Abstract
Sustained, targeted, high-level transgene expression in primary B lymphocytes may be useful for gene therapy in B cell disorders. We developed several candidate B-lineage predominant self-inactivating lentiviral vectors (LV) containing alternative enhancer/promoter elements including: the immunoglobulin β (Igβ) (B29) promoter combined with the immunoglobulin µ enhancer (EµB29); and the endogenous BTK promoter with or without Eµ (EµBtkp or Btkp). LV-driven enhanced green fluorescent protein (eGFP) reporter expression was evaluated in cell lines and primary cells derived from human or murine hematopoietic stem cells (HSC). In murine primary cells, EµB29 and EµBtkp LV-mediated high-level expression in immature and mature B cells compared with all other lineages. Expression increased with B cell maturation and was maintained in peripheral subsets. Expression in T and myeloid cells was much lower in percentage and intensity. Similarly, both EµB29 and EµBtkp LV exhibited high-level activity in human primary B cells. In contrast to EµB29, Btkp and EµBtkp LV also exhibited modest activity in myeloid cells, consistent with the expression profile of endogenous Bruton's tyrosine kinase (Btk). Notably, EµB29 and EµBtkp activity was superior in all expression models to an alternative, B-lineage targeted vector containing the EµS.CD19 enhancer/promoter. In summary, EµB29 and EµBtkp LV comprise efficient delivery platforms for gene expression in B-lineage cells.
Introduction
B cells represent an important target for gene transfer because single gene defects impacting B-lineage function have significant roles in the pathogenesis of immunodeficiency and autoimmunity.1 One B cell disorder predicted to benefit from safe strategies for gene delivery is X-linked agammaglobulinemia (XLA). XLA is an immunodeficiency caused by a recessive gene defect in Bruton's tyrosine kinase (Btk),2 that results in a block in B cell development at the pro-B cell stage, reduced numbers of circulating B cells, and a near absence of antibody responses in affected males.3 XLA is a good candidate for gene therapy for several reasons: a relatively high disease frequency, the ability to treat without interruption of clinical therapy, and a strong selective advantage for gene-corrected cells.4,5 In order to facilitate such therapies, we focused on designing a lentiviral vector (LV) optimized to drive gene expression in primary B cells, with a special emphasis on vectors that mimic the expression profile of endogenous Btk.
Self-inactivating LVs (LV) comprise a promising gene delivery platform for treatment of genetic disorders, autoimmune diseases, and malignancies. In contrast to γ-retroviruses, LVs proficiently target nondividing cells such as multipotent hematopoietic stem cells (HSC) at low viral copy number.6,7,8 Self-inactivating-LV also limit the risk of viral long-terminal repeat enhancer mutagenesis and concurrently permit the use of lineage-specific promoters.9 Furthermore, there is evidence of less transcriptional silencing of internal promoters within integrated LV, and a reduced bias for integration near transcription start sites.10 These combined features likely reduce the overall risk of viral enhancer-mutagenesis responsible for adverse events in several γ-retroviral clinical trials.11,12 Previous work has demonstrated efficient transgene expression with LV utilizing a range of internal promoter and other regulatory elements.8,13,14
To decrease potential side effects of nonspecific transgene expression in HSC-derived lineages, various vectors have been designed to restrict transgene expression to one or several lineages. For example, specific regulatory elements have been used for targeted expression within erythroid, T, antigen-presenting and myeloid cells, respectively.15,16,17,18 Notably, Moreau et al. showed that LV incorporating the regulatory sequences from the human CD19 promoter allowed preferential transgene expression in B-lineage cells.19 Importantly, addition of the immunoglobulin heavy chain µ intronic enhancer (Eµ) and its associated matrix attachment regions both significantly increased gene expression and promoted uniformity of expression compared with either the PGK20 or CD19 minimal promoters alone.21,22 However, the levels of expression obtained with the B-restricted LV reported to date are low in comparison with that mediated by promiscuous γ-retroviral-derived enhancer/promoter elements. Thus, it has remained unclear as to whether candidate B cell-specific LV will mediate transgene expression at levels that will restore function in B-lineage disorders, an important consideration as low levels of B-lineage Btk expression is insufficient to rescue murine models of XLA.23,24
In the studies described here, we sought to design LVs that will mimic the expression pattern of endogenous Btk, with the ultimate goal of utilizing such constructs for gene therapy in XLA patients. We generated LV containing three alternative B cell-specific internal enhancer/promoters and evaluated their ability to drive enhanced green fluorescent protein (eGFP) reporter gene expression. As a reference point, we compared the specificity of expression driven by these elements to a previously described B cell-specific LV, EµS.CD19.21 In parallel, each was compared to a LV containing a strong murine leukemia virus-derived, enhancer/promoter, myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted (MND), capable of driving high-level transgene expression in all hematopoietic lineages,25 and used in a recent successful LV clinical trial.14 LVs were compared with respect to relative expression in cell lines and primary murine and human cells at low viral copy number. We found that both EµB29 and EµBtkp LV preferentially direct high-level, sustained, transgene expression in immature and mature B lymphoid cells, and support future testing of these constructs in XLA gene therapy models.
Results
Design of B-lineage targeted LV
As shown schematically in Figure 1, we generated a number of LV-containing alternative enhancer/promoter elements within the parental pRRL backbone.9 The first, MND-eGFP, incorporated a ubiquitous, γ-retroviral enhancer/promoter. In comparison to other mammalian-derived ubiquitous elements such as elongation factor 1α or PGK, MND drives much higher expression levels in primary murine and human cells providing a benchmark for very high-level gene expression (data not shown). Second, we generated a construct equivalent to the previously reported, B cell-specific vector, EµS.CD19,19 comprised of the minimal CD19 promoter (230 bp) in conjunction with the Eµ enhancer. While it has been reported that inclusion of a larger portion of the CD19 promoter element (1,274 bp—designated EµL.CD19) generates higher expression levels in B cells, we found little difference in expression levels between these promoters when in association with Eµ (Supplementary Figure S1a). Next, we generated EµB29-eGFP LV, which contains the B29 (Igβ, CD79b) minimal promoter element26 in association with the murine immunoglobulin heavy chain, intronic enhancer, Eµ.27 B29, a key signaling component of the B cell antigen receptor, is initially expressed in pro-B cells and its expression increases in immature/mature B cells.26,28,29 Because the B29 promoter alone exhibited only low-level basal expression (data not shown), the Eµ enhancer was added to increase activity in maturing B cells. The total size of the enhancer/promoter elements for EµB29 and EµS.CD19 were nearly identical. Finally, we created two unique LV constructs designated Btkp-eGFP and EµBtkp-eGFP, that contain regulatory elements derived from the human BTK locus either alone or in combination with the Eµ enhancer, respectively. The 37 kb BTK locus is comprised of 19 exons at Xq22.30,31 Transcriptional start sites of human Btk were previously identified within −5 and −30 bp of exon 1 with a minimal promoter critical for lineage-restricted expression between −200 and −1 bp from the transcriptional start site.32,33,34 Additional reporter studies implicated putative regulatory elements between −450 and −200, as well as two conserved NF-κB binding sites within −800 to −600, as positive regulators of Btk expression.35 We therefore used the first 788 bp of the Btk promoter including these elements, hypothesizing that Btkp, in conjunction with the Eµ enhancer, might provide strong B cell-predominant expression and also retain endogenous control elements essential for lineage-appropriate Btk expression.
Figure 1.
Schematic of lentiviral constructs. LV were constructed using a pRRL backbone containing an enhancer-deleted U3 region to generate a self-inactivating (SIN) LTR and differ only in their internal promoter/enhancer region. MND is a retroviral LTR-derived ubiquitous promoter that drives transgene expression in most hematopoietic lineages. Both EµS.CD19-eGFP and EµL.CD19-eGFP employ regulatory sequences from human CD19 and EµB29-eGFP utilizes a murine B29 minimal promoter. Btkp-eGFP LV and EµBtkp-eGFP LV contain a788 bp element derived from endogenous human BTK locus (described in Materials and Methods section). EµB29, EµS.CD19, EµL.CD19, and EµBtkp each also include the murine immunoglobulin intronic enhancer, Eµ. Btk, Bruton's tyrosine kinase; eGFP, enhanced green fluorescent protein; LV, lentiviral vectors; LTR, long-terminal repeats; MND, myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted; wPRE, woodchuck hepatitis virus posttranscriptional regulatory element.
EµB29 LV drives B-lineage predominant expression that increases with cell maturation
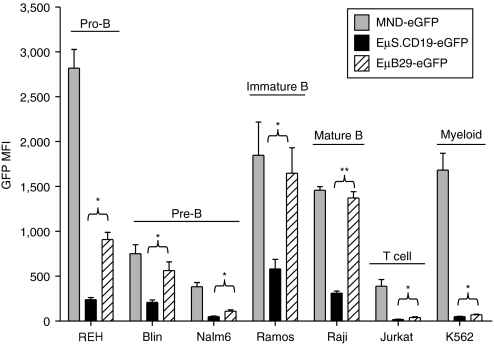
We first compared the expression of eGFP driven by EµB29, EµS.CD19, and MND LV in human T (Jurkat), myeloid (K562), and B cell lines including a panel of different B developmental stages: pro-B (REH), pre-B (Blin and Nalm6), immature B (Ramos), and mature B (Raji) cells. Transduction rates were adjusted via matching eGFP marking to ~10–15% in order to evaluate expression mediated by mostly single viral-integration. In this setting, eGFP mean fluorescent intensity (MFI) provides a direct measure of relative promoter strength. Compared to MND, both EµB29 and EµS.CD19 LV exhibited B cell-predominant eGFP expression with the highest expression levels in more mature B cell lines (Figure 2). Importantly, eGFP MFI was significantly higher in all B cell lines transduced with EµB29 compared to EµS.CD19 LV; with the greatest expression in lines representative of mature stages, e.g., Ramos and Raji B cells. Substitution of the extended CD19 promoter (to generate EµL.CD19 LV) resulted in slightly higher levels of eGFP compared with EµS.CD19 LV (Supplementary Figure S1a). However, the expression level for both constructs remained significantly lower than EµB29 LV (Supplementary Figure S1b).
Figure 2.
EµB29 exhibits increased levels of transgene expression compared to EµS.CD19 in B cell lines. A panel of human cell lines was transduced with MND-eGFP (gray bars), EµS.CD19-eGFP (black bars) or EµB29-eGFP (striped bars) LV to achieve 10–15% eGFP marking. Cells were evaluated on day 4 for eGFP expression by FACS. B cell lines selected were derived from a representative of different developmental stages: REH (pro-B), Blin or Nalm6 (pre-B), Ramos (immature B), and Raji (mature B). Expression was also compared using Jurkat (T) and K562 (myeloid) cell lines. Data shows mean fluorescence intensity (MFI) of eGFP in each line based upon three experiments. *indicate statistically significant results in this and all subsequent figures, with * <0.05, ** <0.01 and *** <0.001. Only the statistically significant data is highlighted in this manner. eGFP, enhanced green fluorescent protein; FACS, fluorescence-activated cell sorting; MND, myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted.
EµB29 drives high-level eGFP expression in primary murine B-lymphocytes
To assess expression and lineage specificity in vivo, congenically marked, B6.Ly5.1 recipient mice were lethally irradiated and transplanted with B6.Ly5.2, Lin− HSC transduced with either: MND-eGFP; EµS.CD19-eGFP; or EµB29-eGFP LV. Mice with donor chimerism of >90% were sacrificed 25 weeks post-transplant and spleen and bone marrow (BM) cells analyzed for relative eGFP expression in myeloid and lymphocyte subsets (Figures 3 and 4). Figure 3a,b shows representative data from a single experiment (3–5 recipients/group). Of note, the MFI of eGFP expression across independent experiments was influenced by differences in the instrument used for analysis and/or the voltage settings of the laser used to detect eGFP (488 nm argon). While this limited our ability to present a combined dataset, we observed consistent relative differences among our panel of LV constructs in multiple experiments. For comparison, the results from a second independent experiment are displayed in Figure 3c and representative fluorescence-activated cell sorting (FACS) plots comparing B-lineage promoter activity for EµB29 versus EµS.CD19 LV from both experiments are shown in Supplementary Figure S2. Analysis of B cells, T cells, neutrophils and macrophages revealed expression by in all lineages. eGFP MFI, however, was markedly lower in non-B versus B cells. EµB29 LV exhibited up to fivefold higher eGFP MFI in B cells compared to all other lineages (Figure 3d). Furthermore, although both LV lead to B cell-predominant expression, nearly all EµB29 recipients exhibited approximately fourfold higher MFI in B cells compared with EµS.CD19 (Figure 3b,c). One EµB29 recipient animal (in experiment #1) exhibited eGFP expression levels similar to that present in EµS.CD19 recipients; and this result correlated with the lowest viral copy number among the eight EµB29 animals shown. By comparison, MND LV exhibited similar, albeit variable, eGFP expression in all lineages. Average viral copy numbers were similar in all LV cohorts (Figure 3e) indicating that differential LV promoter activity did not reflect altered transduction efficiency.
Figure 3.
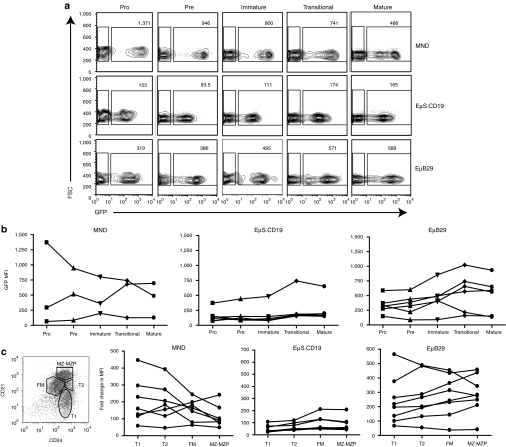
EµB29 drives high-level, B cell-predominant, transgene expression in primary murine B cells. Lin− BM cells were isolated from congenically marked Ly5.1 B6 mice, transduced with MND-eGFP, EµS.CD19-eGFP, or EµB29-GFP LV at an MOI of 10 for 6 hours and 1 × 106 transduced cells transplanted into lethally irradiated Ly5.2 B6 recipients. Twenty-five weeks post-transplantation, mice were sacrificed and splenic cells were stained for B220, CD4, CD8, CD11b, GR1, Ly5.1, and Ly5.2 to identify donor B cells (B220+), T cells (CD4+ or CD8+), neutrophils (B220−CD4−CD8−CD11b+GR1+) and monocytes (B220−CD4−CD8−CD11b+GR1−). (a) Representative FACS plots showing eGFP expression in splenic-derived lineages in one mouse per condition. Numbers indicate MFI for eGFP+ cells. (b) Graphs display eGFP MFI for each lineage in all mice from the experiment shown in (a) (2–5 mice/group). (c) Graphs show eGFP MFI for each lineage in all mice from a second independent experiment (3–4 mice/group). (d) Graphical representation of the ratio of eGFP expression in T cells, myeloid cells or neutrophils verses B cells. (e) Viral copy number in spleen cells. Combined data in d–e are derived from experiments shown in b and c. BM, bone marrow; Btk, Bruton's tyrosine kinase; eGFP, enhanced green fluorescent protein; FACS, fluorescence-activated cell sorting; LV, lentiviral vectors; LTR, long-terminal repeats; MFI, mean fluorescent intensity; MND, myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted; MOI, multiplicity of infection.
Figure 4.
EµB29 facilitates higher transgene expression during B cell maturation. BM from chimeras described in Figure 3 were stained with antibodies against B220, CD43, IgM, IgD, Ly5.1 and Ly5.2 and B cell subsets were identified as: CD43hiIgM−IgD− (pro-B); CD43loIgM−IgD− (pre-B); CD43−IgM+IgDlo (immature); CD43−IgM+IgDint (transitional); and CD43−IgM+IgDhi (mature) B cells. (a) FACS plots show representative MFI of eGFP in BM B cell subsets from one mouse per condition. Numbers in each plot show MFI for eGFP+ cells. (b) Graphs showing relative MFI of eGFP in B cell subsets in all mice from one experiment (3–6 mice per group). Data shown are representative of two independent experiments. (c) Splenic B220+ B cells were subdivided into developmental subsets based upon CD21 and CD24 expression (as in left panel) identify transitional 1 (T1), transitional 2 (T2), follicular mature (FM), and marginal zone/marginal zone precursors (MZ-MZP) B cells. MFI of eGFP expression in each subset is shown for individual mice transduced with each LV. BM, bone marrow; Btk, Bruton's tyrosine kinase; eGFP, enhanced green fluorescent protein; FACS, fluorescence-activated cell sorting; Ig, immunoglobulin; LV, lentiviral vectors; MFI, mean fluorescent intensity; MND, myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted.
We next determined whether LV-mediated expression was modulated during BM and/or peripheral B cell development and selection (Figure 4). Figure 4a shows representative FACS plots of eGFP expression in developing BM B cells. The subsets of developing B cells were gated-based CD43, IgM and IgD expression as described in the figure legend. eGFP MFI was significantly higher in all BM B cells derived from EµB29-eGFP compared to EµS.CD19-eGFP recipients (Figure 4a) and increased as cells matured from the pro- to mature B cell stage in EµB29 LV recipients (Figure 4b). In contrast, there was little or no change in eGFP MFI in EµS.CD19-eGFP recipients and MND-eGFP recipients exhibited greater variability with a trend for reduced expression in mature cells. Splenic transitional 1 and 2 (T1 and T2), follicular mature, and marginal zone/marginal zone precursor B cells were identified based on of CD21 and CD24 expression within B220+ B cells36 and eGFP expression in each subset determined (Figure 4c). Most MND-eGFP LV recipients exhibited a decline, and EµS.CD19 LV recipients little or no change, in relative eGFP MFI in immature versus more mature B cells, respectively. In contrast, the majority of EµB29-LV recipients exhibited a consistent increase in eGFP MFI in more mature, follicular mature, and marginal zone/marginal zone precursor, compared to less mature, T1 B cells. Thus, EµB29-LV drives high-level B predominant expression that increases with B cell maturation.
EµB29 mediates high-level B-lineage expression in primary human B-lymphocytes
We also examined the activity of candidate LV in primary human cells derived from transduced HSC. We utilized a NOD/SCID/γc−/− (NSG) xenograft system in which MND-eGFP, EµS.CD19-eGFP or EµB29-eGFP LV transduced human cord blood CD34+ cells were transplanted into recipient immune-deficient NSG mice.37,38 Engrafted mice were sacrificed at 20–25 weeks post-transplant and human-derived, BM and splenic hematopoietic subsets were analyzed for relative eGFP expression (Figure 5). The level of human cell engraftment in each mouse is depicted as the percentage of DAPI-live cells that express the human leukocyte common antigen marker CD45 (Figure 5b). A large percentage of splenic CD45+ cells were CD19+ B cells similar to other reports using the NSG model. Moderate populations of CD4+ and CD8+ T cells, as well as CD33+ myeloid cells, were also identified in all recipients. MND LV drove high-level eGFP expression in all lineages. In contrast, EµS.CD19 and EµB29 lead to B cell-predominant eGFP expression with lower expression in T and myeloid cells (Figure 5b–d). Although substantial variation was observed among individual recipients, the average level of eGFP expression in B cells was fourfold higher for EµB29 compared to EµS.CD19 LV. Viral copy numbers were similar in all experimental groups (Figure 5e).
Figure 5.
EµB29 drives high-level, lineage predominant eGFP expression in primary human B cells. Human cord blood CD34+ cells were transduced with MND-eGFP, EµS.CD19-eGFP, or EµB29-eGFP LV and transplanted into irradiated NSG recipient mice. Engrafted recipients were sacrificed 20 weeks after transplantation for analysis of human cells. Spleen cells were stained with CD45 to identify human cells with hematopoietic subsets defined as B cells (B220+CD4−CD8− CD33−), T cells (B220−CD4+ or CD8+, CD33−), and myeloid cells (B220−CD4−CD8−CD33+), respectively. (a) Representative FACS plots showing eGFP expression in splenic populations from one mouse per condition. Numbers show MFI for eGFP+ cells. (b) Level of human cell engraftment in the spleen depicted as the percentage of live (DAPI−) cells that express the human leukocyte antigen, CD45. (c) Graph showing MFI of eGFP in human hematopoietic subsets with data from three experiments (7–8 mice per vector). (d) Graphs showing relative fold change in MFI levels between eGFP− and eGFP+ cells with data pooled from four experiments. (e) Graphical representation of the ratio of eGFP expression in T versus B cells or myeloid verse B cells. (f) Viral copy number in spleen cells. BM, bone marrow; Btk, Bruton's tyrosine kinase; eGFP, enhanced green fluorescent protein; FACS, fluorescence-activated cell sorting; Ig, immunoglobulin; LV, lentiviral vectors; MFI, mean fluorescent intensity; MND, myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted.
The cell surface marker, CD10, is expressed specifically on immature human B cells.39 We therefore evaluated relative eGFP expression in immature CD10+ versus more mature CD10− CD19+ BM B cells in recipient NSG mice. EµB29-eGFP LV recipients exhibited a progressive increase in eGFP MFI in CD10− compared to CD10+ B cells (Figure 6a). In contrast, little difference was observed in EµS.CD19 recipients, and MND LV recipients exhibited a decline in eGFP MFI in CD10− cells. Immature, splenic CD24hiCD38hi B cells in NSG recipients also express CD10,40 consistent with the presence of recent BM emigrant B cells. Splenic B cells in EµB29-eGFP LV recipients exhibited a similar increase in eGFP MFI in mature CD10− compared to immature CD10+ B cells (Figure 6b) whereas no increase in transgene expression was observed in EµS.CD19 or MND LV recipients. Together, these data demonstrate that EµB29 LV mediate high-level B predominant expression in primary human B cells and that expression increases with B-lineage maturation.
Figure 6.
EµB29 drives higher eGFP expression in mature versus immature human B cells. Splenic and BM cells were isolated from NSG recipients as shown in Figure 5. Cells were stained with CD45 and CD19 to identify engrafted human B cells and with CD10 to distinguish immature (CD10+) versus more mature (CD10−) B cells. (a–b) Graphs showing change in MFI of eGFP in immature versus mature B cells derived from (a) BM or (b) spleen in mice from one experiment (3–4 mice per group). Data shown are representative of one of three independent experiments. BM, bone marrow; eGFP, enhanced green fluorescent protein; MFI, mean fluorescent intensity; MND, myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted.
EµBtkp LV mediates high-level expression primary B cells as well as modest activity in myeloid subsets
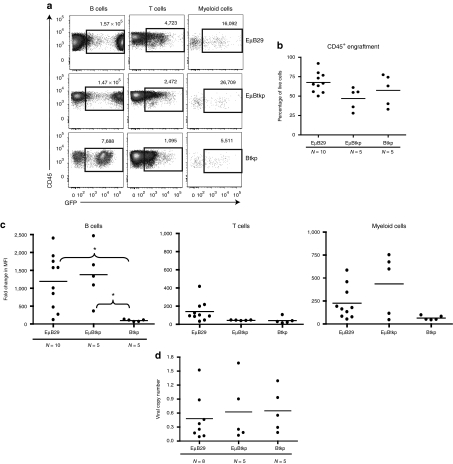
LV containing key endogenous regulatory elements may provide improved safety with regard to nonspecific transgene expression.41 Thus, based upon the potential goal of treating the B-lineage immune disorder, XLA, we generated LV containing regulatory elements derived from the human BTK locus. As described above (Figure 1), we generated Btkp and EµBtkp LV and compared these constructs with our best-performing, B-lineage expression vector, EµB29 LV, in murine and human-based in vivo models. First, we established mouse BM chimeras using HSC transduced with EµB29-eGFP, Btkp-eGFP, or EµBtkp-eGFP LV using the same methods as Figure 3. Recipient mice were sacrificed at 25 weeks post-transplant and the relative percentage and intensity of eGFP expression assessed within various BM (data not shown) and splenic populations (Figure 7). As in Figure 3, due to differences in the 488-nm argon laser voltage settings, the results from two independent experiments are shown (Figure 7b,c). Again, while overall eGFP MFI differed between experiments, we observed consistent results with respect to the panel of LV vectors. Representative FACS plots for both experiments are also shown in Supplementary Figure S2 (as experiment #3 and 4, respectively). EµBtkp LV-mediated high-level eGFP expression in splenic B cells, although by comparison EµB29 LV showed a slightly higher MFI in most animals (Figure 7a–c). In contrast, expression in B cells using Btkp LV was tenfold lower level than Eµ enhancer containing LV. As anticipated, only low-level eGFP expression was present in T cells. Consistent with its lower activity in B cells, the relative T:B cell expression ratio was ~0.35 (approximately threefold less) for Btkp versus ~0.05 (~20-fold less) for EµBtkp and EµB29 (Figure 7d). Consistent with the expression pattern of endogenous Btk, Btkp LV mediated similar levels of eGFP in B and myeloid cells. This feature was most evident when the MFI of eGFP in monocytes or neutrophils was analyzed as a ratio relative to the MFI in B cells (Figure 7d). The monocyte:B and the neutrophil:B expression ratios for Btkp LV were close to 1.0. An increase in myeloid expression ratio was also evident, albeit to a lesser degree, with EµBtkp when compared to EµB29 LV. In contrast, EµB29 expressed eGFP at significantly lower levels in monocytes or neutrophils versus B cells. All recipient animals exhibited similar viral copy numbers (Figure 7e).
Figure 7.
EµBtkp drives high-level eGFP expression in primary murine B cells and also exhibits modest activity in myeloid cells. Mouse BM chimeras were generated, as in Figure 3, using Lin− cells transduced with EµB29-eGFP, EµBtkp-eGFP, or Btkp-eGFP LV. Recipient animals were sacrificed 25 weeks after transplantation. (a) Representative FACS plots showing eGFP expression in splenic populations. Numbers indicate MFI for eGFP+ cells. (b) Graphs display eGFP MFI for splenic subsets in all animals from the experiment shown in a (3–4 mice per group). (c) Graphs show eGFP MFI all animals from a second independent experiment (2–3 mice per group) (d) Graphical representation of the ratio of eGFP MFI in T cells, monocytes, or neutrophils versus B cells. (e) Viral copy number in spleen cells. Combined data shown in d–e are derived from experiments shown in b and c. BM, bone marrow; Btk, Bruton's tyrosine kinase; eGFP, enhanced green fluorescent protein; FACS, fluorescence-activated cell sorting; LV, lentiviral vectors; MND, myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted.
To assess LV function in human primary cells, cord blood CD34+ HSC were transduced with EµB29-eGFP, Btkp-eGFP, or EµBtkp-eGFP LV and transplanted into NSG recipient mice. Overall, the expression pattern in splenic-derived human cells mirrored those from the murine chimeric setting (Figure 8). EµBtkp LV drove high-level eGFP expression in human B cells whereas in Btkp LV eGFP expression was at least fivefold lower. Combined data from several experiments showed high-level, albeit variable, B cell expression for both EµB29 and EµBtkp LV (Figure 8b). T cell expression was very low in all recipients with a B:T cell expression ratio as high as 60 in some EµBtkp recipients. In contrast to EµB29, EµBtkp LV also led to intermediate levels of transgene expression in monocytes. However, because expression was variable this data did not reach statistical significance for the number of animals studied. As in murine studies, Btkp LV exhibited consistent, low-level eGFP expression in both B cells and monocytes. All recipient animals exhibited similar average viral copy numbers (Figure 8c).
Figure 8.
EµBtkp drives high-level transgene expression in primary human B cells and also exhibits activity in myeloid cells. Human cord blood CD34+ cells were transduced with EµB29-eGFP, EµBtkp-eGFP, or Btkp-eGFP LV and transplanted into irradiated NSG recipient mice (as in Figure 6). Engrafted recipients were sacrificed 20 weeks after transplantation for analysis. (a) Representative FACS plots showing eGFP expression in splenic cells with numbers indicating MFI in eGFP+ cells. (b) Level of human cell engraftment in the spleen. (c) Graphs showing relative fold change in MFI levels between eGFP− and eGFP+ cells in CD45+ human hematopoietic subsets (data pooled from four experiments) (d) Relative viral copy number in spleen cells in three experimental cohorts. BM, bone marrow; Btk, Bruton's tyrosine kinase; eGFP, enhanced green fluorescent protein; FACS, fluorescence-activated cell sorting; LV, lentiviral vectors; MFI, mean fluorescent intensity; MND, myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted.
Collectively, these data demonstrate that EµBtkp LV mediates high-level transgene expression in primary murine and human B cells, presumably facilitated by the Eµ element; and also drives intermediate levels of transgene expression in myeloid cells in a manner analogous to the endogenous Btk promoter.
Discussion
Successful LV-based HSC gene therapy requires efficient gene transfer into multipotent HSC followed by sustained, therapeutic levels of transgene expression within key target cell populations. This study focused on developing LV that facilitate gene expression in developing and mature B-lineage cells for use in candidate B-lineage disorders. Our data demonstrate efficient, B cell-predominant gene expression using LV vectors containing two distinct promoter/enhancer combinations. Our initial studies focused on the composite vector, EµB29. This construct mediated high-level reporter transgene expression in multiple human B cell lines and in both human and mouse primary B cells derived from transduced HSC. In contrast to previous observations showing highly variable B-lineage expression following HSC transduction using γ retroviral vectors,42 cell differentiation did not lead to appreciable evidence for silencing or variegation of EµB29 LV. Consistent with this interpretation, sustained eGFP expression was observed in both immature and mature B cells for at least 20 weeks despite low-level viral marking (<1.5 copies/cell). Notably, EµB29 LV exhibited significantly higher levels of reporter gene expression than EµS.CD19 LV, even though both LV contained minimal promoters activated at nearly identical points following B-lineage commitment. EµB29 LV expression also increased progressively in parallel with BM B cell differentiation in both murine and human models and was maintained in the peripheral B lymphoid compartment. Expression levels were further increased during splenic transitional development, leading to highest expression within mature murine marginal zone and follicular mature B cells, and CD10− human B cells, respectively. By contrast, EµS.CD19 LV exhibited lower level, stable expression levels throughout BM and peripheral B cell development.
Together these data suggest that EµB29 LV might be useful for clinical disorders where progressively higher levels of transgene expression are required in developing and mature B cells. Although we observed some variability in expression levels of eGFP driven by EµB29 LV in both the murine and human stem cell models, the overall trend for higher-level transgene expression (compared to EµS.CD19) suggests that this promoter would be a superior choice for B cell-specific expression. Notably, previous work has shown that subendogenous Btk expression levels are insufficient to mediate functional rescue of mature B cells Btk-deficient mice.23,24 Based on these ideas, we recently evaluated ex-vivo gene therapy in a murine model of XLA using the EµB29 LV to deliver human Btk. This approach lead to near-complete developmental and functional B cell rescue.43 Importantly, our findings paralleled earlier work from our group using a highly active, ubiquitously expressed, murine stem cell virus-based γ retroviral vector.5 Thus, equivalent clinical efficacy was achieved via this unique B-lineage targeted, potentially safer, LV delivery platform.
Despite its efficacy in the murine XLA model, the EµB29 enhancer/promoter may not be ideal for cell-specific Btk expression. Notably, while its functional role is most evident in B cells, Btk is also expressed in all myeloid subsets and megakaryocytes; and Btk also impacts signaling via a range of cell surface receptors.3,44 Recent work has demonstrated a requirement for Btk kinases in Toll-like receptor signals45,46,47 implying that Btk deficiency may impact innate, as well as adaptive, immune responses in vivo. Based on these considerations, we generated LV constructs containing the endogenous BTK promoter. As anticipated, Btkp LV exhibited sustained, albeit low-level activity in primary murine and human B and myeloid cells. Importantly, addition of the Eµ enhancer lead to a marked increase in relative B-lineage expression, with activity levels closely mimicking EµB29 LV in both murine and human primary B cells. In contrast to EµB29 LV, however, EµBtkp LV also retained modest transgene expression in myeloid populations suggesting this LV may more faithfully mimic endogenous Btk expression and potentially rescue Btk-dependent myeloid activity.
Taken together, our data indicate that EµB29 LV provides an efficient platform for high-level mammalian B cell gene expression. Furthermore, these data also suggest that EµBtkp LV may represent an optimal system for regulated Btk gene delivery in XLA. This latter possibility is currently being investigated using murine models of XLA. Finally, it is important to note that we observed no evidence for Eµ enhancer-dependent mutagenesis in this study or in >200 primary/secondary/or tertiary recipient mice treated to date using these LV for Btk delivery. Formal studies, however, using genome targeted integration48 and BM immortalization assays49 will be required to more fully address the potential risk(s) of these new LV constructs.
Materials and Methods
LV constructs and viral production. The parental pRRL-SIN-cppt-PGK-eGFP-WPRE vector9 was obtained from Didier Trono (Addgene plasmid #12252) and the internal PGK promoter was replaced to generate pRRL-EµB29-eGFP, pRRL-EµBtkp-eGFP, pRRL-MND-eGFP, or pRRL-EµCD19-eGFP. MND is a retroviral long-terminal repeats-derived promoter.50 The immunoglobulin µ heavy chain enhancer Eµ is comprised of the 1-kb murine Ig heavy chain intronic core enhancer and the 5′ and 3′ matrix attachment region elements27 and was added to all other promoters except MND and Btkp alone. B29 contained the minimal promoter element derived from the B29 gene comprising 184 bp of the murine promoter including −166 to +18 from the major transcriptional start site.26 S.CD19 and L.CD19 contained the minimal (204 bp) versus extended (1,224 bp), human CD19 promoter element and adjacent upstream enhancer region, respectively.19 Btkp contains a 788 bp sequence located directly upstream of exon 1 within the human BTK locus. LV were produced by transient transfection of 293T cells using PEI method, concentrated by low-speed centrifugation, and titered as described previously.43
Cell culture and transduction. The following cell lines were utilized for lentiviral transduction experiments; Blin and Nalm6 (gift from Dr Tucker LeBien, University of Minnesota, Minneapolis, MN), REH, Ramos, Raji, Jurkat, and K562 (ATCC, Manassas, VA). All cells were maintained in complete RPMI (RPMI plus ℓ-glutamine, 10% fetal bovine serum, 0.1% β-mercaptoethanol and 10 µmol/l HEPES) media and transduced with the indicated lentiviral construct overnight with 4 µg/ml polybrene at variable multiplicity of infection's to obtain similar transduction rate (from 10 to 15%). Cells were incubated for 4 days and the MFI of eGFP was determined via flow cytometer.
Murine BM transplantation. C57BL/6 mice were bred and maintained in the specific pathogen-free barrier facilities at Seattle Children's Research Institute. Animal studies were carried out according to the guidelines of Seattle Children's Research Institute Institutional Animal Care and Use Committee. BM from 6 to 8 weeks mice was harvested and Lin− cells were obtained using EasySep Mouse Hematopoietic Progenitor Cell Enrichment kit according to manufacture's instruction (StemCell Technologies, Vancouver, BC), transduced overnight in media (StemSpan SFEM, StemCell Technologies) containing mSCF and mTPO (10 µg/ml; PeproTech, Rocky Hills, NJ) with the indicated LV overnight with 4 µg/ml polybrene. Cells were washed, counted, and 1 × 106 transduced cells were transplanted into mice receiving 2 × 500 Gy total body irradiation conditioning. Recipient mice were evaluated by serial peripheral blood analyses beginning at 5 weeks, and cohorts were sacrificed at 24–26 weeks for detailed analyses.
Cord blood CD34+ cell isolation, transduction, and transplantation of NSG recipients. Human umbilical cord blood was obtained from the Puget Sound Blood Center cord blood donor program and CD34+ cells were isolated using Miltenyi CD34+ beads (Miltenyi, Biotec, Germany) according to the manufacturer's instructions. Purity of CD34+Lineage− cells was >90% in all experiments. CD34+ cells were incubated for 8–12 hours in IMDM with human cytokines (100 ng/ml SCF, TPO, and FLT3-L; PeproTech) and 5% fetal bovine serum. After incubation, cells were transduced with the indicated LV at an multiplicity of infection of 10 for 8 hours in media with cytokines and 4 µg/ml polybrene. Transduced CD34+ cells were washed and transplanted into NOD/SCID/cγ−/− (NSG) mice. For all experiments, 6–12 weeks recipient mice were conditioned with 250 cGy total body irradiation and transplanted with 2–3 × 105 transduced, or mock transduced, CD34+ cells via retro-orbital injection. Peripheral blood samples were obtained from recipients 8 weeks after transplantation and the level of HSC engraftment was accessed via CD45 expression. Cohorts were sacrificed at 20–30 weeks for detailed FACS analysis of BM and spleen.
Flow cytometric analysis. Single-cell suspensions prepared from BM or spleen were incubated in phosphate-buffered saline with 3% fetal calf serum and fluorochrome-conjugated antibodies for 20 minutes at 4 °C, and washed twice before resuspension in phosphate-buffered saline with 3% fetal calf serum for acquisition. The following antibodies were used: antihuman CD19, CD33, CD45, IgM, IgD, 38, CD24, CD10, CD34, anti-mouse CD3, CD4, CD8, B220, CD21, CD24, CD43, IgM, IgD, Gr-1, CD11b, and CD45.1 (BD Biosciences, San Jose, CA and eBioscience, San Diego, CA). Cell analysis were performed using FACSCalibur, Aria II, and LSRII flow cytometers (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR).
Viral copy number determination. Quantification of viral integration was adapted from a previously described protocol.5 Briefly, genomic DNA was obtained from spleen or total BM from sacrificed animals. Gag-specific primer/probe sets were used to amplify viral integrations in both mouse and human cells. To normalize DNA content in mouse cells, a murine β-actin primer/probe set was used; in human cells, a human RNaseP primer probe set was used (Applied Biosytems, Carlsbad, CA). Viral-integration number was determined via a standard curve established using genomic DNA extracted from A20 murine B cell line or Nalm6 human B cell line, each containing a single LV integration.
Statistical analysis. Statistical analyses of relative eGFP marking and viral copy numbers were carried out using a two-tailed paired Student's t test comparing the indicated cell populations from the groups of experimental mice or cell lines.
SUPPLEMENTARY MATERIAL Figure S1. Comparison of EµS.CD19, EµL.CD19, and EµB29 LV activity in human B cell lines. B cell lines were transduced with EμS.CD19-eGFP, EμL.CD19-eGFP or EμB29-eGFP LV for 12 hrs and then evaluated on Day 4 days by FACS. The percentage of eGFP expression was equivalent in all cells lines (between 10-15%). (a) Chart shows the MFI of eGFP for EμS.CD19 (black bars) vs. EμL.CD19 (white bars) for each cell line. Data shows average MFI from three experiments. (b) Chart shows eGFP MFI for three human B cell lines Nalm6, Ramos and Raji, transduced with EμS.CD19, EμL.CD19 or EμB29 LV (striped bars). EμB29 LV mediated eGFP expression was 2-4X higher than either EμCD19 construct in all B cell lines evaluated. Asterisks (*) indicate statistically significant results in all figures, with *<0.05, **<0.01 and ***<0.001. Only the statistically significant data is highlighted in this manner. Figure S2. Representative FACS plots from independent experiments shown in Figures 3 and 7. FACS plots showing the B cell expression pattern for EμB29 vs. EμS.CD19 (upper panels) and EμB29 verses EμBtkp (lower panels). Experiments #1 and 2 correspond to data shown graphically in Fig 3b and 3c, respectively; and Experiments #3 and 4 correspond to data shown in Fig 7b and 7c, respectively. The specific flow cytometer and 488nm argon laser voltage settings used to measure eGFP in each experiment are indicated. Numbers above each panel indicate the MFI for the GFP− or GFP+ gates, and relative fold-change between the GFP− and GFP+ MFI, respectively. As shown, eGFP MFI differed between individual experiments due to differences in the instrument and/or the voltage settings used for data collection. However, the fold change in GFP MFI remained consistent across multiple experiments.
Acknowledgments
We thank Jit Khim for excellent technical assistance with murine transplantation studies, Dr Maria Garcia-Lloret (UCLA) for initial work with first-generation EµB29-GFP vectors, and Angel Hui for administrative support. This work was supported by a CRI fellowship award (to B.D.S.), CMB Training grant, GM07270 (to A.A.), and NIH R01 awards CA081140 and HL075453 (to D.J.R.). The authors declared no conflict of interest.
Supplementary Material
Comparison of EµS.CD19, EµL.CD19, and EµB29 LV activity in human B cell lines. B cell lines were transduced with EμS.CD19-eGFP, EμL.CD19-eGFP or EμB29-eGFP LV for 12 hrs and then evaluated on Day 4 days by FACS. The percentage of eGFP expression was equivalent in all cells lines (between 10-15%). (a) Chart shows the MFI of eGFP for EμS.CD19 (black bars) vs. EμL.CD19 (white bars) for each cell line. Data shows average MFI from three experiments. (b) Chart shows eGFP MFI for three human B cell lines Nalm6, Ramos and Raji, transduced with EμS.CD19, EμL.CD19 or EμB29 LV (striped bars). EμB29 LV mediated eGFP expression was 2-4X higher than either EμCD19 construct in all B cell lines evaluated. Asterisks (*) indicate statistically significant results in all figures, with *<0.05, **<0.01 and ***<0.001. Only the statistically significant data is highlighted in this manner.
Representative FACS plots from independent experiments shown in Figures 3 and 7. FACS plots showing the B cell expression pattern for EμB29 vs. EμS.CD19 (upper panels) and EμB29 verses EμBtkp (lower panels). Experiments #1 and 2 correspond to data shown graphically in Fig 3b and 3c, respectively; and Experiments #3 and 4 correspond to data shown in Fig 7b and 7c, respectively. The specific flow cytometer and 488nm argon laser voltage settings used to measure eGFP in each experiment are indicated. Numbers above each panel indicate the MFI for the GFP− or GFP+ gates, and relative fold-change between the GFP− and GFP+ MFI, respectively. As shown, eGFP MFI differed between individual experiments due to differences in the instrument and/or the voltage settings used for data collection. However, the fold change in GFP MFI remained consistent across multiple experiments.
REFERENCES
- Gaspar HB, Howe S., and, Thrasher AJ. Gene therapy progress and prospects: gene therapy for severe combined immunodeficiency. Gene Ther. 2003;10:1999–2004. doi: 10.1038/sj.gt.3302150. [DOI] [PubMed] [Google Scholar]
- Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- Smith, C., and, Witte ON. Functional aspects of Btk signaling in Primary Immunodeficiency Diseases, a Molecular and Genetic Approach. Oxford University Press; 1999. [Google Scholar]
- Moreau T, Calmels B, Barlogis V, Michel G, Tonnelle C., and, Chabannon C. Potential application of gene therapy to X-linked agammaglobulinemia. Curr Gene Ther. 2007;7:284–294. doi: 10.2174/156652307781369128. [DOI] [PubMed] [Google Scholar]
- Yu PW, Tabuchi RS, Kato RM, Astrakhan A, Humblet-Baron S, Kipp K, et al. Sustained correction of B-cell development and function in a murine model of X-linked agammaglobulinemia (XLA) using retroviral-mediated gene transfer. Blood. 2004;104:1281–1290. doi: 10.1182/blood-2003-09-3044. [DOI] [PubMed] [Google Scholar]
- Uchida N, Sutton RE, Friera AM, He D, Reitsma MJ, Chang WC, et al. HIV, but not murine leukemia virus, vectors mediate high efficiency gene transfer into freshly isolated G0/G1 human hematopoietic stem cells. Proc Natl Acad Sci USA. 1998;95:11939–11944. doi: 10.1073/pnas.95.20.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case SS, Price MA, Jordan CT, Yu XJ, Wang L, Bauer G, et al. Stable transduction of quiescent CD34(+)CD38(-) human hematopoietic cells by HIV-1-based lentiviral vectors. Proc Natl Acad Sci USA. 1999;96:2988–2993. doi: 10.1073/pnas.96.6.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Blömer U, Gage FH, Trono D., and, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder AR, Shinn P, Chen H, Berry C, Ecker JR., and, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivella S, May C, Chadburn A, Rivière I., and, Sadelain M. A novel murine model of Cooley anemia and its rescue by lentiviral-mediated human β-globin gene transfer. Blood. 2003;101:2932–2939. doi: 10.1182/blood-2002-10-3305. [DOI] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- Pawliuk R, Westerman KA, Fabry ME, Payen E, Tighe R, Bouhassira EE, et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294:2368–2371. doi: 10.1126/science.1065806. [DOI] [PubMed] [Google Scholar]
- Indraccolo S, Minuzzo S, Roccaforte F, Zamarchi R, Habeler W, Stievano L, et al. Effects of CD2 locus control region sequences on gene expression by retroviral and lentiviral vectors. Blood. 2001;98:3607–3617. doi: 10.1182/blood.v98.13.3607. [DOI] [PubMed] [Google Scholar]
- Cui Y, Golob J, Kelleher E, Ye Z, Pardoll D., and, Cheng L. Targeting transgene expression to antigen-presenting cells derived from lentivirus-transduced engrafting human hematopoietic stem/progenitor cells. Blood. 2002;99:399–408. doi: 10.1182/blood.v99.2.399. [DOI] [PubMed] [Google Scholar]
- Gough PJ., and, Raines EW. Gene therapy of apolipoprotein E-deficient mice using a novel macrophage-specific retroviral vector. Blood. 2003;101:485–491. doi: 10.1182/blood-2002-07-2131. [DOI] [PubMed] [Google Scholar]
- Moreau T, Bardin F, Imbert J, Chabannon C., and, Tonnelle C. Restriction of transgene expression to the B-lymphoid progeny of human lentivirally transduced CD34+ cells. Mol Ther. 2004;10:45–56. doi: 10.1016/j.ymthe.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Lutzko C, Senadheera D, Skelton D, Petersen D., and, Kohn DB. Lentivirus vectors incorporating the immunoglobulin heavy chain enhancer and matrix attachment regions provide position-independent expression in B lymphocytes. J Virol. 2003;77:7341–7351. doi: 10.1128/JVI.77.13.7341-7351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau T, Barlogis V, Bardin F, Nunes JA, Calmels B, Chabannon C, et al. Development of an enhanced B-specific lentiviral vector expressing BTK: a tool for gene therapy of XLA. Gene Ther. 2008;15:942–952. doi: 10.1038/gt.2008.17. [DOI] [PubMed] [Google Scholar]
- Taher TE, Tulone C, Fatah R, D'Acquisto F, Gould DJ., and, Mageed RA. Repopulation of B-lymphocytes with restricted gene expression using haematopoietic stem cells engineered with lentiviral vectors. Gene Ther. 2008;15:998–1006. doi: 10.1038/gt.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust EA, Rawlings DJ, Saffran DC., and, Witte ON. Development of btk transgenic mice. Curr Top Microbiol Immunol. 1995;194:363–370. doi: 10.1007/978-3-642-79275-5_42. [DOI] [PubMed] [Google Scholar]
- Satterthwaite AB, Cheroutre H, Khan WN, Sideras P., and, Witte ON. Btk dosage determines sensitivity to B cell antigen receptor cross-linking. Proc Natl Acad Sci USA. 1997;94:13152–13157. doi: 10.1073/pnas.94.24.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PB, Yu XJ, Skelton DM, Pepper KA, Wasserman RM, Zhu L, et al. Increased probability of expression from modified retroviral vectors in embryonal stem cells and embryonal carcinoma cells. J Virol. 1997;71:9466–9474. doi: 10.1128/jvi.71.12.9466-9474.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AA, Wood WJ, Jr, Gilly MJ, Damore MA, Omori SA., and, Wall R. The promoter and 5' flanking sequences controlling human B29 gene expression. Blood. 1996;87:666–673. [PubMed] [Google Scholar]
- Banerji J, Olson L., and, Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983;33:729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Hagman J., and, Grosschedl R. Regulation of gene expression at early stages of B-cell differentiation. Curr Opin Immunol. 1994;6:222–230. doi: 10.1016/0952-7915(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Hermanson GG, Eisenberg D, Kincade PW., and, Wall R. B29: a member of the immunoglobulin gene superfamily exclusively expressed on β-lineage cells. Proc Natl Acad Sci USA. 1988;85:6890–6894. doi: 10.1073/pnas.85.18.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J, Parolini O, Belmont JW, Conley ME., and, Parolino O [corrected to Parolini O. The genomic structure of human BTK, the defective gene in X-linked agammaglobulinemia. Immunogenetics. 1994;40:319–324. doi: 10.1007/BF01246672. [DOI] [PubMed] [Google Scholar]
- Oeltjen JC, Malley TM, Muzny DM, Miller W, Gibbs RA., and, Belmont JW. Large-scale comparative sequence analysis of the human and murine Bruton's tyrosine kinase loci reveals conserved regulatory domains. Genome Res. 1997;7:315–329. doi: 10.1101/gr.7.4.315. [DOI] [PubMed] [Google Scholar]
- Müller S, Sideras P, Smith CI., and, Xanthopoulos KG. Cell specific expression of human Bruton's agammaglobulinemia tyrosine kinase gene (Btk) is regulated by Sp1- and Spi-1/PU.1-family members. Oncogene. 1996;13:1955–1964. [PubMed] [Google Scholar]
- Himmelmann A, Thevenin C, Harrison K., and, Kehrl JH. Analysis of the Bruton's tyrosine kinase gene promoter reveals critical PU.1 and SP1 sites. Blood. 1996;87:1036–1044. [PubMed] [Google Scholar]
- Rohrer J., and, Conley ME. Transcriptional regulatory elements within the first intron of Bruton's tyrosine kinase. Blood. 1998;91:214–221. [PubMed] [Google Scholar]
- Yu L, Mohamed AJ, Simonson OE, Vargas L, Blomberg KE, Björkstrand B, et al. Proteasome-dependent autoregulation of Bruton tyrosine kinase (Btk) promoter via NF-kappaB. Blood. 2008;111:4617–4626. doi: 10.1182/blood-2007-10-121137. [DOI] [PubMed] [Google Scholar]
- Pillai S, Cariappa A., and, Moran ST. Marginal zone B cells. Annu Rev Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, et al. NOD/SCID/γ©(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- Hiramatsu H, Nishikomori R, Heike T, Ito M, Kobayashi K, Katamura K, et al. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/γcnull mice model. Blood. 2003;102:873–880. doi: 10.1182/blood-2002-09-2755. [DOI] [PubMed] [Google Scholar]
- Blom B., and, Spits H. Development of human lymphoid cells. Annu Rev Immunol. 2006;24:287–320. doi: 10.1146/annurev.immunol.24.021605.090612. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Takahashi T, Okajima A, Shiokawa M, Ishii N, Katano I, et al. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/γc(null) (NOG) mice (hu-HSC NOG mice) Int Immunol. 2009;21:843–858. doi: 10.1093/intimm/dxp050. [DOI] [PubMed] [Google Scholar]
- Nienhuis AW, Dunbar CE., and, Sorrentino BP. Genotoxicity of retroviral integration in hematopoietic cells. Mol Ther. 2006;13:1031–1049. doi: 10.1016/j.ymthe.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Klug CA, Cheshier S., and, Weissman IL. Inactivation of a GFP retrovirus occurs at multiple levels in long-term repopulating stem cells and their differentiated progeny. Blood. 2000;96:894–901. [PubMed] [Google Scholar]
- Kerns HM, Ryu BY, Stirling BV, Sather BD, Astrakhan A, Humblet-Baron S, et al. B cell-specific lentiviral gene therapy leads to sustained B-cell functional recovery in a murine model of X-linked agammaglobulinemia. Blood. 2010;115:2146–2155. doi: 10.1182/blood-2009-09-241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebani A, Soresina A, Rondelli R, Amato GM, Azzari C, Cardinale F, Italian Pediatric Group for XLA-AIEOP et al. Clinical, immunological, and molecular analysis in a large cohort of patients with X-linked agammaglobulinemia: an Italian multicenter study. Clin Immunol. 2002;104:221–230. doi: 10.1006/clim.2002.5241. [DOI] [PubMed] [Google Scholar]
- O'Neill LA. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Jefferies CA., and, O'Neill LA. Bruton's tyrosine kinase (Btk)-the critical tyrosine kinase in LPS signalling. Immunol Lett. 2004;92:15–22. doi: 10.1016/j.imlet.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Jefferies CA, Doyle S, Brunner C, Dunne A, Brint E, Wietek C, et al. Bruton's tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor kappaB activation by Toll-like receptor 4. J Biol Chem. 2003;278:26258–26264. doi: 10.1074/jbc.M301484200. [DOI] [PubMed] [Google Scholar]
- Ryu BY, Evans-Galea MV, Gray JT, Bodine DM, Persons DA., and, Nienhuis AW. An experimental system for the evaluation of retroviral vector design to diminish the risk for proto-oncogene activation. Blood. 2008;111:1866–1875. doi: 10.1182/blood-2007-04-085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlich U, Navarro S, Zychlinski D, Maetzig T, Knoess S, Brugman MH, et al. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and γ retroviral vectors. Mol Ther. 2009;17:1919–1928. doi: 10.1038/mt.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halene S, Wang L, Cooper RM, Bockstoce DC, Robbins PB., and, Kohn DB. Improved expression in hematopoietic and lymphoid cells in mice after transplantation of bone marrow transduced with a modified retroviral vector. Blood. 1999;94:3349–3357. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of EµS.CD19, EµL.CD19, and EµB29 LV activity in human B cell lines. B cell lines were transduced with EμS.CD19-eGFP, EμL.CD19-eGFP or EμB29-eGFP LV for 12 hrs and then evaluated on Day 4 days by FACS. The percentage of eGFP expression was equivalent in all cells lines (between 10-15%). (a) Chart shows the MFI of eGFP for EμS.CD19 (black bars) vs. EμL.CD19 (white bars) for each cell line. Data shows average MFI from three experiments. (b) Chart shows eGFP MFI for three human B cell lines Nalm6, Ramos and Raji, transduced with EμS.CD19, EμL.CD19 or EμB29 LV (striped bars). EμB29 LV mediated eGFP expression was 2-4X higher than either EμCD19 construct in all B cell lines evaluated. Asterisks (*) indicate statistically significant results in all figures, with *<0.05, **<0.01 and ***<0.001. Only the statistically significant data is highlighted in this manner.
Representative FACS plots from independent experiments shown in Figures 3 and 7. FACS plots showing the B cell expression pattern for EμB29 vs. EμS.CD19 (upper panels) and EμB29 verses EμBtkp (lower panels). Experiments #1 and 2 correspond to data shown graphically in Fig 3b and 3c, respectively; and Experiments #3 and 4 correspond to data shown in Fig 7b and 7c, respectively. The specific flow cytometer and 488nm argon laser voltage settings used to measure eGFP in each experiment are indicated. Numbers above each panel indicate the MFI for the GFP− or GFP+ gates, and relative fold-change between the GFP− and GFP+ MFI, respectively. As shown, eGFP MFI differed between individual experiments due to differences in the instrument and/or the voltage settings used for data collection. However, the fold change in GFP MFI remained consistent across multiple experiments.