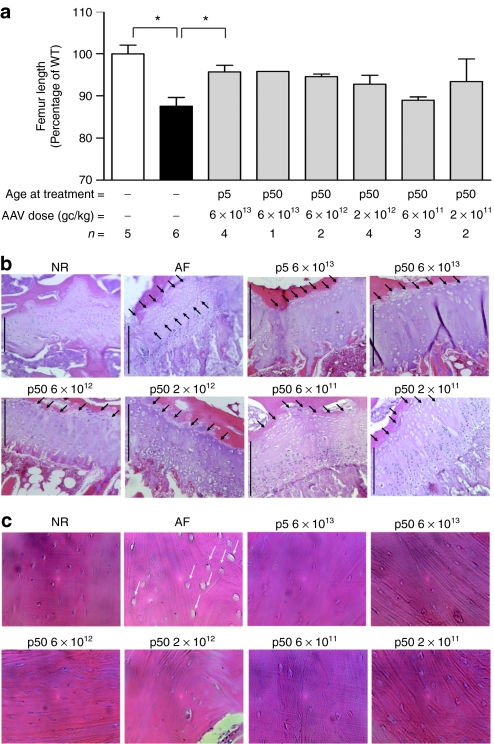
The MPS VI phenotype is characterized by severe skeletal and joint abnormalities both in patients and animal models. We have previously reported that high serum ARSB activity levels (≥50% of NR levels) were required to obtain correction of skeletal abnormalities in MPS VI rats.14 To confirm this and to assess the impact of liver gene transfer on the skeletal phenotype in MPS VI cats, we performed radiographic analysis of long bones from 10- to 12-month-old treated and control animals and measured femur and humerus length. As shown in Figure 3a, AF cats had a significant reduction of femur length compared to age- and sex-matched NR. We observed improved femur length in AAV vector-treated animals (Figure 3a), which was more consistent in cats receiving high vector doses and who showed serum ARSB activity levels in or above the NR range. Significant increases in femur length over AF cats were only observed in the group receiving 6 × 1013 gc/kg at p5, possibly due to the high variability in serum ARSB activity observed among cats injected at p50. Similar results were obtained when analyzing humeri (data not shown). Qualitative analysis of cervical spine, coxofemoral joints, stifle joints, and long bone abnormalities were performed on radiographs from treated and control cats and a score was assigned to each parameter analyzed as previously reported in other MPS animal models (see Materials and Methods section for details).26 Scores resulting from the sum of single scores assigned to various regions of the same pelvic and cervical regions were calculated for each cat and were significantly increased in AF compared to NR cats (Supplementary Figure S2 and data not shown). As observed for long bone length, improvement in pelvic score was observed in AAV vector-treated animals having serum ARSB activity levels similar to or above NR (Supplementary Figure S2). In contrast, no significant improvement was obtained on cervical score (data not shown). GAG storage has been reported in chondrocytes from articular cartilage and growth plate as well as in cortical bone osteocytes in MPS VI.12,14 In addition, long bone growth plates showed variable degrees of structural abnormalities in different MPS VI animal models.12,14,27 In the MPS VI cat, resting zone thickening and abnormal vacuolization of chondrocytes represent a consistent abnormality.27,28 To analyze GAG storage in bone and cartilage and in the growth plate structure, we examined hematoxylin and eosin-stained histological sections of long bones from treated and control MPS VI cats. These sections showed extensive vacuolization, representing lysosomal storage, in AF cortical bone osteocytes and in chondrocytes from the resting zone of the growth plate (Figure 3b,c and Supplementary Figure S3) and from the articular cartilage (data not shown). We observed clearance of GAG storage in cortical bone osteocytes and improvement of the growth plate resting zone thickness and storage in MPS VI cats receiving high vector doses (juvenile administered with 2 × 1012–6 × 1013 gc/kg and newborns administered with 6 × 1013 gc/kg). In contrast, MPS VI cats receiving low vector doses (2 × 1011–6 × 1011, p50) showed minor reduction of osteocyte vacuolization (Figure 3c) and no improvement in the growth plate structure (Figure 3b and Supplementary Figure S3), confirming that high levels of circulating ARSB are required to impact the skeletal phenotype in MPS VI, as we previously reported.14 No reduction of GAG storage in articular cartilage was observed in MPS VI cats independent of the AAV vector dose administered (data not shown). The extent of improvement of long bone anomalies observed was independent of the time of vector administration (p5 or p50), confirming our previous observation that amelioration of skeletal abnormalities in juvenile MPS VI animals is feasible.14
Figure 3.
Improvement of bone size and pathology in MPS VI cats receiving AAV2/8-TBG-fARSB. (a) Femur length in MPS VI cats receiving various doses of AAV2/8-TBG-fARSB vectors. Radiographs were obtained on 10–12-month-old normal (NR), affected (AF), and MPS VI cats treated with AAV vectors and femur length was measured. The femur length, reported as percentage of age- and sex-matched NR cats, was significantly reduced in AF cats and was increased in most animals, independent of the age the cat at treatment. Results are reported as mean ± SE when n > 1; *P value ≤0.05. The number of animals analyzed in each group (n =), the vector doses used and the age at treatment are reported under each bar. White bar: normal cats; black bars: affected cats. (b,c) Representative pictures of (b) femur growth plate and (c) cortical bone histology from NR, AF, and MPS VI cats treated with AAV vectors. GAG storage, evident as vacuoles in cortical bone osteocytes (c, white arrows) and in the growth plate resting zone chondrocytes (b, black arrows) of AF cats was reduced in MPS VI animals receiving AAV vector doses between 6 × 1013 and 2 × 1012 gc/kg (p50) or 6 × 1013 (p5). Cats receiving lower vector doses showed only a minor reduction in osteocyte storage. Black lines in each panel delineate the growth plate. Magnification: ×10 for growth plate, ×40 for cortical bone. AAV, adeno-associated viral; fARSB, feline arylsulfatase B; GAG, glycosaminoglycan; gc, genome copies; MPS VI, mucopolysaccharidosis VI; p5, cats injected at postnatal day 5; p50, cats injected at postnatal day 50; TBG, thyroxin binding globulin; WT, wild type.
Our results suggest that efficient correction of MPS VI visceral and skeletal phenotype can be obtained after liver-directed ARSB gene transfer in MPS VI cats. To further support this observation, we assessed the gross appearance and motor activity of treated versus control animals. As already reported, MPS VI cats had smaller bodies, shortened limbs, deformed backs, and flattened muzzles when compared to NR animals (Figure 4 and Supplementary Figure S4). AAV vector-treated cats receiving AAV vector doses between 2 × 1012 and 6 × 1013 gc/kg (p50) or 6 × 1013 gc/kg (p5) were more similar to NR than to AF animals whereas those receiving lower vector doses showed only minor improvements in gross appearance, as expected from the biochemical and skeletal phenotype data reported above (Figure 4 and Supplementary Figure S4).
Figure 4.
Improved facial dysmorphism in MPS VI cats treated with AAV vectors. Representative pictures of muzzles from control and MPS VI cats receiving AAV2/8-TBG-fARSB at p5 or at p50. Affected cats (AF) showed flattened muzzle and shortened ear (arrows) when compared to normal (NR) animals. Animals receiving high AAV vector doses [6 × 1013–2 × 1012 gc/kg at p50 (AAV-p50) or 6 × 1013 gc/kg at p5 (AAV-p5)] showed amelioration of both ear and muzzle appearence. AAV, adeno-associated viral; gc, genome copies; MPS VI, mucopolysaccharidosis VI.
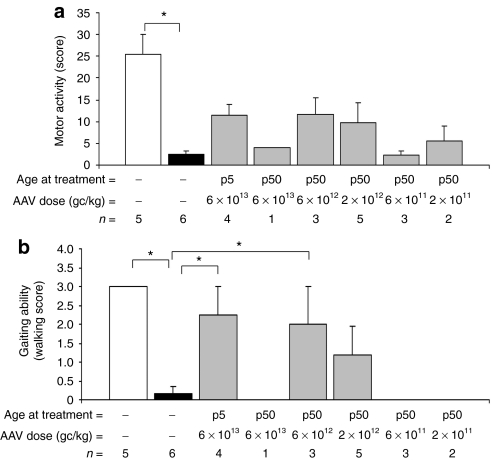
To test whether the biochemical and skeletal improvements we observed following gene transfer impact on the animal spontaneous motility, we set up a behavioral testing session for each cat, and scored various spontaneous and stimulus-elicited behavioral patterns. A motor activity score was assigned to each animal (see Materials and Methods section for details) based on the cats' ability to spontaneously walk, run, or jump. While NR cats performed well in each task without major differences among sexes or animals, AF cats were significantly impaired (Figure 5a). MPS VI cats treated with AAV vectors tend to perform better than AF: despite the lack of statistically significant differences, the motor activity score correlated with circulating ARSB levels (R = 0.63, P = 0.002), with higher scores recorded for cats receiving high vector doses and having circulating ARSB activity levels in or above the NR range. Notably, the total motor activity score takes into account various complex motor activities. When analyzing separately the gaiting ability (i.e., walking, a landmark test in ERT clinical studies3,29,30,31) we observed that MPS VI cats receiving 6 × 1013 gc/kg at p5 and 6 × 1012 gc/kg at p50 have a walking score significantly improved compared to AF animals (6 × 1013 gc/kg at p5; P = 0.006 and 6 × 1012 gc/kg at p50; P = 0.02) whereas not significantly different from NR animals (Figure 5b). Thus, AAV vector-mediated liver gene transfer improves motor ability and exploratory behavior in MPS VI cats, as it is immediately evident from Supplementary Videos S1–S4.
Figure 5.
Improved motor activity in MPS VI cats treated with AAV2/8-TBG-fARSB. (a) Evaluation of spontaneous motor activity in 9–12-month-old control (NR and AF) and AAV vector-treated MPS VI cats. A motor activity score was assigned to each animal based on the ability to spontaneously walk, run, step up, or jump (see Materials and Methods section for details). Animal mobility was significantly reduced in affected cats (AF, black bar) compared to normal (NR, white bar). Higher scores, albeit not significantly different from AF, were observed in cats receiving AAV vector doses between 6 × 1013 and 2 × 1012 gc/kg (p50) or 6 × 1013 gc/kg (p5). (b) Evaluation of walking score in 9–12-month-old control and AAV vector-treated MPS VI cats. A walking score was assigned to each animal based on the ability to spontaneously walk with a normal posture; this is significantly reduced in AF as compared to NR and significantly improved in cats receiving high vector doses. Results are reported as mean ± SE when n > 1; *P value ≤0.05. The number of animals analyzed in each group (n=), the vector doses used and the age at treatment are reported under each bar. AAV, adeno-associated viral; AF, affected cats; fARSB, feline arylsulfatase B; GAG, glycosaminoglycan; gc, genome copies; MPS VI, mucopolysaccharidosis VI; NR, normal untreated cats; p5, cats injected at postnatal day 5; p50, cats injected at postnatal day 50; TBG, thyroxin binding globulin.
Discussion
We have previously reported that AAV vector-mediated, liver-directed ARSB gene transfer can provide high and stable levels of circulating enzyme after a single vector administration in MPS VI animal models13,14 resulting in significant amelioration of the MPS VI phenotype. However, we observed that neutralizing humoral immune responses to the ARSB recombinant protein developed in MPS VI rats with no residual ARSB protein production, limiting therapeutic efficacy.13,14
Here, we show that close to normal ARSB activity levels are obtained after AAV vector-mediated liver-directed gene transfer in the absence of immune responses to the transgene product in MPS VI cats, bearing a homozygous missense ARSB mutation that produces unstable ARSB protein.11 This seems more likely related to the presence of residual endogenous ARSB protein in MPS VI cats than to the immune tolerance to the transgene product reported after AAV vector-mediated liver gene transfer,32,33,34,35 as we observed a systemic neutralizing humoral response to ARSB following gene transfer in ARSB-null MPS VI rats.13,14
Importantly, ≈80% of MPS VI patients bear missense ARSB mutations,36 potentially resulting in production of crossreactive immune material (CRIM). Our data suggest that a single AAV2/8 vector administration may be beneficial in CRIM+ MPS VI patients.
In newborn MPS VI cats treated with AAV2/8 vectors, close to NR serum ARSB activity levels were obtained in animals receiving high vector doses (6 × 1013 gc/kg). In addition, shortly after AAV vectors administration, serum ARSB levels were on average 30-fold higher than normal (ranging from 3.8- to 88-fold the activity measured in NR cats) and shortly dropped to normal levels (Figure 1a). We hypothesized that this could be due to AAV vector dilution as result of hepatocyte proliferation occurring in newborn (p5) liver, as also suggested by other studies.37 To help support this conclusion and to obtain therapeutic circulating ARSB levels at AAV2/8 vector doses lower than 6 × 1013 gc/kg, we injected MPS VI cats at p50 and we obtained higher than NR serum ARSB activity levels with AAV vector doses as low as 2 × 1012 gc/kg. In addition, the MPS VI cat injected at p50 with the highest vector dose (6 × 1013 gc/kg) showed higher serum and liver ARSB activity as well as higher liver AAV vector genome copy number than MPS VI newborn cats injected with the same dose (5.3-fold difference in gc, Table 1, correlating with a 4.5-fold increase in weight between p5 and p50 MPS VI cats). Taken together, these data suggests that vector dilution may occur when administering AAV2/8 vectors to MPS VI cats at p5. This is advantageous as later treatment is more applicable to MPS VI patients who usually will not be diagnosed at birth.
We observed variability in liver gene transfer efficacy (serum ARSB activity) in AAV-injected juvenile cats, which increased at lower vector doses. This variability was not due to AAV vectors preparation differences nor to the presence or absence of the WPRE (Figure 1 and data not shown). In addition, we could not detect anti-ARSB antibodies (Ab) in MPS VI cats treated with AAV vectors (Supplementary Figure S1), nor were the animals with low circulating ARSB levels females, as some studies suggest that AAV vector-mediated liver gene transfer is more efficient in males than females.38,39 Instead, it has been reported that even low titers of circulating neutralizing Ab to AAV8 can significantly reduce the efficacy of AAV vectors-mediated liver gene transfer.40 Unfortunately, we did not collect preinjection sera from our animals to measure neutralizing Ab pre-existing to AAV2/8 vector administration. However, we found some animals in the MPS VI cat colony who had low serum neutralizing activity to AAV8 infection in vitro, particularly in breeding females with kittens treated with AAV vector. Indeed, a queen whose kittens were treated at p5 had low (1:5) titer neutralizing anti-AAV8 serum activity; three subsequent kittens from this same queen were injected at p50 with 2 × 1012 gc/kg and showed low serum ARSB activity levels (Figure 1c). Thus, the presence of pre-existing neutralizing Ab to AAV8 from colostrum from a queen previously exposed to AAV2/8 vector-treated kittens cannot be ruled out in the animals who did not respond to AAV vector treatment. One additional possibility to explain the variability we have observed in AAV vector-mediated transduction is the MPS VI cat mixed genetic background.41
Despite variability, we obtained high (NR or above) circulating ARSB activity levels in 10 MPS VI cats (Figure 1), either injected at birth or at p50, which allowed us to assess the impact of liver ARSB gene transfer on the feline MPS VI phenotype. Liver transduced by AAV vectors efficiently secreted therapeutic levels of ARSB that was taken-up by affected tissues resulting in GAG clearance (Table 1) as well as reduction of urinary GAG excretion (Figure 2a). In some tissues, i.e., kidney and spleen, where we have previously observed AAV2/8 vector distribution upon systemic administration13 and low level gene expression (G. Cotugno, P. Annunziata, and A. Auricchio, unpublished results), ARSB activity and GAG clearance may be the result of both circulating enzyme uptake and low levels local enzyme production.
GAG clearance obtained after AAV vector administration resulted in amelioration of heart anomalies (reduction of mitral valve thickness, Figure 2b) and skeletal abnormalities (improved long bone length and appearance, and reduced GAG storage in osteocytes and chondrocytes, Figure 3a–c and Supplementary Figures S2 and S3) independently of the age the cat at treatment. Interestingly, the amelioration of heart valve abnormalities reported here differs from what we previously reported in MPS VI rats, where serum ARSB activity levels higher than 50% of NR were required to impact on heart valve GAG storage,14 whereas in AAV vector-treated MPS VI cats even low serum enzyme levels resulted in reduced mitral valve thickness (2 × 1011–6 × 1011 group, p50, Figure 2b). However, the analyses we performed in the two models are different and heart valve GAG storage is not always predictive of functional valve abnormalities or mitral valve thickening.42 In addition, variability related to the extent of heart lesions in different MPS VI animal species could explain this difference.
We previously reported that serum ARSB activity levels higher than 50% of NR allowed complete clearance of GAG storage in osteocytes and chondrocytes from both growth plate and articular cartilage in MPS VI rats. In MPS VI cats, despite high serum ARSB activity and reduction of GAG storage in both osteocytes and growth plate chondrocytes, all treated animals showed persistent chondrocyte vacuolization in the articular cartilage (Figure 3b,c, Supplementary Figure S3, and data not shown); this could be due to interspecies differences (i.e., articular cartilage nutrition and ARSB diffusion). Despite persistent articular chondrocyte storage, AAV vector-treated cats showed improved walking posture and general motility likely related to reduced storage in soft tissues surrounding the joints not improvement in articular cartilage lesions.
The improvements of visceral and skeletal abnormalities obtained here in MPS VI cats treated with AAV vectors are similar and in some cases superior to those obtained by repeated enzyme infusions.9,43,44 Indeed, clearance of visceral GAG storage is efficiently obtained with either ERT43 or liver ARSB gene transfer (Table 1), even when low serum enzyme levels are achieved. Interestingly, amelioration of several skeletal anomalies (i.e., radiographic changes of cervical spine and long bones,43 long bones length,44 and gross appearance43) is consistently obtained following gene transfer but not ERT43,44 suggesting that liver ARSB gene transfer may represent an alternative to ERT especially considering the high frequency of enzyme infusions. Some issues like the presence of pre-existing anti-AAV2/8 Ab in the human population45 or the ability to produce 1012–1013 AAV vector gc/kg for human applications will need to be addressed to further progress this strategy toward the clinic.
In conclusion, we report that liver-directed, AAV2/8 vector-mediated ARSB gene transfer resulted in efficient, stable, long-term secretion of therapeutic levels of ARSB in MPS VI cats. This therapy allowed significant improvement of MPS VI in juvenile animals and could potentially be translated to human patients diagnosed beyond the newborn period.
Materials and Methods
Animal colony. The feline MPS VI colony was maintained at the University of Pennsylvania, School of Veterinary Medicine. Animals were raised under National Institutes of Health and US Department of Agriculture guidelines for the care and use of animals in research.
AAV vector production and administration. Therapeutic AAV2/8-TBG-fARSB (with and without the WPRE element13) and control AAV2/8-TBG-eGFP46 vectors were produced by the AAV Vector Core of the Telethon Institute of Genetics and Medicine (TIGEM, Naples, Italy) by triple transfection of 293 cells and purified by CsCl gradients.47 Physical titers of the viral preparations (gc/ml) were determined by real-time PCR (Applied Biosystems, Foster City, CA)48 and dot blot analysis.
MPS VI cats were injected by catheter with various doses (reported in the Results section) of AAV2/8-TBG-fARSB or AAV2/8-TBG-eGFP vectors in a jugular vein at postnatal day 5 (P5) or in a cephalic vein at p50. Two cats in the groups receiving 2 × 1012 and 2 × 1011 gc/kg of AAV2/8-TBG-fARSB at p50 died before the end of the study and thus were not subjected to subsequent analysis.
Blood and tissue collection. Blood was collected every month from treated and control cats and centrifuged at 10,000g in an Eppendorf centrifuge for 10 minutes at 4 °C to obtain the serum.
The cats were sacrificed at the end of the study (12 months following AAV administration) and visceral tissues, urine, and long bones were collected.
Tissues were frozen in dry ice (for ARSB activity, GAG quantitative assays, and measurement of vector gc). Knee joints were fixed in buffered 10% formalin (Sigma-Aldrich, Milan, Italy).
ARSB activity assays and protein levels. Tissues were homogenized in water and protein concentrations were determined using the BCA protein assay reagent (Pierce Chemical, Boston, MA).
The ARSB assay was performed as described previously.13,49 For tissue lysates, 30 µg of protein were incubated with 40 µl of the fluorogenic substrate, 4-methylumbelliferyl-sulfate (12.5 mmol/l; Sigma-Aldrich), for 3 hours at 37 °C in the presence of 40 µl of silver nitrate to inhibit activity by other sulfatases (0.75 mmol/l; Carlo Erba, Milan, Italy). The reaction was stopped by adding 200 µl of the carbonate stop buffer (0.5 mol/l NaHCO3/0.5 mol/l Na2CO3, pH 10.7), and the fluorescence of the 4-methylumbelliferone produced was measured in a multiplate fluorimeter (Tecan, Mannedorf, Switzerland) using 365 nm excitation and 460 nm emission. The enzyme activities were calculated using a standard curve of the fluorogenic product, 4-methylumbelliferone (Sigma-Aldrich). For tissue lysates, the activity is expressed as nmol/mg protein/hour. For serum samples, enzyme activity was measured as follows: 15 µl serum was diluted with 285 µl H2O and 100 µl of diluted serum was incubated in the following solution: 5 mmol/l 4-methylumbelliferyl sulfate, 0.05 mol/l sodium acetate pH 5.6, 0.3 mmol/l silver nitrate, 3 mmol/l lead acetate (Sigma-Aldrich). Samples were incubated for 2 hours at 37 °C and the reaction was stopped by addition of 2 ml of carbonate stop buffer. Fluorescence was then measured with a VersaFluor Fluorometer (BioRad, Hercules, CA), Serum ARSB activity is expressed as nmol/ml/hour. For comparison of ARSB activity among experimental groups the serum enzyme activity measured over time was averaged for each cat and the resulting value was then averaged for each group when n > 1.
Assay for the detection of anti-ARSB Ab. The presence of circulating Ab against the fARSB protein in MPS VI cats was determined as follows: cellular lysates containing fARSB protein were produced by transfecting 293 cells with an expression plasmid encoding for fARSB; sera from control uninjected cats and AAV vector-treated animals were diluted 1:100 in 0.2 mol/l sodium acetate containing 0.05% Tween 20 and incubated with fARSB lysates overnight at 4 °C. As a positive control anti-hARSB antibody50 was incubated with fARSB lysates; a negative control was obtained by incubating the cellular lysate with buffer solution (0.2 mol/l sodium acetate, 0.05% Tween 20). To precipitate fARSB-antibody complexes, 20 µl of protein A/G-agarose (Santa Cruz Biotechnology, Santa Cruz, CA) was added and samples incubated under rotation for 4 hours at 4 °C. ARSB-Ab complexes were precipitated by centrifugation at 1,000g for 5′. Supernatant was removed and the pellet resuspended in 50 µl of 0.2 mol/l sodium acetate. ARSB activity was then measured as described. The ARSB activity measured in each sample is reported. Increased activity in pellet represents an indirect measure of the presence of anti-ARSB Ab in the sample.
AAV vector genome distribution. Genomic DNA was extracted from snap frozen tissues after lysis in a buffer containing 10 mmol/l Tris, 10 mmol/l EDTA, 0.6% SDS, 200 ng/µl proteinase k (Qiagen, Washington, DC). RNAseA (Qiagen) was added at a final concentration of 10 µg/µl and samples incubated at 37 °C for 1 hour followed by two phenol/clorophorm extractions. DNA was then precipitated by adding 2.5 volumes of EtOH and incubating at −80 °C for 2 hours. Samples were centrifuged at 14,000 r.p.m. for 1 hour, DNA pellets were washed in 70% EtOH and resuspended in water.
Real-time PCR analysis was performed on 100-ng genomic DNA using a set of primers/probe-specific for the viral genome and Taqman universal PCR master mix (Applied Biosystems).13 Amplification was run on a 7300 Real-Time PCR system (Applied Biosystems) with standard cycles. All the reactions were performed in triplicate.
Quantitative analysis of GAG accumulation in tissues and urine. Urine samples from treated and control cats were collected at the end of the study and were diluted in water tenfold for measurement of GAG content.
Fifty micro liter of diluted urine or 250 µg of protein extract from the liver, spleen, and kidney were used for the GAG assay, as previously described.13 The GAG concentrations were determined using the dermatan sulfate standard curve (Sigma-Aldrich). Tissue GAGs are expressed as µg GAG/µg protein. Urine GAGs were normalized to the creatinine content. Creatinine was measured using a Creatinine Assay Kit (Quidel, San Diego, CA). The urinary GAGs are expressed as µg GAG/µmol creatinine. The correlation between serum ARSB activity and urinary GAG levels was evaluated with simple regression; the correlation coefficient R with the corresponding P value is reported.
Echocardiographic analysis. Complete echocardiograms were obtained (including 2-D, M-mode, and color flow Doppler of all valves) by an expert Board-certified veterinary cardiologist (Meg. M. Sleeper) using a Philips 7500 Sonos ultrasound machine (Wilmington, MA) and a 12 MHz transducer. The mitral and aortic valves were interrogated with color flow Doppler. A subjective score was assigned for thickness of the mitral valve and increased echogenicity and/or thickening of the valve leaflets was used to develop a subjective valve thickening grade. The subjective scores ranged from 0 to 4 with 0 = normal/none; 4 = severe. Two cats in the groups receiving 2 × 1012 and 6 × 1012 gc/kg of AAV2/8-TBG-fARSB at p50 were not subjected to echocardiographic analysis because of technical problems.
Radiograph analyses. Radiographs were taken on 10–12-month-old treated and control MPS VI cats using a General Electric MPH high frequency multipulse generator with automatic exposure control radiology machine. The length of the femur and humerus were measured on digital radiograph with the iSite Enterprise 3.5 software (Stentor; Philips Medical System, Foster City, CA) and reported as % of the length of each bone in sex- and age-matched normal cats.
Qualitative analysis of cervical and pelvic anomalies was also performed on digital radiographs by a Board-certified veterinary radiologist (Van W. Knox) who was blinded to the genotype and treatment of the animals. For most categories, radiographs were scored on a 0–2 scale, where 0 represented normal, 1 represented a moderate abnormality, and 2 represented a severe abnormality. The coxofemoral joint was scored for laxity, dysplasia, articular cartilage erosions, and degenerative joint disease. The stifle joint was scored for patellar luxation, effusions, dysplasia, and articular cartilage erosions. The cervical spine was scored for beaking, tipping, thickening, articular facet fusion/proliferation, and vertebral body shortening/dysplasia. Two cats in the groups receiving 2 × 1012 and 6 × 1012 gc/kg of AAV2/8-TBG-fARSB at p50 were not subjected to radiographic analysis because of technical problems.
Bone histology. Formalin-fixed bones were decalcified in 8% formic acid (Sigma-Aldrich), dehydrated, embedded in paraffin, and sectioned in 7 µm sections. For histological analysis, sections were stained with hematoxylin and eosin (Richard-Allen Scientific, Kalamazoo, MI; Sigma-Aldrich) with standard procedures.
Spontaneous mobility assessment. For assessment of spontaneous mobility, cats were recorded in a vacant animal housing room with fluorescent lighting. The size of the room was 4.5 m wide and 6 m long. A platform (1 m wide, 2 m long) was placed in the room and covered with a cloth backdrop. Multiple video sessions occurred throughout the year to match the ages of the cats as close to each other as possible (about 9 months of age). During these sessions, there was one operator controlling the camera and another operator stimulating the cats to either play, walk, or climb on a platform. To stimulate cats a small cat toy (filled with catnip and cotton) was used. The toy was attached to a 1-m long plastic pole via a 1 m long string and was used to initiate forward movement, stimulate the cats to step up onto the platform and observe the stretching capability of the cat's forelimb. On a few occasions, another cat was used to bait animals across the platform. The length of the video was about 5–10 minutes total per cat.
Videos were evaluated by an expert in animal behavior (Fabio Russo) who was blinded to the genotype and treatment of the animals. Ten behavioral patterns were analyzed: walking (that is the animal walks with the right posture standing up both hind limbs), running, spontaneous leaning, stepping up, step-up jumping, spontaneous head movement, tailing up, two forelimbs movement while sitting, two forelimbs while standing, self-body rotations. These behavioral categories were selected because commonly occurring in all normal animals during the behavioral session. For each task, a score was assigned as follows:
never: the cat does not perform the movement (score = 0)
sometimes: the cat performs once or twice the movement (score = 1)
often: the cat performs the movement three or more times (score = 3)
For each animal, the motor activity score was calculated as the sum of the single scores assigned to each task and values for all cats in each group were analyzed with one-way ANOVA for statistically significant differences. The Fisher least significant difference post hoc was used to make comparison among groups. Correlation between circulating ARSB (average of serum ARSB activity measured during all the study) and motor activity score was made using simple regression; the correlation coefficient R with the corresponding P value is reported.
Statistical analyses. An unpaired t-test between the various data sets, except for the motor activity score analysis (see previous paragraph), was performed using the Microsoft Excel (Microsoft, Redmond, VA) t-test function. Significance at P ≤ 0.05 is indicated by a single asterisk in the figures and table.
SUPPLEMENTARY MATERIAL Figure S1. MPS VI cats treated with AAV vectors do not develop anti-ARSB antibodies. To assess the presence of anti-ARSB antibodies in the serum of cats treated with AAV vectors, sera from MPS VI animals receiving AAV vectors either at p5 or at p50 (INJ cats) were incubated with lysates from 293 cells transfected with a plasmid expressing the fARSB protein. Samples were immunoprecipitated with protein A/G and ARSB activity was measured on the immunopurified complexes. As controls, sera from NR and AF cats that did not receive AAV vectors were used (CTR cats). No significant increase in serum anti-ARSB antibodies levels was observed in cats treated with AAV vectors compared to controls. pos CTR: fARSB lysates incubated with anti-ARSB antibodies; neg CTR: fARSB lysates incubated with buffer solution. Results are reported as mean ± SE. Figure S2. Long bone and joint abnormalities in MPS VI cats treated with AAV2/8-TBG-fARSB. Hind-limb pathological score in control and MPS VI cats treated with AAV vectors. Hind-limb abnormalities were evaluated by analysis of digital radiographs. The coxofemoral joint was scored for laxity, dysplasia, articular cartilage erosions, and degenerative joint disease. The stifle joint was scored for patellar luxation, effusions, dysplasia, and articular cartilage erosions. For each parameter, a score between 0 (normal) and 2 (severely abnormal) was assigned. The sum of all scores was calculated for each animal and averaged for each group. Results are reported as mean ± SE when n>1; *: p-value ≤ 0.05. The number of animals analyzed in each group (n=), the vector doses used and the age at treatment are reported under each bar. White bar: normal cats, black bars: affected cats. Figure S3. Reduced GAG storage in growth plate chondrocytes in MPS VI cats treated with AAV vectors. High magnification (40x) of femur growth plate histology from NR, AF, and MPS VI cats treated with AAV vectors shown in figure 3b. GAG storage, evident as vacuoles in the growth plate resting zone chondrocytes of AF cats was reduced in MPS VI animals receiving AAV vector doses between 6x1013 and 2x1012 gc/kg (p50) or 6x1013 (p5). Figure S4. Improved gross appearance in MPS VI cats treated with AAV vectors. Representative pictures of control and MPS VI cats receiving various doses of AAV2/8-TBG-fARSB (reported under each panel). Affected cats (AF) showed smaller bodies, shortened limbs, deformed backs, and flattened muzzles (black arrows) when compared to normal (NR) animals. Animals receiving high AAV vector doses (6x1013 − 2x1012 gc/kg at p50 or 6x1013 gc/kg at p5, reported under each panel) appeared more similar to NR than to AF cats. Video S1. Representative video from a normal (NR) cat. Video S2. Representative video from an affected (AF) MPS VI cat. Video S3. Representative video from an MPS VI cat injected at p5 with 6×1013 gc/kg of AAV2/8-TBG-fARSB. The best performing cat from this group is shown. Video S4. Representative video from an MPS VI cat injected at p50 with 6×1012 gc/kg of AAV2/8-TBG-fARSB. The best performing cat from this group is shown.
Acknowledgments
The TIGEM AAV Vector Core produced the vectors used in this study. This work was supported by the European Union, 7th Frame Program “Euclyd—a European Consortium for Lysosomal Storage Diseases” grant 201678, and NIH grants DK25759 and RR02512. This work was done in Naples, Italy and in Philadelphia, PA, USA. The authors declared no conflict of interest.
Supplementary Material
MPS VI cats treated with AAV vectors do not develop anti-ARSB antibodies. To assess the presence of anti-ARSB antibodies in the serum of cats treated with AAV vectors, sera from MPS VI animals receiving AAV vectors either at p5 or at p50 (INJ cats) were incubated with lysates from 293 cells transfected with a plasmid expressing the fARSB protein. Samples were immunoprecipitated with protein A/G and ARSB activity was measured on the immunopurified complexes. As controls, sera from NR and AF cats that did not receive AAV vectors were used (CTR cats). No significant increase in serum anti-ARSB antibodies levels was observed in cats treated with AAV vectors compared to controls. pos CTR: fARSB lysates incubated with anti-ARSB antibodies; neg CTR: fARSB lysates incubated with buffer solution. Results are reported as mean ± SE.
Long bone and joint abnormalities in MPS VI cats treated with AAV2/8-TBG-fARSB. Hind-limb pathological score in control and MPS VI cats treated with AAV vectors. Hind-limb abnormalities were evaluated by analysis of digital radiographs. The coxofemoral joint was scored for laxity, dysplasia, articular cartilage erosions, and degenerative joint disease. The stifle joint was scored for patellar luxation, effusions, dysplasia, and articular cartilage erosions. For each parameter, a score between 0 (normal) and 2 (severely abnormal) was assigned. The sum of all scores was calculated for each animal and averaged for each group. Results are reported as mean ± SE when n>1; *: p-value ≤ 0.05. The number of animals analyzed in each group (n=), the vector doses used and the age at treatment are reported under each bar. White bar: normal cats, black bars: affected cats.
Reduced GAG storage in growth plate chondrocytes in MPS VI cats treated with AAV vectors. High magnification (40x) of femur growth plate histology from NR, AF, and MPS VI cats treated with AAV vectors shown in figure 3b. GAG storage, evident as vacuoles in the growth plate resting zone chondrocytes of AF cats was reduced in MPS VI animals receiving AAV vector doses between 6x1013 and 2x1012 gc/kg (p50) or 6x1013 (p5).
Improved gross appearance in MPS VI cats treated with AAV vectors. Representative pictures of control and MPS VI cats receiving various doses of AAV2/8-TBG-fARSB (reported under each panel). Affected cats (AF) showed smaller bodies, shortened limbs, deformed backs, and flattened muzzles (black arrows) when compared to normal (NR) animals. Animals receiving high AAV vector doses (6x1013 − 2x1012 gc/kg at p50 or 6x1013 gc/kg at p5, reported under each panel) appeared more similar to NR than to AF cats.
Representative video from a normal (NR) cat.
Representative video from an affected (AF) MPS VI cat.
Representative video from an MPS VI cat injected at p5 with 6×1013 gc/kg of AAV2/8-TBG-fARSB. The best performing cat from this group is shown.
Representative video from an MPS VI cat injected at p50 with 6×1012 gc/kg of AAV2/8-TBG-fARSB. The best performing cat from this group is shown.
REFERENCES
- Neufeld E., and, Muenzer J. Scriver. McGraw-Hill: New York; 2001. The mucopolysaccharidoses. [Google Scholar]
- Hopwood JJ., and, Morris CP. The mucopolysaccharidoses. Diagnosis, molecular genetics and treatment. Mol Biol Med. 1990;7:381–404. [PubMed] [Google Scholar]
- Harmatz P, Giugliani R, Schwartz I, Guffon N, Teles EL, Miranda MC, et al. Enzyme replacement therapy for mucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J Pediatr. 2006;148:533–539. doi: 10.1016/j.jpeds.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Desnick RJ., and, Schuchman EH. Enzyme replacement and enhancement therapies: lessons from lysosomal disorders. Nat Rev Genet. 2002;3:954–966. doi: 10.1038/nrg963. [DOI] [PubMed] [Google Scholar]
- Dahms NM, Lobel P., and, Kornfeld S. Mannose 6-phosphate receptors and lysosomal enzyme targeting. J Biol Chem. 1989;264:12115–12118. [PubMed] [Google Scholar]
- Schlander M., and, Beck M. Expensive drugs for rare disorders: to treat or not to treat? The case of enzyme replacement therapy for mucopolysaccharidosis VI. Curr Med Res Opin. 2009;25:1285–1293. doi: 10.1185/03007990902892633. [DOI] [PubMed] [Google Scholar]
- Pitz S, Ogun O, Arash L, Miebach E., and, Beck M. Does enzyme replacement therapy influence the ocular changes in type VI mucopolysaccharidosis. Graefes Arch Clin Exp Ophthalmol. 2009;247:975–980. doi: 10.1007/s00417-008-1030-1. [DOI] [PubMed] [Google Scholar]
- McGill JJ, Inwood AC, Coman DJ, Lipke ML, de Lore D, Swiedler SJ, et al. Enzyme replacement therapy for mucopolysaccharidosis VI from 8 weeks of age–a sibling control study. Clin Genet. 2010;77:492–498. doi: 10.1111/j.1399-0004.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- Crawley AC, Niedzielski KH, Isaac EL, Davey RC, Byers S., and, Hopwood JJ. Enzyme replacement therapy from birth in a feline model of mucopolysaccharidosis type VI. J Clin Invest. 1997;99:651–662. doi: 10.1172/JCI119208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Noguchi J, Ikadai H, Takahashi M., and, Nagase S. Arylsulfatase B-deficient mucopolysaccharidosis in rats. J Clin Invest. 1993;91:1099–1104. doi: 10.1172/JCI116268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogalingam G, Litjens T, Bielicki J, Crawley AC, Muller V, Anson DS, et al. Feline mucopolysaccharidosis type VI. Characterization of recombinant N-acetylgalactosamine 4-sulfatase and identification of a mutation causing the disease. J Biol Chem. 1996;271:27259–27265. doi: 10.1074/jbc.271.44.27259. [DOI] [PubMed] [Google Scholar]
- Evers M, Saftig P, Schmidt P, Hafner A, McLoghlin DB, Schmahl W, et al. Targeted disruption of the arylsulfatase B gene results in mice resembling the phenotype of mucopolysaccharidosis VI. Proc Natl Acad Sci USA. 1996;93:8214–8219. doi: 10.1073/pnas.93.16.8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Faella A, O'Malley T, Cotugno G, Doria M, Kunieda T, et al. Biochemical, pathological, and skeletal improvement of mucopolysaccharidosis VI after gene transfer to liver but not to muscle. Mol Ther. 2008;16:30–37. doi: 10.1038/sj.mt.6300325. [DOI] [PubMed] [Google Scholar]
- Cotugno G, Tessitore A, Capalbo A, Annunziata P, Strisciuglio C, Faella A, et al. Different serum enzyme levels are required to rescue the various systemic features of the mucopolysaccharidoses. Hum Gene Ther. 2010;21:555–569. doi: 10.1089/hum.2009.189. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J., and, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Lu Y, Calcedo R, Grant RL, Bell P, Wang L, et al. Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Mol Ther. 2006;13:77–87. doi: 10.1016/j.ymthe.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Mango RL, Sands MS, Haskins ME, Ellinwood NM., and, Ponder KP. Evaluation of pathological manifestations of disease in mucopolysaccharidosis VII mice after neonatal hepatic gene therapy. Mol Ther. 2002;6:745–758. doi: 10.1006/mthe.2002.0809. [DOI] [PubMed] [Google Scholar]
- Liu Y, Xu L, Hennig AK, Kovacs A, Fu A, Chung S, et al. Liver-directed neonatal gene therapy prevents cardiac, bone, ear, and eye disease in mucopolysaccharidosis I mice. Mol Ther. 2005;11:35–47. doi: 10.1016/j.ymthe.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Ma X, Liu Y, Tittiger M, Hennig A, Kovacs A, Popelka S, et al. Improvements in mucopolysaccharidosis I mice after adult retroviral vector-mediated gene therapy with immunomodulation. Mol Ther. 2007;15:889–902. doi: 10.1038/sj.mt.6300112. [DOI] [PubMed] [Google Scholar]
- Ill CR, Yang CQ, Bidlingmaier SM, Gonzales JN, Burns DS, Bartholomew RM, et al. Optimization of the human factor VIII complementary DNA expression plasmid for gene therapy of hemophilia A. Blood Coagul Fibrinolysis. 1997;8 Suppl 2:S23–S30. [PubMed] [Google Scholar]
- Loeb JE, Cordier WS, Harris ME, Weitzman MD., and, Hope TJ. Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Hum Gene Ther. 1999;10:2295–2305. doi: 10.1089/10430349950016942. [DOI] [PubMed] [Google Scholar]
- Cosma MP, Pepe S, Annunziata I, Newbold RF, Grompe M, Parenti G, et al. The multiple sulfatase deficiency gene encodes an essential and limiting factor for the activity of sulfatases. Cell. 2003;113:445–456. doi: 10.1016/s0092-8674(03)00348-9. [DOI] [PubMed] [Google Scholar]
- Sleeper MM, Kusiak CM, Shofer FS, O'Donnell P, Bryan C, Ponder KP, et al. Clinical characterization of cardiovascular abnormalities associated with feline mucopolysaccharidosis I and VI. J Inherit Metab Dis. 2008;31:424–431. doi: 10.1007/s10545-008-0821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangel JH. Cardiovascular changes in children with mucopolysaccharide storage diseases and related disorders–clinical and echocardiographic findings in 64 patients. Eur J Pediatr. 1998;157:534–538. doi: 10.1007/s004310050872. [DOI] [PubMed] [Google Scholar]
- Herati RS, Knox VW, O'Donnell P, D'Angelo M, Haskins ME., and, Ponder KP. Radiographic evaluation of bones and joints in mucopolysaccharidosis I and VII dogs after neonatal gene therapy. Mol Genet Metab. 2008;95:142–151. doi: 10.1016/j.ymgme.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall JD, Brumfield LK, Fazzalari NL, Hopwood JJ., and, Byers S. Histomorphometric analysis of the tibial growth plate in a feline model of mucopolysaccharidosis type VI. Calcif Tissue Int. 1999;65:47–52. doi: 10.1007/s002239900656. [DOI] [PubMed] [Google Scholar]
- Abreu S, Hayden J, Berthold P, Shapiro IM, Decker S, Patterson D, et al. Growth plate pathology in feline mucopolysaccharidosis VI. Calcif Tissue Int. 1995;57:185–190. doi: 10.1007/BF00310256. [DOI] [PubMed] [Google Scholar]
- Harmatz P, Giugliani R, Schwartz IV, Guffon N, Teles EL, Miranda MC, et al. Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: Final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase. Mol Genet Metab. 2008;94:469–475. doi: 10.1016/j.ymgme.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Harmatz P, Ketteridge D, Giugliani R, Guffon N, Teles EL, Miranda MC, et al. Direct comparison of measures of endurance, mobility, and joint function during enzyme-replacement therapy of mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): results after 48 weeks in a phase 2 open-label clinical study of recombinant human N-acetylgalactosamine 4-sulfatase. Pediatrics. 2005;115:e681–e689. doi: 10.1542/peds.2004-1023. [DOI] [PubMed] [Google Scholar]
- Harmatz P, Whitley CB, Waber L, Pais R, Steiner R, Plecko B, et al. Enzyme replacement therapy in mucopolysaccharidosis VI (Maroteaux-Lamy syndrome) J Pediatr. 2004;144:574–580. doi: 10.1016/j.jpeds.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Cooper M, Nayak S, Hoffman BE, Terhorst C, Cao O., and, Herzog RW. Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Hum Gene Ther. 2009;20:767–776. doi: 10.1089/hum.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeberl DD, Sun BD, Damodaran TV, Brown T, Millington DS, Benjamin DK, Jr, et al. Early, sustained efficacy of adeno-associated virus vector-mediated gene therapy in glycogen storage disease type Ia. Gene Ther. 2006;13:1281–1289. doi: 10.1038/sj.gt.3302774. [DOI] [PubMed] [Google Scholar]
- Franco LM, Sun B, Yang X, Bird A, Zhang H, Schneider A, et al. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther. 2005;12:876–884. doi: 10.1016/j.ymthe.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litjens T., and, Hopwood JJ. Mucopolysaccharidosis type VI: Structural and clinical implications of mutations in N-acetylgalactosamine-4-sulfatase. Hum Mutat. 2001;18:282–295. doi: 10.1002/humu.1190. [DOI] [PubMed] [Google Scholar]
- Flageul M, Aubert D, Pichard V, Nguyen TH, Nowrouzi A, Schmidt M, et al. Transient expression of genes delivered to newborn rat liver using recombinant adeno-associated virus 2/8 vectors. J Gene Med. 2009;11:689–696. doi: 10.1002/jgm.1343. [DOI] [PubMed] [Google Scholar]
- Pañeda A, Vanrell L, Mauleon I, Crettaz JS, Berraondo P, Timmermans EJ, et al. Effect of adeno-associated virus serotype and genomic structure on liver transduction and biodistribution in mice of both genders. Hum Gene Ther. 2009;20:908–917. doi: 10.1089/hum.2009.031. [DOI] [PubMed] [Google Scholar]
- Davidoff AM, Ng CY, Zhou J, Spence Y., and, Nathwani AC. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood. 2003;102:480–488. doi: 10.1182/blood-2002-09-2889. [DOI] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Wang H, Bell P, Grant R, Vandenberghe LH, et al. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol Ther. 2010;18:126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins ME, Jezyk PF., and, Patterson DF. Mucopolysaccharide storage disease in three families of cats with arylsulfatase B deficiency: leukocyte studies and carrier identification. Pediatr Res. 1979;13:1203–1210. doi: 10.1203/00006450-197911000-00001. [DOI] [PubMed] [Google Scholar]
- Strauch OF, Stypmann J, Reinheckel T, Martinez E, Haverkamp W., and, Peters C. Cardiac and ocular pathologies in a mouse model of mucopolysaccharidosis type VI. Pediatr Res. 2003;54:701–708. doi: 10.1203/01.PDR.0000084085.65972.3F. [DOI] [PubMed] [Google Scholar]
- Crawley AC, Brooks DA, Muller VJ, Petersen BA, Isaac EL, Bielicki J, et al. Enzyme replacement therapy in a feline model of Maroteaux-Lamy syndrome. J Clin Invest. 1996;97:1864–1873. doi: 10.1172/JCI118617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers S, Nuttall JD, Crawley AC, Hopwood JJ, Smith K., and, Fazzalari NL. Effect of enzyme replacement therapy on bone formation in a feline model of mucopolysaccharidosis type VI. Bone. 1997;21:425–431. doi: 10.1016/s8756-3282(97)00175-0. [DOI] [PubMed] [Google Scholar]
- Calcedo R, Vandenberghe LH, Gao G, Lin J., and, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auricchio A, Hildinger M, O'Connor E, Gao GP., and, Wilson JM. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum Gene Ther. 2001;12:71–76. doi: 10.1089/104303401450988. [DOI] [PubMed] [Google Scholar]
- Xiao W, Chirmule N, Berta SC, McCullough B, Gao G., and, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Qu G, Burnham MS, Huang J, Chirmule N, Joshi B, et al. Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum Gene Ther. 2000;11:2079–2091. doi: 10.1089/104303400750001390. [DOI] [PubMed] [Google Scholar]
- Chang PL, Rosa NE., and, Davidson RG. Differential assay of arylsulfatase A and B activities: a sensitive method for cultured human cells. Anal Biochem. 1981;117:382–389. doi: 10.1016/0003-2697(81)90795-8. [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Grasby DJ, Dean CJ, Lang DL, Bockmann M, Whittle AM, et al. Newborn screening for lysosomal storage disorders. Mol Genet Metab. 2006;88:307–314. doi: 10.1016/j.ymgme.2006.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MPS VI cats treated with AAV vectors do not develop anti-ARSB antibodies. To assess the presence of anti-ARSB antibodies in the serum of cats treated with AAV vectors, sera from MPS VI animals receiving AAV vectors either at p5 or at p50 (INJ cats) were incubated with lysates from 293 cells transfected with a plasmid expressing the fARSB protein. Samples were immunoprecipitated with protein A/G and ARSB activity was measured on the immunopurified complexes. As controls, sera from NR and AF cats that did not receive AAV vectors were used (CTR cats). No significant increase in serum anti-ARSB antibodies levels was observed in cats treated with AAV vectors compared to controls. pos CTR: fARSB lysates incubated with anti-ARSB antibodies; neg CTR: fARSB lysates incubated with buffer solution. Results are reported as mean ± SE.
Long bone and joint abnormalities in MPS VI cats treated with AAV2/8-TBG-fARSB. Hind-limb pathological score in control and MPS VI cats treated with AAV vectors. Hind-limb abnormalities were evaluated by analysis of digital radiographs. The coxofemoral joint was scored for laxity, dysplasia, articular cartilage erosions, and degenerative joint disease. The stifle joint was scored for patellar luxation, effusions, dysplasia, and articular cartilage erosions. For each parameter, a score between 0 (normal) and 2 (severely abnormal) was assigned. The sum of all scores was calculated for each animal and averaged for each group. Results are reported as mean ± SE when n>1; *: p-value ≤ 0.05. The number of animals analyzed in each group (n=), the vector doses used and the age at treatment are reported under each bar. White bar: normal cats, black bars: affected cats.
Reduced GAG storage in growth plate chondrocytes in MPS VI cats treated with AAV vectors. High magnification (40x) of femur growth plate histology from NR, AF, and MPS VI cats treated with AAV vectors shown in figure 3b. GAG storage, evident as vacuoles in the growth plate resting zone chondrocytes of AF cats was reduced in MPS VI animals receiving AAV vector doses between 6x1013 and 2x1012 gc/kg (p50) or 6x1013 (p5).
Improved gross appearance in MPS VI cats treated with AAV vectors. Representative pictures of control and MPS VI cats receiving various doses of AAV2/8-TBG-fARSB (reported under each panel). Affected cats (AF) showed smaller bodies, shortened limbs, deformed backs, and flattened muzzles (black arrows) when compared to normal (NR) animals. Animals receiving high AAV vector doses (6x1013 − 2x1012 gc/kg at p50 or 6x1013 gc/kg at p5, reported under each panel) appeared more similar to NR than to AF cats.
Representative video from a normal (NR) cat.
Representative video from an affected (AF) MPS VI cat.
Representative video from an MPS VI cat injected at p5 with 6×1013 gc/kg of AAV2/8-TBG-fARSB. The best performing cat from this group is shown.
Representative video from an MPS VI cat injected at p50 with 6×1012 gc/kg of AAV2/8-TBG-fARSB. The best performing cat from this group is shown.