Abstract
Autologous T lymphocytes genetically engineered to express a murine T cell receptor (TCR) against human carcinoembryonic antigen (CEA) were administered to three patients with metastatic colorectal cancer refractory to standard treatments. All patients experienced profound decreases in serum CEA levels (74–99%), and one patient had an objective regression of cancer metastatic to the lung and liver. However, a severe transient inflammatory colitis that represented a dose limiting toxicity was induced in all three patients. This report represents the first example of objective regression of metastatic colorectal cancer mediated by adoptive T cell transfer and illustrates the successful use of a TCR, raised in human leukocyte antigen (HLA) transgenic mice, against a human tumor associated antigen. It also emphasizes the destructive power of small numbers of highly avid T cells and the limitations of using CEA as a target for cancer immunotherapy.
Introduction
Carcinoembryonic antigen (CEA) is a 180 kDa glycoprotein belonging to the immunoglobulin superfamily and is a tumor associated protein overexpressed in many epithelial cancers, most notably in colorectal adenocarcinoma. However, it is also expressed in a variety of normal epithelial cells throughout the gastrointestinal tract, most prominently in highly differentiated epithelial cells in the upper third of colonic crypts.1 Cancer immunotherapies using vaccines and antibodies targeting CEA are actively being explored.2,3,4,5,6 We have developed an approach to treating patients with metastatic colorectal cancer using autologous T cells genetically engineered to express a high avidity T cell receptor (TCR) that mediates recognition of CEA. This TCR was isolated by immunizing HLA-A*0201 transgenic mice with the peptide epitope CEA:691–699, and its functional avidity was enhanced by introducing a single amino acid substitution in the complementarity-determining region 3 of the α chain.7,8 This murine TCR specifically mediated recognition of peptide-loaded HLA-A*0201+ targets (T2 cells) and HLA-A*0201+ CEA+ human colon cancer cell lines in vitro.7 This report describes three patients with metastatic colorectal cancer treated with adoptively transferred autologous T cells retrovirally transduced to express this TCR.
Results
A retrovirus encoding the high avidity murine CEA-reactive TCR was used to transduce peripheral blood lymphocytes (PBL) from three HLA-A*0201+ patients with metastatic colorectal cancer who had elevated levels of serum CEA protein (Table 1) and tumors that expressed high levels of CEA. All three patients had progressive disease refractory to a minimum of four different chemotherapy regimens that included agents and antibodies with known activity against colorectal cancer. Genetically modified autologous T lymphocytes were adoptively transferred into these patients with interleukin-2 after a lymphodepleting chemotherapy regimen as previously described.9,10,11 Patient and cell characteristics are presented in Table 1. Transduction efficiencies in the adoptively transferred cells ranged from 79 to 90% as evaluated by fluorescence-activated cell sorting (FACS) using an antibody against the murine TCR β chain constant region. These T cells were functionally active as assessed by their ability to secrete interferon (IFN)-γ and form IFN-γ enzyme-linked immunosorbent spots specifically in response to CEA:691-699 peptide-loaded T2 cells and HLA-A*0201+ CEA+ human colon cancer cells in vitro (Table 1).
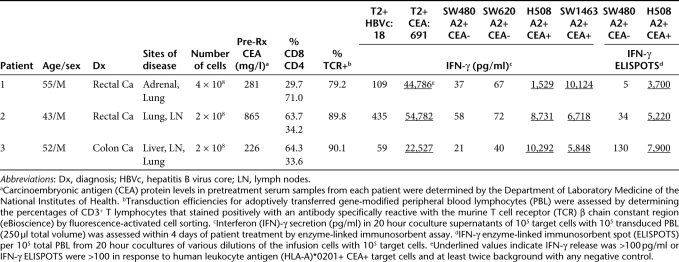
Table 1. Summary of patients treated with anti-carcinoembryonic antigen (CEA) reactive T cell receptor (TCR) transduced autologous peripheral blood lymphocytes (PBL).
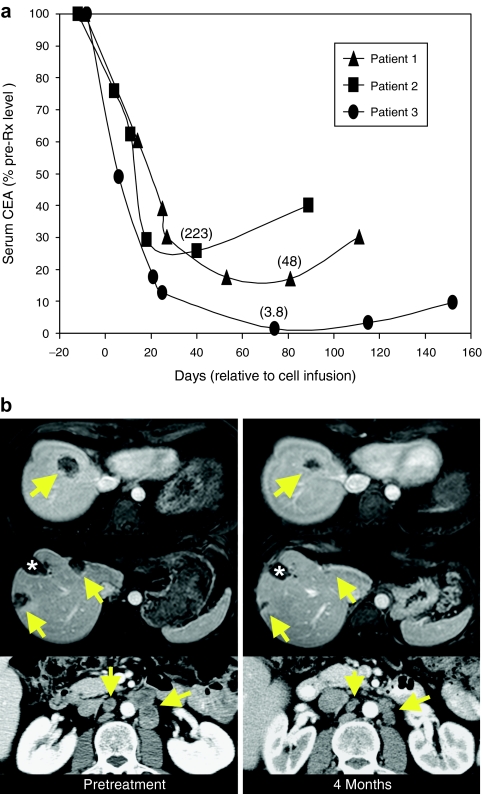
Serum CEA protein levels, which ranged between 226 and 865 µg/l prior to treatment (Table 1), dropped by 74–99% after adoptive cell transfer in all patients (Figure 1c). However, these decreases were transient, and CEA serum levels began to increase from their lowest levels at 3–4 months post-treatment. Patient 1 had a 17% reduction in metastatic cancer to the lung at 2 months after adoptive cell transfer but had progressive disease by 5 months (data not shown). Patient 2 had no response. Patient 3 had a 34% reduction in metastatic cancer to the liver, lung, and paraaortic lymph nodes assessed by Response Evaluation Criteria In Solid Tumors (RECIST) criteria at his 3 month follow-up evaluation, and that reduction improved to 49% at 4 months post-treatment (Figure 1b). As such, he met the criteria for a confirmed partial response as specified by RECIST; however, he had progressive disease by 6 months.
Figure 1.
Cancer-related responses to treatment. (a) Carcinoembryonic antigen (CEA) protein levels in sequential serum samples from each patient. Levels are expressed as the percent of the pretreatment concentrations shown in Table 1, and values in parentheses are the lowest concentrations achieved in each patient in µg/l. (b) Computed tomography scans for patient 3 prior to treatment and 4 months post-treatment. Arrows represent locations of colorectal cancer metastases, and the asterisk indicates a liver defect at a site of liver metastasis previously treated with radiofrequency ablation (RFA). Top and middle rows: patient 3 liver. Bottom row: patient 3 paraaortic lymph nodes.
Hematologic recovery in these patients was similar to that observed in our previous gene therapy patients receiving lymphodepleting chemotherapy. Their clinical courses were unique, however, because of the onset of grade 2 diarrhea in patient 1 and grade 3 diarrhea in patients 2 and 3. Diarrhea started on days 5–8 and persisted for ~2 weeks before slowly resolving to normal by 4–6 weeks. All three patients were febrile between days 7 and 9 and were hemodynamically stable but required fluid replacement. The two incidents of grade 3 diarrhea represented a dose limiting toxicity, and accrual to this clinical trial was halted in accord with requirements from the U.S. Food and Drug Administration.
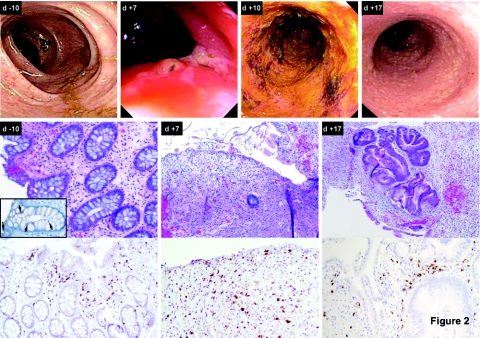
Sequential colonoscopies revealed the development of inflammatory colitis in all three patients. Characteristic findings are presented in Figure 2. Prior to treatment, the appearance of the colon was normal (Figure 2, top row, d-10), and biopsy revealed typical mucosal architecture (Figure 2, middle and bottom rows, d-10) with weak, albeit positive, expression of CEA in crypt epithelial cells (inset). However, ~1 week later, the mucosa was edematous with areas of ulceration and loss of mucosal folds (Figure 2, top row, d+7). Biopsy at this time point revealed virtually complete denudation of the glandular epithelium, replaced by granulation tissue (Figure 2, middle row, d+7) and infiltration of CD3+ (Figure 2, bottom row, d+7) T cells. Over the next several days, an inflammatory colitis evolved, and the colonic mucosa appeared ulcerated, denuded, and exudative (Figure 2, top row, d+10). By 2–3 weeks, the appearance of the colon was normalizing (Figure 2, top row, d+17), and biopsy showed atypical glandular epithelium consistent with repair/regenerative changes with continued granulation tissue and acute and chronic inflammation (Figure 2, middle and bottom rows, d+17). Immunohistochemical staining for CEA in these biopsies demonstrated near complete loss of CEA in the denuded colon specimens, but this protein was re-expressed in the regenerating tissue (data not shown).
Figure 2.
Sequential colonoscopy findings for patient 2 during treatment. Top row: sequential images from colonoscopies performed prior to treatment (d-10) as well as 7, 10, and 17 days post-treatment. Middle row: hematoxylin and eosin staining of consecutive colon biopsies obtained prior to treatment (d-10) as well as 7 and 17 days post-treatment. The inset in the first panel shows staining of the pretreatment biopsy with a monoclonal antibody against carcinoembryonic antigen, and arrows indicate positive expression. Bottom row: staining of consecutive colon biopsies obtained prior to treatment (d-10) as well as 7 and 17 days post-treatment with a monoclonal antibody against human CD3.
Since these patients were neutropenic, stool cultures were tested, and Clostridium difficile was checked when diarrhea began to determine whether the colitis might have an infectious cause (C. difficle toxin assay × 3; C. difficle culture; Ova and Parasite; Cryptosporidium; Salmonella; Shigella; Campylobacter; Enteric Culture). All of these tests were negative in all three patients. Patients 1 and 2 received Budesonide and Mesalamine, but patient 3 did not, so it is unclear to what, if any extent, these steroids aided in resolving the colitis. In a previously published report describing the adoptive transfer of allogeneic T cells specifically reactive with minor histocompatibility antigens, toxicities were observed due to expression of these antigens in normal tissues.12 In that study, systemic steroids were administered that eradicated the adoptively transferred cells and aided in the resolution of symptoms. However, we opted to administer only steroids with limited local, rather than systemic activity in an attempt to eliminate the T cells causing the colitis without eliminating those that might have induced tumor regression.
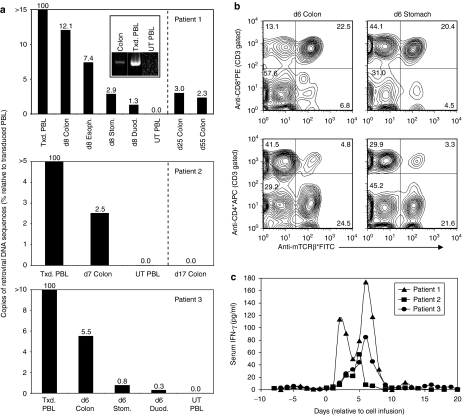
Genetic and cellular analyses of biopsy samples obtained during upper and lower endoscopies 6–11 days post-treatment using PCR and FACS analyses indicated the presence of substantial numbers of the adoptively transferred lymphocytes in all patients (Figure 3a and b). As one example, DNA samples were extracted from four different gastrointestinal biopsies from the esophagus, stomach, duodenum, and colon of patient 1 and were analyzed by PCR to quantify the number of retroviral DNA copies, normalized to the number of β-actin copies, relative to the amounts present in the adoptively transferred PBL. DNA was isolated from whole biopsy specimens, not from selected T cell populations. The colon specimen contained 12% as many retroviral DNA copies as the infused transduced PBL population. Lesser numbers, but still significant, were found in the esophagus, stomach, and duodenum at this time point in patient 1 (Figure 3a top panel). Colon biopsies obtained from patient 1 at later time points including days 25 and 55 post-treatment also contained significant numbers of the adoptively transferred cells as determined by the presence of retroviral DNA sequences (Figure 3a top panel). In addition, nonquantitative PCR which amplified the full-length murine CEA reactive TCR αβ construct but not any human TCR sequences clearly indicated the presence of the murine CEA-reactive TCR in the colon biopsy specimen from patient 1 obtained 8 days post-treatment (Figure 3a top panel inset). Colon biopsies from patients 2 and 3 obtained 7 and 6 days post-treatment respectively also contained significant numbers of retroviral DNA sequences indicating the presence of the adoptively transferred cells (Figure 3a middle and bottom panels). However, we did not obtain colon biopsies at later time points from these two patients. Colon and gastric biopsy samples from patient 3 were also disrupted to form single cell suspensions, and FACS analyses revealed that nearly 30% of the T cells present in the colon biopsy expressed the murine CEA TCR as did ~25% of those in the gastric specimen. Both of these populations were predominantly CD8+ (75–86%) (Figure 3b). Although we considered attempting to expand lymphocytes from gastric and colon biopsies in vitro to examine these cells functionally, we did not as the samples were very small, and we had doubts about maintaining sterility in culture. In addition, serum IFN-γ levels increased from baselines near zero pg/ml to maximum levels of 57–174 pg/ml 5–6 days after cell infusion (Figure 3c) which was similar to observations from previous adoptive cell transfer protocols in patients with melanoma.9
Figure 3.
Analyses of sequential biopsies and peripheral blood samples during treatment. (a) Biopsies from each patient were obtained during colonoscopy and endoscopy procedures at the indicated time points after adoptive transfer of autologous peripheral blood lymphocytes (PBL) transduced to express a carcinoembryonic antigen (CEA) reactive T cell receptor (TCR). DNA was extracted from these specimens, and quantitative PCR was then performed to determine the amount of retroviral DNA present in biopsy specimens. The number of retroviral DNA sequences present in each sample was normalized to the amount of β-actin DNA and expressed as a percent relative to that present in the adoptively transferred PBL using the comparative CT method. In addition, nonquantitative PCR was also performed on samples from patient 1 using specific distal 5′ (α chain V region) and 3′ (β chain C region) primers to amplify the full-length TCR αβ construct encoding the murine CEA-reactive TCR. The PCR products were then separated using a precast 2% agarose ethidium bromide gel (Invitrogen) (top panel inset). Txd, transduced; UT, untransduced; Esoph, esophagus; Stom, stomach; Duod, duodenum. (b) Biopsies from patient 3 were obtained during colonoscopy and endoscopy procedures 7 days after adoptive transfer of autologous PBL transduced to express the CEA reactive TCR. Biopsy specimens were disrupted using a BD Medimachine and/or passage through a 40 µm nylon filter to obtain single cell suspensions. These were then stained with anti-CD3, -CD8, -CD4, and –murine TCR β chain constant region antibodies and analyzed by fluorescence-activated cell sorting. The results shown were gated on CD3+ T cells, and the numbers indicate the percentages of cells in each quadrant. (c) Interferon (IFN)-γ protein levels in consecutive serum samples from each patient were determined by enzyme-linked immunosorbent assay. APC, antigen presenting cells; FITC, fluorescein isothiocyanate.
Persistence of the adoptively transferred cells in the peripheral circulation was evaluated for each patient using FACS and IFN-γ ELISPOT assays as previously described9 (Supplementary Figure S1). Analysis carried out using an anti-murine TCR β chain antibody revealed that 23, 6, and 1% of all T lymphocytes in patient peripheral blood ~1 month post-treatment in patients 1, 2, and 3 respectively expressed the CEA-reactive TCR. We also evaluated the phenotype of the adoptively transferred cells and those that persisted in the peripheral circulation using antibodies against CD45RA, CD45RO, CD27, and CD28. The T cells given to patient 1 that expressed the CEA reactive TCR appeared more differentiated13 than those given to the other two patients in that they were predominantly negative for CD45RA and CD27 (Supplementary Figure S1). This is consistent with the fact that these cells underwent two rounds of in vitro stimulation whereas T cells from patients 2 and 3 only underwent one. The CEA reactive T cells given to patients 2 and 3 appeared to be more heterogenous, containing mixtures of naive, central, and effector memory cells.13 None of the patients received fully differentiated effector cells based on significant expression of CD28, and the persistent TCR+ cells in all three patients appeared to be heterogenous populations based on expression of these markers.
Specific ELISPOT reactivity to the CEA peptide was also observed in peripheral blood mononuclear cell samples obtained 10 and 26 days following transfer from patient 1, and 11 days post-transfer from patient 3. However, these cells did not recognize tumor cells expressing HLA-A*0201 and CEA. This is also likely due to T cell quiescence observed in previous clinical trials.14 In this clinical trial, patients received a lymphodepleting chemotherapy regimen prior to cell transfer. This regimen was required in order to see persistence of the transduced cells as we reported in two prior publications.15,16 We have recently confirmed these results in a new clinical trial in 44 patients with melanoma who received cell transfer without a lymphodepleting chemotherapy regimen. No persistence of transferred cells was observed (unpublished observations).
A unique feature of this protocol was the use of a high avidity TCR raised in HLA transgenic mice to overcome the natural tolerance in humans to a self-tumor associated antigen. Since the murine TCR may appear as a foreign antigen to the human immune system, we evaluated the development of antibodies against the murine CEA-reactive TCR in patient serum samples at multiple time points after adoptive cell transfer (Supplementary Figure S2 and Supplementary Table S1). No such antibodies were detected in serum samples from patient 1 at any time point up to 6 months post-treatment. However, IgG antibodies capable of binding to the CEA TCR were detected in serum samples from the second and third patients at 3 and 4 months post-treatment, respectively (Supplementary Figure S2). In addition to binding, these antibodies inhibited the function of PBL expressing the CEA-reactive TCR as indicated by their ability to inhibit antigen-specific IFN-γ secretion (Supplementary Table S1). It is unclear if the development of these antibodies negatively impacted on tumor response; however, these antibodies did not appear until 3 to 4 months after adoptive cell transfer.
Discussion
Adoptive cell transfer using tumor reactive T lymphocytes represents an effective immunotherapy capable of mediating durable complete regressions in patients with metastatic melanoma.17 Genetic modification of the transferred cells holds the potential to increase the antitumor effectiveness of this approach.18,19 The current study represents the first application of cell transfer therapy for the treatment of a common epithelial cancer such as metastatic colorectal cancer in patients refractory to all standard treatments. All three patients treated experienced profound drops in serum CEA levels, and one patient had an objective clinical response.
It is unclear why decreases in serum CEA protein levels did not correlate with significant reductions in tumor burden; however, this phenomenon has been observed in several previous clinical trials in which patients with gastrointestinal cancers were treated with several different chemotherapy regimens.20,21,22 Although the TCR on cells can not directly bind to whole CEA protein, it seems possible in these trials and in ours that the therapies may have had some minor impact causing destruction or impairment of a limited number of tumor cells. Since only viable tumor cells can produce CEA, it seems possible that such an effect on even fairly small numbers of tumor cells could result in transient decreases in CEA serum protein levels without significant clinical benefit. In addition, although the combination of cyclophosphamide and fludarabine have not been previously shown to have significant clinical impact on metastatic colorectal cancer, we can not formally exclude the possibility that these chemotherapeutic reagents played some role in the observed CEA serum protein level decreases and/or tumor regressions.
In this trial, T cell persistence in the circulation did not correlate with clinical response. In particular, the greatest persistence and function of the adoptively transferred cells at 1 month post-treatment was observed in patient 1, but the only objective tumor response occurred in patient 3 who had the lowest level of persistence. This discordance may have been due to differences in CEA expression and T cell persistence within the tumors. However, since these patients had visceral metastases, we were unable to obtain sufficient biopsies to evaluate this possibility. Also in this trial, no correlation was observed between the phenotypes of the adoptively transferred or persistent cells and the transient nature, or the severity, of the colitis. We speculate that the colitis resolved despite the presence of persistent CEA-reactive T cells because these cells became quiescent over time as has been observed in other adoptive T cell transfer protocols.14
Previous studies have suggested that mispairing of genetically introduced TCR α and/or β chains with endogenous TCR chains may result in mixed TCR dimers with new, and potentially harmful autoimmune reactivities.23,24 Although we can not conclusively disprove the idea that expression of mixed TCR dimers may have accounted for the colitis observed in this clinical trial, it seems unlikely for two reasons. First, several previous studies have suggested that murine TCR chains do not effectively pair with human chains. In fact, several investigators have grafted murine TCR constant regions onto human variable chains specifically to prevent mispairing of endogenous and introduced TCR chains.25,26,27 Second, in a previous clinical trial, we adoptively transferred T cells expressing a murine TCR specifically reactive with gp100 into 16 patients with metastatic melanoma.9 In that trial, no autoimmune colitis was observed in any patient. Furthermore, we have treated 106 patients in the Surgery Branch of the National Cancer Institute with autologous T cells transduced with seven different γ retroviral vectors encoding tumor reactive TCRs and have seen no evidence of graft-versus-host disease in any patient.28
In this trial 100-fold fewer cells were transferred than the numbers of tumor infiltrating lymphocytes we have administered to patients with melanoma in other trials.11 The observation that this small number of adoptively transferred lymphocytes with high functional avidity for a single epitope can mediate potent damage to tumor and normal tissues bearing the relevant self-antigen emphasizes the power of adoptive cell transfer and the importance of future immunotherapy strategies that target antigens whose normal tissue expression is limited (e.g., cancer-testis or unique tumor antigens), or antigens whose expression on cancers is shared by nonessential organs such as prostate, thyroid, ovary, or breast. Our observations of transient mucosal destruction by CEA-reactive T cells represent an autoimmune colitis probably due to lymphocyte recognition of the normal levels of CEA present in colonic mucosa. It is currently unclear why there was a significant discord between the robust anti-CEA reactions observed in the colons of all three patients and the lack of significant tumor responses in patients 1 and 2. However, one possible explanation is that factors within the tumor microenvironment suppress T cell function as has been previously described.29 In addition, cyclophosphamide administration is associated with mucositis which may have exacerbated the induction of colitis by the adoptively transferred CEA-reactive lymphocytes. However, we have seen no evidence of mucositis in over 100 patients given 60 mg/kg cyclophosphamide for 2 days prior to receiving adoptively transferred T cells expressing tumor reactive TCRs in other clinical trials. Therefore, we speculate that the functional reactivity of the adoptively transferred T cells under lymphopenic conditions in the colon may have been intensified by enhanced local immunologic activity resulting from Toll-like receptor stimulation by colonic commensal bacteria.30 Hence, the severity of this inflammatory colitis may be lessened by maneuvers that reduce the load of commensal flora or the local application of steroids to reduce T cell activity in the colon.
Concomitant induction of inflammatory colitis and tumor regression was previously described in a murine model in which CEA-reactive T cells were adoptively transferred into tumor-bearing lymphopenic CEA transgenic mice in which CEA expression patterns were similar to that in humans.31 In this model, pretreatment of irradiated mice with anti-CD25 preserved the tumor response while eliminating the colonic toxicities, probably due to elimination of T regulatory cells prior to treatment as well as to dampening the in vivo function of the adoptively transferred activated T cells. These results suggest that manipulation of the host may enable a more effective tumor treatment while lessening the severity of the colitis.
Clinicaltrials.gov currently lists 31 active clinical immunotherapy vaccine trials that target CEA, 18 of which are actively recruiting patients as of August 2010. Eleven trials targeting CEA have completed accrual, and the lack of tumor responses and the lack of concomitant toxicities in these trials were likely due to the weak immunologic reactivity mediated by these vaccine strategies.
Materials and Methods
Cell lines and peptides. The following cell lines were routinely cultured in DMEM supplemented with 25 mmol/l HEPES, 2 mmol/l -glutamine, and 10% heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA): colon cancer cell lines (SW620, SW480, SW1463, and H508) and COS-7 cells. T2 cells were routinely cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mmol/l -glutamine, 50 U/ml penicillin, and 50 µg/ml streptomycin (Invitrogen). COS-7 cells stably expressing HLA-A*0201 and either full-length CEA (COS-A2-CEA) or NY-ESO-1 (COS-A2-ESO) were previously generated by retroviral transduction with vectors encoding these proteins. The colon cancer cell lines were purchased from ATCC (Manassas, VA) in 2007, and the T2 cells and COS-7 cells were obtained from Dr Paul Robbins (National Institutes of Health/National Cancer Institute (NCI)/Surgery Branch) in the mid-1990's. Prior to the initiation of this clinical trial, for authentication purposes, cell lines were characterized for expression of HLA-A2.1 and CEA (CEACAM5; CD66e) using a fluorescein isothiocyanate-conjugated monoclonal antibody against HLA-A2 (One Lambda; Canoga Park, CA) and a phycoerythrin-conjugated anti-CEA monoclonal antibody (Chemicon; Temecula, CA).
Three of four peptides used in this study were synthesized and purified to >95% purity by commercial sources (PiProteomics, Huntsville, AL; Macromolecular Resources Colorado State University, Fort Collins, CO; Multiple Peptide Systems, San Diego, CA) including CEA:691–699 (IMIGVLVGV) and the negative control peptides, HBVc:18–27 (23Y) (FLPSDYFPSV) and gp100:280–288 (YLEPGPVTA). The negative control peptide p53:264–272 (LLGRNSFEV) was synthesized in-house using a solid-phase method based on fluorenylmethoxycarbonyl (Fmoc) chemistry on a multiple peptide synthesizer (model AMS 422; Gilson, Worthington, OH), and molecular weight was verified by laser desorption mass spectrometry (Tufts University Core Facility, Boston, MA).
Retroviral vector, clinical trial, and transduction of patient PBL. A retroviral vector encoding both α and β chains of the CEA-reactive murine TCR separated by a picornavirus ribosomal skip element was constructed using an MSGV1 backbone as previously described.7 Clinical grade good manufacturing practices-quality retroviral supernatants were produced in the Surgery Branch of the NCI. This retrovirus was used to transduce PBL from three HLA-A*0201+ patients with metastatic colorectal cancer who had elevated levels of serum CEA protein (Table 1) and tumors that expressed high levels of CEA. This clinical trial (registry #NCT00923806 at http://clinicaltrials.gov) was conducted in the Surgery Branch of the NCI and was reviewed and approved by the National Institutes of Health Institutional Biosafety Committee, the NCI Institutional Review Board, the National Institutes of Health Office of Biotechnology Activities, and the Food and Drug Administration (all Bethesda, MD). Written informed consent was obtained from all three patients prior to inclusion in this clinical trial. Patient peripheral blood mononuclear cells were stimulated with anti-CD3 monoclonal antibody (OKT-3) and were transduced 2 days later with retroviral particles immobilized on retronectin (Takara Bio, Japan)-coated plates as previously described.9 2–4 × 108 genetically modified autologous T lymphocytes were adoptively transferred into these patients with interleukin-2 after a lymphodepleting chemotherapy regimen as previously described in our gene therapy trials in patients with melanoma that received autologous T cells transduced with TCRs against melanoma/melanocyte antigens.9,10,11
Quantitative PCR for retroviral DNA sequences and nonquantitative PCR for the CEA-reactive TCR. DNA was extracted from biopsy specimens and PBL using either the Maxwell 16 System (Promega, Madison, WI) or the Allprep DNA/RNA isolation kit (Invitrogen). DNA samples were subjected to quantitative PCR using a CFX96 Real Time System (Bio-Rad, Hercules, CA) with Taqman Fast Universal PCR Master Mix (Applied Biosystems, Foster City, CA) and a primer-probe mixture incorporating long-terminal repeats sequences designed to amplify MSGV1 retroviral DNA sequences as previously described.10,32 Quantitative PCR was also performed to determine the number of β-actin DNA copies in each sample. The number of retroviral DNA copies, normalized to the number of β-actin copies, was calculated relative to the number present in the adoptively transferred PBL using the ΔΔCT relative quantification method (Applied Biosystems). Nonquantitative PCR was also performed on DNA samples from patient 1 using specific distal 5′ (mTCR TRAV8-1; 5′-CACCATGCACAGCCTCCTGGGG-3′) and 3′ (mTCR CB2; 5′-TCAGGAATTTTTTTTCTTGACCATGGCCATCAGCACCAGG-3′) primers to amplify the full-length TCR αβ construct encoding the murine CEA-reactive TCR. The PCR products were then separated using a precast agarose ethidium bromide gel (Invitrogen).
FACS analyses. Biopsy specimens were disrupted using a BD Medimachine and 50 µm Medicon (BD Biosciences, San Jose, CA) and/or passage through a 40 µm nylon filter to obtain single cell suspensions. These samples, transduced PBL, and post-treatment peripheral blood mononuclear cell were stained with propidium iodide, anti-CD3, -CD8, -CD4, and –murine TCR β chain constant region antibodies (BD Biosciences and eBioscience, San Diego, CA), and analyzed by FACS using a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed using FlowJo software (Tree Star, Ashland, OR) after gating for live (propidium iodide-) CD3+ T cells.
IFN-γ secretion and ELISPOT assays. IFN-γ secretion (pg/ml) in 20 hour coculture supernatants of 105 target cells with 0.5–1 × 105 transduced PBL (250 µl total volume) was assessed within 4 days of patient treatment by enzyme-linked immunosorbent assay as previously described.7 IFN-γ enzyme-linked immunosorbent spots per 105 total transduced PBL or post-treatment peripheral blood mononuclear cell samples from 20 hour cocultures with various dilutions of the particular sample with 105 target cells were determined as previously described.9
Evaluation of anti-murine CEA TCR antibody responses. Post-treatment antibody responses to the murine CEA TCR were assessed by determining whether human IgGs capable of binding to CEA TCR transduced PBL were present in patient sera. Patient serum samples were collected at various time points before and after adoptive cell transfer and were stored at −80 °C prior to use. Thawed serum samples, diluted 1:1 with phosphate-buffered saline, were incubated with CEA TCR transduced PBL (50 µl per 5 × 105 cells in ~100 µl total volume) with gentle rocking at 4 °C for 1 hour. Cells were then washed three times and subsequently stained with FITC-conjugated antihuman IgG (BD Biosciences). Cells were also stained with anti-CD3 and anti-murine TCR β chain constant region antibody to determine transduction efficiency, and FACS analyses were performed as described above.
Serum inhibition of CEA TCR function was also assessed in vitro by evaluating antigen-specific IFN-γ secretion (pg/ml) by transduced PBL after incubation with pre and post-treatment serum samples. As described above, thawed serum samples were incubated with CEA TCR transduced PBL at 4 °C for 1 hour. Cells were then washed three times, and IFN-γ secretion was measured in 20 hour coculture supernatants by enzyme-linked immunosorbent assay. Percent inhibition of IFN-γ secretion by CEA TCR transduced PBL after incubation with post-treatment serum was calculated as follows:
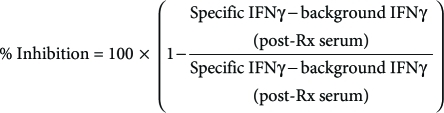 |
The pairs of specific and background target cells used for the calculations were as follows: (i) T2+CEA:691 versus T2+HBVc:18, (ii) COS-A2-CEA (HLA-A2+ CEA+) versus COS-A2-ESO (HLA-A2+ CEA-), and (iii) H508 (HLA-A2+ CEA+) versus SW480 (HLA-A2+ CEA-). Values less than zero were interpreted as no inhibition (0%), and values greater than 100 were interpreted as complete inhibition (100%).
SUPPLEMENTARY MATERIAL Figure S1. Persistence, function, and phenotype of adoptively transferred peripheral blood lymphocytes transduced to express the murine carcinoembryonic antigen reactive T cell receptor. IFNγ ELISPOTS per 105 total PBMC from 20 hr cocultures with various dilutions of the indicated samples with 105 target cells were determined as previously described (Johnson et al. 2009). Persistence was assessed by determining the percentage of CD3+ T lymphocytes that stained positively with an antibody specifically reactive with the murine TCR β chain constant region (eBioscience) by FACS. Phenotypes of the adoptively transferred and persistent cells were evaluated by FACS using antibodies against CD8, CD4, CD45RA, CD45RO, CD27, and CD28 after gating on CD3+ T cells expressing the murine TCR. *PBMC samples at early time points were limited in number. Therefore, persistence data for patient 2 corresponds to day 7 post-treatment rather than day 10 from which the ELISPOT data was derived. Figure S2. Development of anti-carcinoembryonic antigen T cell receptor IgG in patient sera after adoptive cell transfer. Transduced PBL were incubated with pre- and post-treatment serum samples and were subsequently stained with FITC-conjugated anti-human IgG. Transduced PBL were analyzed by FACS, and the values indicate the percentage of gated cells in the population pre-incubated with the latest post-treatment serum sample from each patient. Transduction efficiency in this experiment was 86% as evaluated by staining with anti-CD3 and anti-murine TCR b chain constant region antibodies. Table S1. Blocking of interferon-γ secretion (pg/ml) by anti-T cell receptor antibodies from patient serum.
Acknowledgments
This work was funded through the NCI intramural program. The authors thank Arnold Mixon and Shawn Farid for performing FACS analyses; Linda Parker, Azam Nahvi, and Laura Deviller for expanding the CEA TCR transduced T cells in vitro for adoptive transfer; Hui Xu and Mary A. Black for producing GMP quality retrovirus encoding the CEA TCR; Yong Li for assisting with DNA analyses; Patricia Fetsch for immunohistochemical staining; Susan Schwarz and John Riley for performing ELISPOT assays; and all the clinical fellows and nursing staff in the Clinical Center of The National Institutes of Health who provided these patients with outstanding care.
Supplementary Material
Persistence, function, and phenotype of adoptively transferred peripheral blood lymphocytes transduced to express the murine carcinoembryonic antigen reactive T cell receptor. IFNγ ELISPOTS per 105 total PBMC from 20 hr cocultures with various dilutions of the indicated samples with 105 target cells were determined as previously described (Johnson et al. 2009). Persistence was assessed by determining the percentage of CD3+ T lymphocytes that stained positively with an antibody specifically reactive with the murine TCR β chain constant region (eBioscience) by FACS. Phenotypes of the adoptively transferred and persistent cells were evaluated by FACS using antibodies against CD8, CD4, CD45RA, CD45RO, CD27, and CD28 after gating on CD3+ T cells expressing the murine TCR. *PBMC samples at early time points were limited in number. Therefore, persistence data for patient 2 corresponds to day 7 post-treatment rather than day 10 from which the ELISPOT data was derived.
Development of anti-carcinoembryonic antigen T cell receptor IgG in patient sera after adoptive cell transfer. Transduced PBL were incubated with pre- and post-treatment serum samples and were subsequently stained with FITC-conjugated anti-human IgG. Transduced PBL were analyzed by FACS, and the values indicate the percentage of gated cells in the population pre-incubated with the latest post-treatment serum sample from each patient. Transduction efficiency in this experiment was 86% as evaluated by staining with anti-CD3 and anti-murine TCR b chain constant region antibodies.
Blocking of interferon-γ secretion (pg/ml) by anti-T cell receptor antibodies from patient serum.
REFERENCES
- Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- Hance KW, Zeytin HE., and, Greiner JW. Mouse models expressing human carcinoembryonic antigen (CEA) as a transgene: evaluation of CEA-based cancer vaccines. Mutat Res. 2005;576:132–154. doi: 10.1016/j.mrfmmm.2004.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foon KA, John WJ, Chakraborty M, Das R, Teitelbaum A, Garrison J, et al. Clinical and immune responses in resected colon cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics the carcinoembryonic antigen. J Clin Oncol. 1999;17:2889–2885. doi: 10.1200/JCO.1999.17.9.2889. [DOI] [PubMed] [Google Scholar]
- Hörig H, Lee DS, Conkright W, Divito J, Hasson H, LaMare M, et al. Phase I clinical trial of a recombinant canarypoxvirus (ALVAC) vaccine expressing human carcinoembryonic antigen and the B7.1 costimulatory molecule. Cancer Immunol Immunother. 2000;49:504–514. doi: 10.1007/s002620000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JL, Hoyer RJ, Toomey MA, Faraguna K, Chang P, Richmond E, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–3973. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- Smith CL, Dulphy N, Salio M., and, Cerundolo V. Immunotherapy of colorectal cancer. Br Med Bull. 2002;64:181–200. doi: 10.1093/bmb/64.1.181. [DOI] [PubMed] [Google Scholar]
- Parkhurst MR, Joo J, Riley JP, Yu Z, Li Y, Robbins PF, et al. Characterization of genetically modified T-cell receptors that recognize the CEA:691-699 peptide in the context of HLA-A2.1 on human colorectal cancer cells. Clin Cancer Res. 2009;15:169–180. doi: 10.1158/1078-0432.CCR-08-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Li YF, El-Gamil M, Zhao Y, Wargo JA, Zheng Z, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180:6116–6131. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren EH, Fujii N, Akatsuka Y, Chaney CN, Mito JK, Loeb KR, et al. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010;115:3869–3878. doi: 10.1182/blood-2009-10-248997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Gattinoni L., and, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns WR, Zheng Z, Rosenberg SA., and, Morgan RA. Lack of specific γ-retroviral vector long terminal repeat promoter silencing in patients receiving genetically engineered lymphocytes and activation upon lymphocyte restimulation. Blood. 2009;114:2888–2899. doi: 10.1182/blood-2009-01-199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA., and, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner MK., and, Heslop HE. Adoptive T cell therapy of cancer. Curr Opin Immunol. 2010;22:251–257. doi: 10.1016/j.coi.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, Blazar BR., and, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol. 2009;9:704–716. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Raubitschek A, Leong L, Koczywas M, Williams L, Zhan J, et al. A phase I study of a combination of yttrium-90-labeled anti-carcinoembryonic antigen (CEA) antibody and gemcitabine in patients with CEA-producing advanced malignancies. Clin Cancer Res. 2009;15:2935–2941. doi: 10.1158/1078-0432.CCR-08-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeh HJ, 3rd, Brown CK, Holtzman MP, Egorin MJ, Holleran JL, Potter DM, et al. A phase I study of hyperthermic isolated hepatic perfusion with oxaliplatin in the treatment of unresectable liver metastases from colorectal cancer. Ann Surg Oncol. 2009;16:385–394. doi: 10.1245/s10434-008-0179-5. [DOI] [PubMed] [Google Scholar]
- Blesa JM., and, Pulido EG. Colorectal cancer: response to sunitinib in a heavily pretreated colorectal cancer patient. Anticancer Drugs. 2010;21 Suppl 1:S23–S26. doi: 10.1097/01.cad.0000361533.36428.0b. [DOI] [PubMed] [Google Scholar]
- van Loenen MM, de Boer R, Amir AL, Hagedoorn RS, Volbeda GL, Willemze R, et al. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc Natl Acad Sci USA. 2010;107:10972–10977. doi: 10.1073/pnas.1005802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010;16:565–70, 1p following 570. doi: 10.1038/nm.2128. [DOI] [PubMed] [Google Scholar]
- Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA., and, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss RH, Kuball J, Engel R, Guillaume P, Romero P, Huber C, et al. Redirection of T cells by delivering a transgenic mouse-derived MDM2 tumor antigen-specific TCR and its humanized derivative is governed by the CD8 coreceptor and affects natural human TCR expression. Immunol Res. 2006;34:67–87. doi: 10.1385/IR:34:1:67. [DOI] [PubMed] [Google Scholar]
- Stanislawski T, Voss RH, Lotz C, Sadovnikova E, Willemsen RA, Kuball J, et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat Immunol. 2001;2:962–970. doi: 10.1038/ni1001-962. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA. Of mice, not men: no evidence for graft-versus-host disease in humans receiving T-cell receptor-transduced autologous T cells. Mol Ther. 2010;18:1744–1745. doi: 10.1038/mt.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulos CM, Kaiser A, Wrzesinski C, Hinrichs CS, Cassard L, Boni A, et al. Toll-like receptors in tumor immunotherapy. Clin Cancer Res. 2007;13 18 Pt 1:5280–5289. doi: 10.1158/1078-0432.CCR-07-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos R, van Duikeren S, Morreau H, Franken K, Schumacher TN, Haanen JB, et al. Balancing between antitumor efficacy and autoimmune pathology in T-cell-mediated targeting of carcinoembryonic antigen. Cancer Res. 2008;68:8446–8455. doi: 10.1158/0008-5472.CAN-08-1864. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183:5563–5574. doi: 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Persistence, function, and phenotype of adoptively transferred peripheral blood lymphocytes transduced to express the murine carcinoembryonic antigen reactive T cell receptor. IFNγ ELISPOTS per 105 total PBMC from 20 hr cocultures with various dilutions of the indicated samples with 105 target cells were determined as previously described (Johnson et al. 2009). Persistence was assessed by determining the percentage of CD3+ T lymphocytes that stained positively with an antibody specifically reactive with the murine TCR β chain constant region (eBioscience) by FACS. Phenotypes of the adoptively transferred and persistent cells were evaluated by FACS using antibodies against CD8, CD4, CD45RA, CD45RO, CD27, and CD28 after gating on CD3+ T cells expressing the murine TCR. *PBMC samples at early time points were limited in number. Therefore, persistence data for patient 2 corresponds to day 7 post-treatment rather than day 10 from which the ELISPOT data was derived.
Development of anti-carcinoembryonic antigen T cell receptor IgG in patient sera after adoptive cell transfer. Transduced PBL were incubated with pre- and post-treatment serum samples and were subsequently stained with FITC-conjugated anti-human IgG. Transduced PBL were analyzed by FACS, and the values indicate the percentage of gated cells in the population pre-incubated with the latest post-treatment serum sample from each patient. Transduction efficiency in this experiment was 86% as evaluated by staining with anti-CD3 and anti-murine TCR b chain constant region antibodies.
Blocking of interferon-γ secretion (pg/ml) by anti-T cell receptor antibodies from patient serum.