Abstract
Nonintegrating lentiviral vectors present a means of reducing the risk of insertional mutagenesis in nondividing cells and enabling short-term expression of potentially hazardous gene products. However, residual, integrase-independent integration raises a concern that may limit the usefulness of this system. Here we present a novel 3′ polypurine tract (PPT)-deleted lentiviral vector that demonstrates impaired integration efficiency and, when packaged into integrase-deficient particles, significantly reduced illegitimate integration. Cells transduced with PPT-deleted vectors exhibited predominantly 1–long terminal repeat (LTR) circles and a low level of linear genomes after reverse transcription (RT). Importantly, the PPT-deleted vector exhibited titers and in vitro and in vivo expression levels matching those of conventional nonintegrating lentiviral vectors. This safer nonintegrating lentiviral vector system will support emerging technologies, such as those based on transient expression of zinc–finger nucleases (ZFNs) for gene editing, as well as reprogramming factors for inducing pluripotency.
Introduction
The notion that there is an inherent risk of insertional mutagenesis in all integrating vectors1,2 gives impetus for the development and use of nonintegrating lentiviral vectors in vitro and in vivo. The nonintegrating lentiviral vectors most commonly used in current studies are generated by packaging vectors with a mutated integrase gene bearing one of several nonpleiotropic mutations at the integrase's catalytic-core domain that specifically disrupt integration.3
To date, nonintegrating lentiviral vectors have been used to deliver transgenes to a number of target organs, including the eye, liver, brain, muscle, and lymph nodes.4,5,6,7,8 Importantly, the transient transgene expression of nonintegrating lentiviral vectors in rapidly dividing cells would be highly advantageous in preclinical settings where short-term expression of potentially genotoxic transgenes is required, such as the delivery of zinc–finger nucleases (ZFNs) to dividing cells to mediate site-specific, double-strand DNA break-induced recombination.9 Notwithstanding these advances, the phenomenon of integrase-independent (illegitimate) integration poses a safety concern to nonintegrating lentiviral vectors that may restrict their utilization.10,11 Indeed, a recent study reported that the frequency of illegitimate integration ranged between 0.35% and 2.3%.9
Structures of retroviral DNA found in the nucleus include linear episomes, formed directly by reverse transcription (RT);12 2-long terminal repeat (LTR) circular episomes, generated by cellular nonhomologous end-joining DNA double-strand break repair machinery;13 and heterogeneous circular episomes, formed, at least in part, by self-integration.14 Interestingly, 1-LTR circular episomes are formed either by the host cell's homologous recombination (HR) double-strand break repair pathway15 or as abortive RT products.16,17 The specific episomal form contributing to illegitimate integration has not been established, although studies conducted on plasmid DNA suggest that linear DNA integrates much more efficiently than supercoiled circular DNA.18 To extend and refine these observations, we hypothesized that minimizing the formation of linear episomes may be a strategy for reducing the magnitude of illegitimate integration. In this regard, we perceived the possibility of enhanced 1-LTR circle formation prior to the completion of RT as a means of altering the natural formation of linear episomes, and, consequently, reducing illegitimate integration. This approach was premised on the notion that 1-LTR circles are formed, in part, as natural by-products of incomplete RT, which occurs in the absence of strand displacement.12,19 We hypothesized that this phenomenon could be enhanced by deleting the vector's 3′ polypurine tract (PPT), thus forcing initiation of plus-strand synthesis from non-PPT sequences.19,20 Indeed, we describe here novel PPT-deleted HIV-1 vectors, which produce predominantly 1-LTR circular genomes and reduced levels of linear genomes. Consequently, integrase-mediated integration and 2-LTR circle formation, which require linear genomes as substrates,13 were reduced tenfold and threefold, respectively. Interestingly, analysis of 2-LTR circles formed by PPT-deleted vectors revealed unique cryptic plus-strand initiation sites. Furthermore, PPT-deleted vectors packaged into integrase-deficient particles exhibited in vitro and in vivo titers and expression levels comparable to the levels of conventional nonintegrating vectors. Importantly, the level of illegitimate (integrase-independent) integration from PPT-deleted vectors was approximately threefold lower than the levels of illegitimate integration from conventional integrase-deficient vectors.
Results
Efficient formation of 1-LTR circles under conditions that do not favor HR
We hypothesized that aberrant RT, which does not complete the final strand-displacement step, may account for the formation of some 1-LTR circles, and that inhibiting HR would, therefore, only partially reduce the formation of 1-LTR circles in vector-transduced cells. To this end, we transduced either cell lines mutant for the HR factors BRCA121 or Ercc1.22 Also, to address the possibility that HR enzymes other than BRCA1 and Ercc1 are responsible for 1-LTR circle formation, we transduced cultured cells arrested in G1 phase of the cell-cycle, in which HR is not active.23,24 Furthermore, to analyze episome formation in a physiologically relevant, nondividing cell type, we examined 1-LTR circle formation in liver tissue (a major target organ for gene therapy), which is primarily in G1/G0 phase and, thus, is not expected to exhibit a high level of HR activity. Relative abundance of episomal forms was determined by Southern-blot analysis of total DNA from transduced cells or through a shuttle–vector assay. As described earlier,5,25 shuttle vectors contain a drug resistance gene and bacterial origin of replication upstream of the vectors' 3′ LTR, which allows the Hirt extraction of circular vector genomes from transduced cells, individual-clone amplification in bacteria, and restriction-digest analysis of the vectors' circular episomes (Figure 1, Supplementary Figure S1). The data presented in Figure 1 demonstrated that transduced HR factor-deficient cell lines, mutated for either BRCA1 or Ercc1, did not exhibit a reduction in 1-LTR circles relative to linear episomes (Figure 1a,d). Similarly, transduced G1-arrested cells also did not show a decrease in the 1-LTR circular forms (Figure 1c,d, Supplementary Figure S2 confirms G1 arrest). Consistent with these results, analysis of episome formation in the liver (Figure 1b) was in the range of normal episome formation, as observed in a previous study.5 These data suggest that incomplete RT could be the primary source of 1-LTR circles in transduced cells (Figure 2).
Figure 1.
Analysis of lentiviral vector episome formation under conditions not conducive to homologous recombination. Shuttle vector analysis (see Materials and Methods) was employed on Hirt-extracted episomes from lentiviral shuttle vector-transduced (a) Ercc1-defective Chinese hamster ovary (E1KO7-5) cells, (b) liver cells, and (c) cycle-arrested Chinese hamster ovary (AA8 cells). Panels a–c represent episome formation as a percentage of total episomes; each experiment was performed in triplicate. The term “mutant” refers to circular vector genomes generated by autointegration, as determined by restriction products that do not fit the expected band sizes corresponding to 2-long terminal repeat (LTR) and 1-LTR circular episomes. (d) Episome formation was examined by Southern-blot analysis on DNA extracted 2 days after transduction with an integrating, polypurine tract+ vector (vTK945) from Ercc1-deficient (E1K07-5) (lane 1), Ercc1-wild-type (ATStg) (lane 2), cycling AA8 (lane 3), arrested AA8 (lane 4), human breast cancer BRCA1-deficient (SUM149) cells (lane 5), and human breast cancer (ME16C2) cells containing wild-type BRCA1 (lane 6). DNA was digested with EcoNI and PflMI and probed with the probe complementary to the 3′ region of vTK945, as shown in Figure 3b.
Figure 2.
Proposed model for episome formation and integration in cells transduced with polypurine tract (PPT)-positive or PPT-deleted vectors. (a) The RNA genomes of PPT-positive vectors undergo plus-strand synthesis (depicted as a dashed line) initiation at the PPT, leading to efficient strand displacement and producing primarily linear episomes, with a small number of 1-long terminal repeat (LTR) circles formed by failed strand displacement. Linear episomes may then serve as the substrates for homologous recombination (HR), forming 1-LTR circles; nonhomologous end-joining (NHEJ), forming 2-LTR circles; and integrase-mediated integration, forming integrated provirus. (b) The RNA genomes of PPT-deleted (ΔPPT) vectors undergo plus-strand synthesis initiation at cryptic sites upstream or downstream of the PPT, leading to inefficient strand displacement and producing primarily 1-LTR circles (plus-strand synthesis is represented by dashed lines). However, in the few instances in which initiation of plus-strand synthesis from non-PPT sites (from sites located either upstream to the deleted PPT or in the 3′U3) is followed by strand displacement, the aberrant episomal linear forms contained either additions to their 5′ ends or deletions in the 5′ U3 region. NHEJ-mediated circularization of these aberrant linear forms generate 2-LTR circles containing either an insertion between the LTRs or a deletion in the 5′ U3 region. The aberrant linear forms that lack the 5′ integrase attachment (att) site do not support efficient integrase-mediated integration.
PPT deletion decreases the levels of linear vector forms and enhances the formation of 1-LTR circles
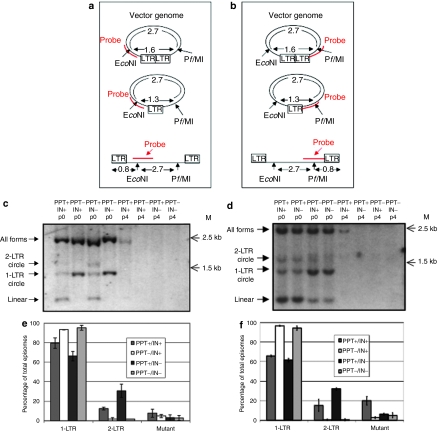
We hypothesized that initiating plus-strand synthesis at non-PPT (cryptic) sites may inhibit strand displacement, thereby altering the RT process and leading to an increase in the levels of 1-LTR circles. Accordingly, we deleted the 3′ PPT, which borders the 3′LTR and serves as a primer for plus-strand synthesis, and characterized the effect of the deletion on episome formation. However, the modified vectors retained the central PPT (cPPT) (Supplementary Figure S1). We used either PPT-positive (vTK945) or PPT-deleted (vTK1179) lentiviral vectors (outlined in Supplementary Figures S1 and S3), packaged with or without functional integrase, to transduce human embryonic kidney 293T cells. To characterize episome formation, we employed Southern-blot analysis with probes complementary to either the vectors' 5′ or 3′ regions (Figure 3). Importantly, as observed when using the probe complementary to the vectors' 5′ regions (Figure 3a,c), the PPT-deleted vector significantly increased 1-LTR circle formation and eliminated linear episome formation. Interestingly, employing a probe complementary to the 3′ end of the vectors' genomes (Figure 3b) showed that PPT-deleted vectors produce detectable levels of linear episomes, though at roughly a threefold lower level than PPT-positive vectors (Figure 3d) (Supplementary Table S1). These data support our notion that the aberrant RT induced by the PPT deletion mediates plus-strand synthesis from several cryptic sites in the vector genome, producing linear episomes with a uniform (detectable) 3′ end, but with a heterogeneous 5′ end, which could not be detected by the 5′ probe (Supplementary Figure S4). Interestingly, this data was supported by a Southern-blot analysis of undigested genomic DNA extracted from cells transduced with PPT+ (vTK945) and PPT-deleted (vTK1179) vectors, which exhibited predominantly linear and 1-LTR episomal forms, respectively (Supplementary Figure S5). Furthermore, concomitant with the reduction of linear episomes, PPT-deleted vectors exhibited a corresponding reduction of 2-LTR circle formation (Figure 3c–f), consistent with the previously established model that 2-LTR circles are formed from linear episomes by the process of nonhomologous end joining.13 The effect of the PPT deletion on episome formation was further confirmed by employing novel PPT-deleted shuttle vectors. In keeping with the result of the Southern-blot analysis, we found that the relative proportion of 1-LTR circles produced by non-self-inactivating PPT-deleted vectors increased from 79.5% to 93.3% (IN+) and from 66.2% to 95.3% (IN−) (Figure 3e). Furthermore, the relative abundance of 2-LTR circles was concomitantly reduced by the PPT deletion, to 1.77% (integrase-proficient (IN+)) and 1.87% (integrase-mutant (IN−)), compared with 12.6% and 30.7%, respectively, in PPT-positive vectors (Figure 3e), which was in keeping with the reduction in linear vector genomes, possibly due to inefficient strand displacement caused by cryptic plus-strand initiation in PPT-deleted vectors, as observed in Figure 3d and outlined in Figure 2. Not surprisingly, the relative proportion of 1-LTR circles produced by a SIN PPT-deleted vector was increased relative to its PPT-wild-type counterpart, while the relative proportion of 2-LTR circles generated by the PPT-deleted SIN vector was found to be strongly reduced (Figure 3f). Intrigued by the formation of linear and 2-LTR genomes produced by aberrant plus-strand synthesis during RT, we characterized the structure of the 2-LTR circles' junctions of these non-self-inactivating vectors by sequence analysis. Interestingly, all of the 2-LTR circles sequenced from a PPT-deleted, non-self-inactivating vector exhibited wild-type 3′ LTRs, and a 5′ LTR bearing a ~180 bp deletion at the 5′ end of the U3; this finding indicates that positive-strand synthesis initiation took place within the 5′ U3 (Supplementary Figure S6). Next, we used shuttle–vector analysis of episome formation by a SIN vector to investigate the effect of deleting the alternative plus-strand synthesis initiation sites in the U3 on the mechanism of 2-LTR circle formation. Interestingly, we found that 2-LTR circle formation from the SIN PPT-deleted vector, as determined by shuttle–vector assay, was reduced up to threefold in comparison with that of a non-self-inactivating PPT-deleted vector (Figure 3e,f). Sequence analysis of a 2-LTR circle formed by a SIN PPT-deleted vector (vTK1046) revealed intact SIN LTRs flanking an insert of 43bp derived from the 3′ end of the vector genome, adjacent to the 3′ LTR (Supplementary Figure S6), which indicates plus-strand initiation at a cryptic site 5′ of the 3′ LTR (Figure 2). Intrigued by this finding, we sought to characterize the effect of the cPPT on the episome formation of PPT-deleted vectors. Southern-blot analysis of SIN vectors either bearing deletions of the cPPT and PPT, of the PPT alone, or bearing no PPT deletions indicated that the dual deletion of the cPPT and PPT did not alter the relative abundances of episomes exhibited by vectors bearing only the PPT deletion (Supplementary Figure S7).
Figure 3.
Analyzing the effect of the polypurine tract (PPT) deletion on lentiviral vector episome formation. (a,b) Outline of Southern-blot analysis used to characterize vector provirus and episomes by probing at the (a) 5′ and (b) 3′ regions of the genome. After extracting vector DNA from transduced cells, digestion with EcoNI and PflMI, and probing with either (a) a KasI/BamHI fragment of at PPT+ vector (vTK945) or (b) a AfeI/EcoRV fragment of vTK945, bands indicative of total episomes and integrated provirus (2.7 kb), 2-long terminal repeat (LTR) circles (1.6 kb), 1-LTR circles (1.3 kb), and linear episomes (0.8 kb) were determined. (c) Cells were transduced with a PPT+ vector (vTK945), integrating (lanes 1 and 5) or nonintegrating (lanes 3 and 7), or with a PPT− vector (vTK1179), integrating (lanes 2 and 6) or nonintegrating (lanes 4 and 8) and total DNA was extracted from transduced cells 3 days (no passages) (lanes 1–4) or ~14 days (four passages (p4)) (lanes 5–8) post-transduction, digested as shown in a and b, and analyzed by Southern blot, using the EcoNI-spanning probe complementary to the 5′ region of the virus. (d) Cells were transduced with the PPT+ vector, integrating (lanes 1 and 5) or nonintegrating (lanes 2 and 6), or with the PPT− vector, integrating (lanes 3 and 7) or nonintegrating (lanes 4 and 8) and total DNA was extracted from transduced cells 3 days (no passages) (lanes 1–4) or ~14 days (p4) (lanes 5–8) post-transduction, digested as in (a,b), and analyzed by Southern blot, using the PflMI-spanning probe complementary to the 3′ region of the virus. For c,d, bands corresponding to total vector genomes, 2-LTR circular, 1-LTR circular, and linear episomal genomes, as outlined in a,b, respectively, are shown. (e) Sixteen hours after transduction with a non-self-inactivating (non-SIN), PPT-deleted shuttle vector (vTK1074) and its PPT-positive equivalent (vTK459), which were packaged with both functional integrase (IN+) and mutant integrase (IN−), episomes were harvested from 293T cells and analyzed by shuttle–vector assay; each transduction was performed in triplicate. (f) Episomes were harvested from 293T cells 16 hours after transduction with a SIN, PPT-deleted shuttle vector (vTK1046) and its PPT-positive equivalent (vTK1054), which were packaged with IN+ and IN−, and analyzed by shuttle–vector assay; each transduction was performed in triplicate. For e,f, the data are presented as percentages of 1-LTR, 2-LTR, and mutant forms out of the number of total episomes.
PPT-deleted vectors exhibit impaired integrase-mediated integration and reduced illegitimate, integrase-independent integration
We next sought to explore the possibility that the decrease in linear genomes generated by PPT-deleted vectors would result in a concomitant reduction in illegitimate (integrase-independent) integration of vector genomes packaged into integrase-deficient particles. To test this hypothesis, 293T cells were transduced with either wild-type or PPT-deleted vectors, as shown above (Figure 3c), and, after four passages (p4), were analyzed by Southern blotting to determine the relative abundances of episomal and integrated vector genomes. As shown in Figure 3c, the PPT-deleted vectors did not generate an integrated-provirus signal, even in the presence of functional integrase. Hence integration was either nonexistent or below the threshold of detection by Southern-blot analysis using a radiolabeled probe complementary to a sequence in the vector genome's 5′ region. To confirm and quantify these results with greater sensitivity, we employed fluorescence-activated cell sorting analysis and quantitative PCR to measure vector-derived green fluorescent protein (GFP) expression and vector–genome persistence, respectively. As shown in Figure 4a, the integrase-proficient, PPT-deleted (PPT-/IN+) vector produced a notably low level of GFP expression, with a mean fluorescence intensity of 40, which was typical of nonintegrating lentiviral vectors previously examined by this group and others5,9 and comparable to the GFP expression generated by the integrase-mutant vectors, which produced mean fluorescence intensities of 30 (PPT+/IN−) and 39 (PPT-IN−); conversely, the conventional, PPT-positive, integrase-proficient vector exhibited a high level of GFP expression (mean fluorescence intensity of 280). The low level of GFP expression, which is a hallmark of nonintegrating vectors, produced by the PPT-deleted vectors indicates that they fail to integrate efficiently, even in the presence of functional integrase. The idea that PPT-deleted vectors integrate poorly was further supported by the finding that only 2.4% of cells transduced with the PPT−/IN+ vector (out of 63% 3 days after transduction) still expressed GFP after p4, while approximately a third of cells transduced with the conventional PPT+/IN+ (vTK1187) vector continued to express GFP at p4 (Figure 4b), which is in line with previously measured levels of integrase-mediated integration.5,26 Similar to earlier studies demonstrating minimal vector integration in the absence of integrase activity,5,27 0.5% of cells transduced with integrase-deficient vectors, either with or without the PPT, were GFP-positive after p4 (compared to 50% GFP-positive cells at 3 days post-transduction). This low level of GFP-positive cells, generated by illegitimate integration, which is near the level of background signal detected by fluorescence-activated cell sorting, constituted a technical limitation necessitating the use of a more sensitive, qPCR-based assay in order to characterize illegitimate integration more accurately. Accordingly, qPCR analysis of integration efficiency was determined as the ratio of vector genome copies per cell measured p4 post-transduction to those detected after 3 days. Indeed, as shown in Figure 4c, employing the qPCR assay showed that the integration efficiency of the PPT-deleted, integrase-proficient vector was 2.7%, in comparison to 27% for the PPT+, integrase-proficient vector, indicating that the PPT deletion alone causes a tenfold reduction in integration efficiency. Indeed, these results are in line with the results of the fluorescence-activated cell sorting analysis (Figure 4b), which also indicate a roughly tenfold difference in integration between PPT+ and PPT-deleted, integrase-competent vectors. Most importantly, the qPCR assay provided direct evidence that the PPT deletion reduced illegitimate integration from integrase-deficient lentiviral vectors. As shown in Figure 4c, the PPT−/IN− vector exhibited an integration level of 0.08%. In comparison, the PPT+/IN− vector's integration level was 0.2%, which was in line with previously measured frequencies of integrase-independent integration.9 Furthermore, these results were confirmed with similar vectors expressing luciferase, in which the PPT deletion reduced illegitimate integration by 2.8-fold (Supplementary Figure S8). These results are concordant with the Southern-blot analysis using a 3′ probe, which indicated that the PPT deletion reduces linear episome formation threefold, suggesting that the linear form is, indeed, the major substrate for illegitimate integration.
Figure 4.
Analyzing the effect of the polypurine tract (PPT) deletion on in vitro expression and integrase-mediated or integrase-independent integration from lentiviral vectors. (a,b) Fluorescence-activated cell sorting analysis of green fluorescent protein (GFP) expression generated by PPT+ (vTK1187) (left) or PPT− (vTK1188) (right), with (IN+) (upper) or without (lower) functional integrase (IN−), in 293T cells was carried out either (a) at 3 days (passage 0 (p0)) or (b) four passages (p4) post-transduction. The percentage of GFP-positive cells and mean fluorescence intensity (MFI) are shown. (c) Quantification in 293T cells of vector integration by PPT+ (vTK1187) (dark gray) or PPT− (vTK1188) (light gray), with (IN+) (left side) or without (IN−) (right side) functional integrase. Integration percentage was calculated for each vector as a ratio of vector copy number per cell at p4 and p0. Samples subjected to the qPCR assay were analyzed in triplicate, and error bars are presented as ± SD.
PPT-deleted vectors exhibit titers comparable to those of conventional nonintegrating HIV-1 vectors
We sought to characterize the effects of the PPT deletion on HIV-1 vector titers. To this end, the various PPT-deleted and PPT positive vectors were packaged into either integrase-proficient or integrase-deficient particles. Vector titers were determined by scoring GFP-positive cells following serial dilutions on 293T cells. As shown in Supplementary Table S2, the PPT-deleted vectors exhibited titers comparable to those of PPT-positive vectors, indicating that the PPT deletion did not hinder the production of vector particles.
Efficient expression of transgene expression in the rat brain
To test the efficacy of PPT-deleted vectors in vivo, we examined the expression of wild-type and PPT-deleted vectors, packaged with and without functional integrase, 2 months after striatal infusion into the rat brain. As in the in vitro expression assays, we found that expression from the PPT-deleted vectors was comparable to the expression produced by the PPT+/IN− vector (Figure 5). These findings indicate that PPT-deleted lentiviral vectors are as capable of forming particles and effecting target-cell transduction in vivo as conventional nonintegrating vectors.
Figure 5.
The effect of the polypurine tract (PPT) deletion on transgene expression from lentiviral vectors in vivo. Green fluorescent protein expression mediated by PPT+ (vTK945) or PPT− (vTK1023) vectors, packaged into particles containing either functional (IN+) (upper) or deficient (IN−) (lower) integrase, in the striatum of the rat brain, was measured 2 months after vector infusion. The indicated scale bar is equal to 20 µm.
Discussion
This study was aimed at reducing illegitimate integration, which can limit the applications of nonintegrating vectors, as demonstrated in a recent study on reprogramming somatic cells into stem cells.28 Attempts to reduce illegitimate integration by solely targeting the integration pathway through mutating the vector's attachment sites in combination with integrase mutations have not been successful.6,27 In a different approach, we sought to reduce the illegitimate integration of lentiviral vectors by enhancing the formation of 1-LTR circles, which are not substrates for integration. This strategy was premised on earlier publications indicating that 1-LTR circles are natural products of RT,29 generated in the absence of strand displacement.19 This scheme was further predicated on the notion that the PPT deletion could alter the RT process, directing the plus-strand initiation site to cryptic sites (Figure 2); indeed, previous in vitro and in vivo studies with HIV-1 have indicated that, in the course of RT, HIV-1 second-strand synthesis can be initiated from non-PPT sites.20,30,31,32 Furthermore, premised on earlier studies18 suggesting that linear DNA is more prone to integrase-independent integration than circular DNA, we postulated that the decrease in the formation of linear genomes would correspond with a decrease in illegitimate integration of vector genomes. Indeed, the data presented here indicated a threefold reduction in linear genomes after deletion of the PPT, with a comparable reduction in illegitimate integration of HIV vectors packaged without functional integrase. Interestingly, the relative reduction of 2-LTR circle formation, as measured by the shuttle–vector assay, was more pronounced than the reduction in the linear form, as measured by Southern-blot analysis. Furthermore, the PPT-deleted SIN vectors showed even lower 2-LTR circle formation. This finding may be attributed to difficulties in transforming bacteria with oversized episomal genomes generated following plus-strand synthesis initiation at cryptic sites located significantly upstream of the vector's 3′ LTR (Figure 2 and Supplementary Figure S4). These putative linear RT products may contain more than one bacterial origin of replication, which would not be conducive to colony formation, as required by the shuttle–vector assay. Overall, our results support the idea that reducing integration at two different steps of the viral life cycle, by (i) using a novel PPT deletion to decrease linear episome formation during RT, and (ii) inhibiting integrase activity using nonpleiotropic integrase mutations, decreases illegitimate integration significantly. The data presented here demonstrate a more efficacious method of reducing illegitimate integration than utilizing integrase mutations alone. Indeed, this combinatorial approach generates a safer gene-therapy platform for clinical and research applications than currently used nonintegrating lentiviral vector systems, especially in settings requiring large doses of vector particles in vivo or transient expression of hazardous genes ex vivo.
To date, the relative efficiency of transgene expression from the different HIV-1 vector episomes has not been determined. Here, we demonstrated that in vitro and in vivo expression from PPT-deleted vectors matched the levels of expression from conventional nonintegrating vectors (Figures 4 and 5), indicating that the dramatic alteration in the relative amounts of episomal HIV-1 vectors induced by the PPT deletion does not affect episomal transgene expression. Taken together, these data suggest that transgene expression efficiency from linear episomal HIV-1 vector genomes (the predominant episomal vector form in cells transduced with conventional HIV-1 vectors) is comparable to the levels of transgene expression from 1-LTR circles (the predominant form generated following transduction with PPT-deleted vectors).
The development of ZFNs to introduce site-specific DNA double-strand breaks as a means to enhance host DNA repair-mediated gene editing is considered a major biotechnological breakthrough. To avoid genotoxicity associated with continuous expression of ZFNs, Lombardo et al. successfully employed nonintegrating HIV-1 vectors to express ZFNs transiently, as well as to deliver the donor DNA template required for DNA repair-mediated gene editing in vitro.33 Although illegitimate integration of nonintegrating HIV-1 vectors carrying either the ZFNs or the donor DNA has not been detected by Lombardo et al., recent studies by Cornu et al. indicated that >0.35% of the nonintegrating HIV-1 vector genomes are expected to illegitimately integrate into the host cell genomes.9 Furthermore, the fact that the incorporation of donor DNA into double-stranded DNA breaks can be mediated by either HR or nonhomologous end-joining suggests that the nature of the template DNA could affect the gene editing process. In this regard, ZFNs and donor DNA template delivery by PPT-deleted vectors could reduce the risks associated with illegitimate integration of either the donor DNA template or the ZFN-expression cassettes. Furthermore, the low levels of linear vector forms generated by PPT-deleted vectors could reduce the occurrence of nonhomologous endjoining-based gene correction.
Overall, we present here the development of novel PPT-deleted vectors that primarily generate 1-LTR circles. The novel vectors exhibit impaired integration in the presence of proficient HIV-1 integrase and reduced illegitimate integration when packaged into integrase-deficient particles. These features render the PPT-deleted vectors safer and more suitable for gene replacement therapy and for research and clinical applications, including ZFN-based gene editing.
Materials and Methods
Lentiviral vector construction. vTK945 and vTK1054 were described previously.5 vTK1023 was generated by deleting an NheI fragment from vTK945, eliminating the PPT, then inserting an NheI fragment from vTK978. vTK1046 was generated by deleting a KpnI/ApaI fragment from vTK459 and replacing it with a KpnI/ApaI fragment from vTK1023. vTK1179 was constructed by deleting a PflMI/EcoRV fragment from vTK945 and replacing it with a PflMI/EcoRV fragment from vTK1074, which contains a precise PPT deletion. vTK1074 was generated by performing two separate PCR experiments with two sets of primers, both using vTK459 as template: CCTGGTTGCTGTCTCTTTATGAGG (Fw) and GAATTAGCCCTTCCAGTAAAAAGTGGCTAAG (Rev), and CTTAGCCACTTTTTACTGGAAGGGCTAATTC (Fw) and CAATGTCAACGCGTATATCTGGCCCG (Rev). The resulting amplicons served as templates in a second PCR experiment, in which CCTGGTTGCTGTCTCTTTATGAGG (Fw) and CAATGTCAACGCGTATATCTGGCCCG (Rev) were the primers. The resulting single amplicon was cloned into vTK459 with SacII/ApaI to create vTK1074. vTK1187 was created by cloning vTK529, a BSD-GFP-containing vector, into vTK945 with AfeI/XhoI, while vTK1188 was made by cloning vTK529 into vTK1179 with AfeI/XhoI. vTK993 was described previously,5 and vTK1034 was generated by cloning vTK1023 into vTK464, a luciferase-containing vector, with KpnI/PmeI. The term vTK refers to a particular DNA construct and the corresponding lentiviral vector.
Viral vector production. All lentiviral vectors were prepared as previously described,34 transiently transfecting 107 293T cells with 15 µg vector cassette, 10 µg packaging cassette, and 5 µg envelope cassette. Integrating vectors were made using the packaging cassette ΔNRF,34 which expresses functional integrase, while nonintegrating vectors were made using the packaging cassette vTK939, which was made by inserting the D64E-mutant integrase from pD64E into ΔNRF by standard cloning procedures. All vectors were pseudotyped with the vesicular stomatitis virus-glycoprotein envelope cassette. For vectors constitutively expressing GFP, titers were assessed by serial dilution in 293T cells followed by visual analysis and counting of GFP-positive cells by fluorescence microscope. For other vectors, concentrations were determined by p24 gag enzyme-linked immunosorbent assay. The absence of replication-competent retroviruses was determined by three independent methods: tat transfer assay, vector rescue assay, and p24 gag enzyme-linked immunosorbent assay, as described previously.35
Cell culture. The following cell lines were used: 293T, AA8, E1KO7-5, and ATStg (kind gift from Rodney Nairn, University of Texas), SUM149 and ME16C2 (kind gift from William Kaufmann, University of North Carolina). AA8, E1KO7-5, and ATStg cells were grown in α-MEM (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum. SUM149 were grown in HuMEC basal serum free medium (Invitrogen), supplemented with 5% fetal bovine serum, HuMEC supplement, and 25 mg bovine pituitary extract. ME16C2 cells were grown in HuMEC basal serum free medium (Invitrogen), supplemented with HuMEC supplement, and 25 mg bovine pituitary extract. All media were supplemented with 1% penicillin/streptomycin solution.
In vivo experiments in mouse liver. BALB/c mice (Jackson Laboratory, Bar Harbor, ME) were injected intraperitoneally at 8 weeks of age with 40 µg p24 of lentiviral vector. At 21 days postinjection, livers were harvested, strained into single-cell suspensions, and subjected to shuttle–vector analysis of episome formation.5
FACScan analysis. 293T cells were transduced and analyzed as previously described.5 Cell-cycle arrest was achieved by growing AA8 cells to confluence for 4 days in media containing 1% dimethyl sulfoxide. For cell-cycle analysis, cells were harvested, fixed in 70% ethanol (Pharmco-AAPER, Brookfield, CT), and incubated in a solution containing 50 µg/ml propidium iodide (Molecular Probes, Eugene, OR), 100 units/ml RNase A (Sigma, St. Louis, MO), 100 mg bovine serum albumin fraction V (Roche, Mannheim, Germany), and 10 ml phosphate buffered saline.
Shuttle–vector assay. 293T cells were transduced with integrating or nonintegrating vTK459, vTK1054, or vTK1074 (see above for construction details), and mouse livers were transduced with integrating or nonintegrating vTK1054. Transduced cells were harvested 16 hours post-transduction using 0.85 ml Hirt lysis buffer and 0.25 ml 5 mol/l NaCl per 10 cm plate of transduced cells, and livers were harvested 21 days post-transduction using 14.56 ml sodium dodecyl sulfate-free Hirt lysis buffer, 0.92 ml 10% sodium dodecyl sulfate, and 4.36 ml 5 mol/l NaCl per liver. Episomal DNA was subsequently subjected to phenol/chloroform extraction and DpnI digestion and electroporated into bacteria. Bacterial colonies were cultured individually in 2 ml volumes of lysogeny broth medium containing 100 µg/ml ampicillin and pelleted, and their episomes were extracted by boiling in a hotprep buffer made with 5 g sucrose, 4 ml 0.5 mol/l Tris/EDTA solution, 40 ml 10% Triton X-100 (Sigma), 5 ml 1 mol/l Tris/HCl pH 8.0 and water to 100 ml. 700 µl hotprep buffer and 25 µl lysozyme solution (10 mg/ml lysozyme (Sigma) in 0.25 mol/l Tris/HCl pH 8.0) were added to each bacterial pellet, which was resuspended and placed on ice for 5–10 minutes before boiling for 1 minute. After boiling, samples were centrifuged at 14 K for 10 minutes to dispose of cell debris. The supernatant was then mixed with 700 µl isopropanol and centrifuged at 14 K for 10 minutes, then the DNA pellet was washed with 70% ethanol, dried, and resuspended in 50–200 µl water containing 10 µg/ml RNase A. Episomal DNA clones were digested with NotI and SacII and electrophoresed in a 1% agarose gel to be characterized as 1-LTR, 2-LTR, or mutant circular episomes. For every transduction, between 100 and 112 episome-bearing bacterial colonies were characterized.
Southern-blot analysis. 293T, E1KO7-5, ATStg, SUM149, ME16C2, and AA8 cells were harvested 3 days and p4 after transduction with vTK945 or vTK1179, and their total DNA was extracted as previously described.36 10 µg total DNA was digested with DpnI, EcoNI and PflMI and probed with either the 1.4 kb KasI/BamHI fragment or the 1.6 kb AfeI/EcoRV fragment of vTK945, except in the case of Supplementary Figure S5, in which 10 µg total DNA was digested with DpnI (to eliminate plasmid DNA), SphI (to cut cellular genomic DNA), and NheI (to cut cellular genomic DNA) or XbaI (to singly cut vector DNA). Samples were probed with the 1.3 kb BssHII/XmaI fragment of vTK459. Quantitative analysis of DNA density in the Southern blot presented in Figure 3d was achieved using ImageJ software.
Quantitative PCR analysis. In this study, vector titers were quantified through direct counting of GFP-positive cells and/or p24gag enzyme-linked immunosorbent assay of vector stocks, and equal amounts of the lentiviral vectors were used for transduction of 293T cells. 293T cells were transduced at an multiplicity of infection of 0.5 with PPT+ and PPT-deleted vectors expressing GFP or luciferase. After 48 hours or p4 post-transduction cells were harvested, and, subsequently, total genomic DNA was extracted and treated with DpnI (New England Biolabs, Ipswich, MA). Normalization of amounts of genomic DNA was achieved by PCR amplification of the human β-globin gene, as previously described.36 β-Globin was targeted with the following primers: 5′-cagagccatctattgcttac-3′(forward) and 5′-gcctcaccaccaacttcatc-3′(reverse). The WPRE was analyzed with the following primers: 5′-ACGTCCTTCTGCTACGTCC-3′ (forward), 5′-AAAGGGAGATCCGACCGACTCGTC-3′ (reverse). Flp9 cells (293T cells containing one copy of the lentiviral vector vTK113 per diploid genome, as previously explained36) were used to establish a standard curve. A range of 3.03 × 104 to 15 β-globin cell-equivalent of the Flp9 cells (two copies per diploid cell) was used in duplicate to establish the standard curve for the β-globin region amplification. A range of 1.5 × 104 to 7.5 of the Flp9 cells (one copy of the viral DNA per diploid genome) was used in duplicate to establish the standard curve for the viral sequence's amplification. Reactions were labeled with SYBR Green I (Cambrex, East Rutherford, NJ) and performed in triplicate on a 7300 Real-Time PCR System (Applied Biosystems). Reactions were analyzed using the 7300 Real-Time PCR System RQ Study Software Version 1.4 (Applied Biosystems, Foster City, CA). 10 µl of each PCR sample was subjected to gel electrophoresis and visualized by staining with ethidium bromide.
Quantification of integration by qPCR. 293T cells were transduced at an multiplicity of infection of 0.5 with PPT+ and PPT-deleted vectors expressing GFP or luciferase. After 48 hours or p4 post-transduction (four 1:5 passages being the minimal number required to dilute out unintegrated vector genomes, as previously shown36), cells were harvested and genomic DNA was isolated by the phenol/chloroform extraction method. Samples were analyzed by qPCR, as outlined above, and each vector's efficiency of integration was calculated as a percentage by comparing vector copy number after p4 with vector copy number after 48 hours.
In vivo experiments in rat brain. All animals were pathogen-free male Sprague–Dawley rats obtained from Charles Rivers. All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals (DHHS Publication No. (National Institutes of Health)85–23), and all procedures received prior approval by the University of North Carolina Institutional Animal Care and Usage Committee. Virus vector infusions were performed as previously described.37 Briefly, rats (N = 4/group) first were anesthetized with 50 mg/kg pentobarbital and then placed into a stereotaxic frame. Using a 32 gauge stainless steel injector and a Sage infusion pump, the rats received 1 µl (at 1 × 109 GFP transducing units/ml) of the integrating and nonintegrating vTK945 and vTK1023 vectors over a 10-minute period into the striatum (1.0 mm anterior to bregma, 3.0 mm lateral, 5.5 mm vertical) according to the atlas of Paxinos and Watson.38 In all cases, the injector was left in place 3-minutes postinfusion to allow diffusion from the injector.
GFP visualization. Two months after the vector infusion, rats received an overdose of pentobarbital (100 mg/kg pentobarbital, i.p.) and subsequently were perfused transcardially with ice-cold 100 mmol/l sodium phosphate buffered saline (pH = 7.4), followed by 4% paraformaldehyde in 100 mmol/l phosphate buffer (pH = 7.4). After overnight fixation in paraformaldehyde-phosphate buffer, vibratome sections (40 µm thick) were taken through the striatum and rinsed in phosphate buffered saline. Following three rinses in phosphate buffered saline, the sections were mounted on slides and coverslipped with fluorescent mounting media. eGFP fluorescence was visualized on an Olympus IX 71 fluorescence microscope (Olympus, Center Valley, PA), and digital pictures were taken. For details on luciferase assay see Supplementary Materials and Methods.
SUPPLEMENTARY MATERIAL Figure S1. Diagram of the vectors used in the study. Schematic of the lentiviral vectors used in the present study. cPPT: central polypurine tract, CMV: cytomegalovirus immediate-early promoter, GFP: green fluorescent protein, PPT: 3' polypurine tract, luc.: luciferase, GFP-BSD: green fluorescent protein-blasticidin resistance fusion protein, ampR: ampicillin-resistance gene, ori: bacterial origin of replication. Figure S2. PI FACS figure verifying cell-cycle arrest. Verification of cell-cycle arrest by FACS analysis of propidium iodide-stained cells. The DNA content of assayed cells is presented for cycling cells, which are shown in a, while the DNA content of arrested cells is presented in b. Figure S3. Sequence comparison of deleted U3 and PPT sequences in vectors. Sequence alterations at the 3' PPT and U3 region for the vectors used in the present study. Vectors were generated with deletions in the 3' vector sequence (shown in red), in the PPT (shown in green), and in the U3 region, either individually or in combination. Figure S4. Diagram of failed strand displacement caused by the PPT deletion. Aberrant reverse transcription induced by the PPT deletion mediates plus-strand synthesis from potential cryptic sites in the vector genome, possibly inhibiting strand displacement and promoting 1-LTR circle formation. Linear episomes generated as the minor product of RT from a PPT-deleted substrate would have a uniform 3' end, but a heterogeneous 5' end. Figure S5. Southern-blot analysis of the effect of the PPT deletion on episome formation, performed on undigested DNA from transduced cells. PPT deletion leads to reduced linear episome formation and increased 1-LTR circular episome formation in transduced cells. The effect of the PPT deletion on episome formation was examined by Southern-blot analysis. Undigested DNA isolated from 293T cells transduced with PPT+ (vTK459), integrating (lane 1) and nonintegrating (lane 2), or with PPT- (vTK1074), integrating (lane 3) and nonintegrating (lane 4), or Xba I-digested, PPT-, integrating (lane 5) and nonintegrating (lane 6) vectors were analyzed by Southern blot, using the Bss HII/Xma I DNA fragment from the PPT+ vector (vTK459) plasmid as a probe. A DNA-size marker ladder (M) verified the size of tested DNA samples. Lanes 3 and 4 may represent supercoiled DNA. Figure S6. Sequence analysis of deletions and an insertion at the 2-LTR junction of 2-LTR circles produced by PPT-deleted vectors. 2-LTR circles formed by the non-SIN, PPT-deleted vector (vTK1074) exhibited a ~180 bp deletion at the 5' end of the 5' U3, while a 2-LTR circle formed by the SIN, PPT-deleted vector (VTK1046) exhibited a ~40 bp insertion 5' of the 5' U3, consisting of a sequence duplicated from the 3' end of the vector genome. Figure S7. Southern-blot analysis of the effect of the cPPT deletion on integration and episomal formation. (a) Cells were transduced with a PPT+, cPPT+ vector (vTK945) (lanes 1 and 4), a PPT-, cPPT+ vector (vTK1023) (lanes 2 and 5), and a PPT-, cPPT- vector (vTK1039) (lanes 3 and 6), and total DNA was extracted from transduced cells three days (no passages) (lanes 1-3) or ~14 days (four passages) (lanes 4-6) posttransduction, digested as shown in Fig. 3a, and analyzed by Southern blot, using a Kas I/Bam HI fragment from the 5' region of the PPT+/cPPT+ vector's genome as a probe. All vectors were packaged with functional integrase. Figure S8. Comparison of the reductions in illegitimate integration achieved by two PPT-deleted vectors. Fold change in illegitimate integration between the GFP-expressing, PPT-positive vector (vTK1187) and its PPT-deleted equivalent (vTK1188), as measured by qPCR, is shown on the left. Fold change in illegitimate integration between the luciferase-expressing, PPT-positive vector (vTK993) and its PPT-deleted equivalent (vTK1034), as measured by qPCR, is shown on the right. All vectors shown in this figure were packaged with defective integrase. Table S1. Quantification of the effect of the PPT deletion on integration and episomal formation. Quantification of relative amounts of integrated lentiviral genomes and linear, 1-LTR, and 2-LTR episomal genomes produced by PPT-positive (vTK945), PPT-deleted (vTK1179), integrating, and nonintegrating lentivectors in 293T cells. Quantitative analysis of DNA density of the Southern blot presented in Fig. 3d was achieved using ImageJ software. Table S2. Titering of vectors packaged with and without functional integrase, with and without the PPT. Titers were determined by scoring GFP expression following serial dilution in 293T cells. Materials and Methods.
Acknowledgments
B.K., M.B., and T.K. designed the research, M.B., B.K., T.M., H.M., C.L., and R.J.S. carried out experiments and analyzed results, and B.K., M.B., and T.K. wrote the manuscript. M.B. and B.K. contributed equally to this study. The following reagents were obtained through the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: pD64E from Dr Vinay K. Pathak and HIV-1 p24 monoclonal antibody (183-H12-5C) from Dr Bruce Chesebro and Kathy Wehrly. We are grateful to the White Rose, Dr McKeon, Aristides de Sousa Mendes, and the efforts on Musa Dagh. We would like to thank Nikunj Somia for useful discussions. The study was supported by the UNC Center for AIDS Research (CFAR) and by NIH grants 2-R01-DK058702-06 to B.K. and T.K., 5-R21-HL086406 to R.J.S. and T.K., and 5-PO1-HL066973-05 to T.K. The authors have no competing interests to disclose.
Supplementary Material
Diagram of the vectors used in the study. Schematic of the lentiviral vectors used in the present study. cPPT: central polypurine tract, CMV: cytomegalovirus immediate-early promoter, GFP: green fluorescent protein, PPT: 3' polypurine tract, luc.: luciferase, GFP-BSD: green fluorescent protein-blasticidin resistance fusion protein, ampR: ampicillin-resistance gene, ori: bacterial origin of replication.
PI FACS figure verifying cell-cycle arrest. Verification of cell-cycle arrest by FACS analysis of propidium iodide-stained cells. The DNA content of assayed cells is presented for cycling cells, which are shown in a, while the DNA content of arrested cells is presented in b.
Sequence comparison of deleted U3 and PPT sequences in vectors. Sequence alterations at the 3' PPT and U3 region for the vectors used in the present study. Vectors were generated with deletions in the 3' vector sequence (shown in red), in the PPT (shown in green), and in the U3 region, either individually or in combination.
Diagram of failed strand displacement caused by the PPT deletion. Aberrant reverse transcription induced by the PPT deletion mediates plus-strand synthesis from potential cryptic sites in the vector genome, possibly inhibiting strand displacement and promoting 1-LTR circle formation. Linear episomes generated as the minor product of RT from a PPT-deleted substrate would have a uniform 3' end, but a heterogeneous 5' end.
Southern-blot analysis of the effect of the PPT deletion on episome formation, performed on undigested DNA from transduced cells. PPT deletion leads to reduced linear episome formation and increased 1-LTR circular episome formation in transduced cells. The effect of the PPT deletion on episome formation was examined by Southern-blot analysis. Undigested DNA isolated from 293T cells transduced with PPT+ (vTK459), integrating (lane 1) and nonintegrating (lane 2), or with PPT- (vTK1074), integrating (lane 3) and nonintegrating (lane 4), or Xba I-digested, PPT-, integrating (lane 5) and nonintegrating (lane 6) vectors were analyzed by Southern blot, using the Bss HII/Xma I DNA fragment from the PPT+ vector (vTK459) plasmid as a probe. A DNA-size marker ladder (M) verified the size of tested DNA samples. Lanes 3 and 4 may represent supercoiled DNA.
Sequence analysis of deletions and an insertion at the 2-LTR junction of 2-LTR circles produced by PPT-deleted vectors. 2-LTR circles formed by the non-SIN, PPT-deleted vector (vTK1074) exhibited a ~180 bp deletion at the 5' end of the 5' U3, while a 2-LTR circle formed by the SIN, PPT-deleted vector (VTK1046) exhibited a ~40 bp insertion 5' of the 5' U3, consisting of a sequence duplicated from the 3' end of the vector genome.
Southern-blot analysis of the effect of the cPPT deletion on integration and episomal formation. (a) Cells were transduced with a PPT+, cPPT+ vector (vTK945) (lanes 1 and 4), a PPT-, cPPT+ vector (vTK1023) (lanes 2 and 5), and a PPT-, cPPT- vector (vTK1039) (lanes 3 and 6), and total DNA was extracted from transduced cells three days (no passages) (lanes 1-3) or ~14 days (four passages) (lanes 4-6) posttransduction, digested as shown in Fig. 3a, and analyzed by Southern blot, using a Kas I/Bam HI fragment from the 5' region of the PPT+/cPPT+ vector's genome as a probe. All vectors were packaged with functional integrase.
Comparison of the reductions in illegitimate integration achieved by two PPT-deleted vectors. Fold change in illegitimate integration between the GFP-expressing, PPT-positive vector (vTK1187) and its PPT-deleted equivalent (vTK1188), as measured by qPCR, is shown on the left. Fold change in illegitimate integration between the luciferase-expressing, PPT-positive vector (vTK993) and its PPT-deleted equivalent (vTK1034), as measured by qPCR, is shown on the right. All vectors shown in this figure were packaged with defective integrase.
Quantification of the effect of the PPT deletion on integration and episomal formation. Quantification of relative amounts of integrated lentiviral genomes and linear, 1-LTR, and 2-LTR episomal genomes produced by PPT-positive (vTK945), PPT-deleted (vTK1179), integrating, and nonintegrating lentivectors in 293T cells. Quantitative analysis of DNA density of the Southern blot presented in Fig. 3d was achieved using ImageJ software.
Titering of vectors packaged with and without functional integrase, with and without the PPT. Titers were determined by scoring GFP expression following serial dilution in 293T cells.
REFERENCES
- Zaiss AK, Son S., and, Chang LJ. RNA 3' readthrough of oncoretrovirus and lentivirus: implications for vector safety and efficacy. J Virol. 2002;76:7209–7219. doi: 10.1128/JVI.76.14.7209-7219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Leavitt AD, Shiue L., and, Varmus HE. Site-directed mutagenesis of HIV-1 integrase demonstrates differential effects on integrase functions in vitro. J Biol Chem. 1993;268:2113–2119. [PubMed] [Google Scholar]
- Yáñez-Muñoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ, et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med. 2006;12:348–353. doi: 10.1038/nm1365. [DOI] [PubMed] [Google Scholar]
- Bayer M, Kantor B, Cockrell A, Ma H, Zeithaml B, Li X, et al. A large U3 deletion causes increased in vivo expression from a nonintegrating lentiviral vector. Mol Ther. 2008;16:1968–1976. doi: 10.1038/mt.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolonia L, Waddington SN, Fernandes C, Ward NJ, Bouma G, Blundell MP, et al. Stable gene transfer to muscle using non-integrating lentiviral vectors. Mol Ther. 2007;15:1947–1954. doi: 10.1038/sj.mt.6300281. [DOI] [PubMed] [Google Scholar]
- Philippe S, Sarkis C, Barkats M, Mammeri H, Ladroue C, Petit C, et al. Lentiviral vectors with a defective integrase allow efficient and sustained transgene expression in vitro and in vivo. Proc Natl Acad Sci USA. 2006;103:17684–17689. doi: 10.1073/pnas.0606197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Yang H, Dai B, Tai A., and, Wang P. Nonintegrating lentiviral vectors can effectively deliver ovalbumin antigen for induction of antitumor immunity. Hum Gene Ther. 2009;20:1652–1664. doi: 10.1089/hum.2009.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu TI., and, Cathomen T. Targeted genome modifications using integrase-deficient lentiviral vectors. Mol Ther. 2007;15:2107–2113. doi: 10.1038/sj.mt.6300345. [DOI] [PubMed] [Google Scholar]
- Leavitt AD, Robles G, Alesandro N., and, Varmus HE. Human immunodeficiency virus type 1 integrase mutants retain in vitro integrase activity yet fail to integrate viral DNA efficiently during infection. J Virol. 1996;70:721–728. doi: 10.1128/jvi.70.2.721-728.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima N, Lu R., and, Engelman A. Human immunodeficiency virus type 1 replication in the absence of integrase-mediated dna recombination: definition of permissive and nonpermissive T-cell lines. J Virol. 2001;75:7944–7955. doi: 10.1128/JVI.75.17.7944-7955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin JM, Hughes SM., and, Varmus HE. Retroviruses. Cold Spring Harbor Laboratory Press: Plainview, New York; 1997. [PubMed] [Google Scholar]
- Li L, Olvera JM, Yoder KE, Mitchell RS, Butler SL, Lieber M, et al. Role of the non-homologous DNA end joining pathway in the early steps of retroviral infection. EMBO J. 2001;20:3272–3281. doi: 10.1093/emboj/20.12.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker C, Goff S, Gilboa E, Paskind M, Mitra SW., and, Baltimore D. Structure of a cloned circular Moloney murine leukemia virus DNA molecule containing an inverted segment: implications for retrovirus integration. Proc Natl Acad Sci USA. 1980;77:3932–3936. doi: 10.1073/pnas.77.7.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnet CM., and, Haseltine WA. Circularization of human immunodeficiency virus type 1 DNA in vitro. J Virol. 1991;65:6942–6952. doi: 10.1128/jvi.65.12.6942-6952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina D., and, Benz EW., Jr Structure of murine sarcoma virus DNA replicative intermediates synthesized in vitro. J Virol. 1980;33:377–389. doi: 10.1128/jvi.33.1.377-389.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold GM, Yamamoto KR, Shank PR., and, Varmus HE. Mouse mammary tumor virus DNA in infected rat cells: characterization of unintegrated forms. Cell. 1977;10:19–26. doi: 10.1016/0092-8674(77)90135-0. [DOI] [PubMed] [Google Scholar]
- Folger KR, Wong EA, Wahl G., and, Capecchi MR. Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol. 1982;2:1372–1387. doi: 10.1128/mcb.2.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint SJ, American Society for Microbiology . Principles of Virology. ASM Press: Washington, DC; 2009. [Google Scholar]
- Klarmann GJ, Yu H, Chen X, Dougherty JP., and, Preston BD. Discontinuous plus-strand DNA synthesis in human immunodeficiency virus type 1-infected cells and in a partially reconstituted cell-free system. J Virol. 1997;71:9259–9269. doi: 10.1128/jvi.71.12.9259-9269.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstrodt F, Hollestelle A, Nagel JH, Gorin M, Wasielewski M, van den Ouweland A, et al. BRCA1 mutation analysis of 41 human breast cancer cell lines reveals three new deleterious mutants. Cancer Res. 2006;66:41–45. doi: 10.1158/0008-5472.CAN-05-2853. [DOI] [PubMed] [Google Scholar]
- Adair GM, Rolig RL, Moore-Faver D, Zabelshansky M, Wilson JH., and, Nairn RS. Role of ERCC1 in removal of long non-homologous tails during targeted homologous recombination. EMBO J. 2000;19:5552–5561. doi: 10.1093/emboj/19.20.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, et al. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- Al-Minawi AZ, Saleh-Gohari N., and, Helleday T. The ERCC1/XPF endonuclease is required for efficient single-strand annealing and gene conversion in mammalian cells. Nucleic Acids Res. 2008;36:1–9. doi: 10.1093/nar/gkm888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., and, Kafri T. A single-LTR HIV-1 vector optimized for functional genomics applications. Mol Ther. 2004;10:139–149. doi: 10.1016/j.ymthe.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Butler SL, Hansen MS., and, Bushman FD. A quantitative assay for HIV DNA integration in vivo. Nat Med. 2001;7:631–634. doi: 10.1038/87979. [DOI] [PubMed] [Google Scholar]
- Nightingale SJ, Hollis RP, Pepper KA, Petersen D, Yu XJ, Yang C.et al. (2006Transient gene expression by nonintegrating lentiviral vectors Mol Ther 131121–1132. [DOI] [PubMed] [Google Scholar]
- Mali P, Ye Z, Hommond HH, Yu X, Lin J, Chen G, et al. Improved efficiency and pace of generating induced pluripotent stem cells from human adult and fetal fibroblasts. Stem Cells. 2008;26:1998–2005. doi: 10.1634/stemcells.2008-0346. [DOI] [PubMed] [Google Scholar]
- Gilboa E, Mitra SW, Goff S., and, Baltimore D. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979;18:93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Hungnes O, Tjotta E., and, Grinde B. The plus strand is discontinuous in a subpopulation of unintegrated HIV-1 DNA. Arch Virol. 1991;116:133–141. doi: 10.1007/BF01319237. [DOI] [PubMed] [Google Scholar]
- Miles LR, Agresta BE, Khan MB, Tang S, Levin JG., and, Powell MD. Effect of polypurine tract (PPT) mutations on human immunodeficiency virus type 1 replication: a virus with a completely randomized PPT retains low infectivity. J Virol. 2005;79:6859–6867. doi: 10.1128/JVI.79.11.6859-6867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MD, Wang B., and, Bushman FD. Human immunodeficiency virus type 1 preintegration complexes containing discontinuous plus strands are competent to integrate in vitro. J Virol. 1995;69:3938–3944. doi: 10.1128/jvi.69.6.3938-3944.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo A, Genovese P, Beausejour CM, Colleoni S, Lee YL, Kim KA, et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol. 2007;25:1298–1306. doi: 10.1038/nbt1353. [DOI] [PubMed] [Google Scholar]
- Xu K, Ma H, McCown TJ, Verma IM., and, Kafri T. Generation of a stable cell line producing high-titer self-inactivating lentiviral vectors. Mol Ther. 2001;3:97–104. doi: 10.1006/mthe.2000.0238. [DOI] [PubMed] [Google Scholar]
- Kafri T, van Praag H, Gage FH., and, Verma IM. Lentiviral vectors: regulated gene expression. Mol Ther. 2000;1:516–521. doi: 10.1006/mthe.2000.0083. [DOI] [PubMed] [Google Scholar]
- Kantor B, Ma H, Webster-Cyriaque J, Monahan PE., and, Kafri T. Epigenetic activation of unintegrated HIV-1 genomes by gut-associated short chain fatty acids and its implications for HIV infection. Proc Natl Acad Sci USA. 2009;106:18786–18791. doi: 10.1073/pnas.0905859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown TJ. Adeno-associated virus-mediated expression and constitutive secretion of galanin suppresses limbic seizure activity in vivo. Mol Ther. 2006;14:63–68. doi: 10.1016/j.ymthe.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Paxinos G., and, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press: Orlando, FL; 1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diagram of the vectors used in the study. Schematic of the lentiviral vectors used in the present study. cPPT: central polypurine tract, CMV: cytomegalovirus immediate-early promoter, GFP: green fluorescent protein, PPT: 3' polypurine tract, luc.: luciferase, GFP-BSD: green fluorescent protein-blasticidin resistance fusion protein, ampR: ampicillin-resistance gene, ori: bacterial origin of replication.
PI FACS figure verifying cell-cycle arrest. Verification of cell-cycle arrest by FACS analysis of propidium iodide-stained cells. The DNA content of assayed cells is presented for cycling cells, which are shown in a, while the DNA content of arrested cells is presented in b.
Sequence comparison of deleted U3 and PPT sequences in vectors. Sequence alterations at the 3' PPT and U3 region for the vectors used in the present study. Vectors were generated with deletions in the 3' vector sequence (shown in red), in the PPT (shown in green), and in the U3 region, either individually or in combination.
Diagram of failed strand displacement caused by the PPT deletion. Aberrant reverse transcription induced by the PPT deletion mediates plus-strand synthesis from potential cryptic sites in the vector genome, possibly inhibiting strand displacement and promoting 1-LTR circle formation. Linear episomes generated as the minor product of RT from a PPT-deleted substrate would have a uniform 3' end, but a heterogeneous 5' end.
Southern-blot analysis of the effect of the PPT deletion on episome formation, performed on undigested DNA from transduced cells. PPT deletion leads to reduced linear episome formation and increased 1-LTR circular episome formation in transduced cells. The effect of the PPT deletion on episome formation was examined by Southern-blot analysis. Undigested DNA isolated from 293T cells transduced with PPT+ (vTK459), integrating (lane 1) and nonintegrating (lane 2), or with PPT- (vTK1074), integrating (lane 3) and nonintegrating (lane 4), or Xba I-digested, PPT-, integrating (lane 5) and nonintegrating (lane 6) vectors were analyzed by Southern blot, using the Bss HII/Xma I DNA fragment from the PPT+ vector (vTK459) plasmid as a probe. A DNA-size marker ladder (M) verified the size of tested DNA samples. Lanes 3 and 4 may represent supercoiled DNA.
Sequence analysis of deletions and an insertion at the 2-LTR junction of 2-LTR circles produced by PPT-deleted vectors. 2-LTR circles formed by the non-SIN, PPT-deleted vector (vTK1074) exhibited a ~180 bp deletion at the 5' end of the 5' U3, while a 2-LTR circle formed by the SIN, PPT-deleted vector (VTK1046) exhibited a ~40 bp insertion 5' of the 5' U3, consisting of a sequence duplicated from the 3' end of the vector genome.
Southern-blot analysis of the effect of the cPPT deletion on integration and episomal formation. (a) Cells were transduced with a PPT+, cPPT+ vector (vTK945) (lanes 1 and 4), a PPT-, cPPT+ vector (vTK1023) (lanes 2 and 5), and a PPT-, cPPT- vector (vTK1039) (lanes 3 and 6), and total DNA was extracted from transduced cells three days (no passages) (lanes 1-3) or ~14 days (four passages) (lanes 4-6) posttransduction, digested as shown in Fig. 3a, and analyzed by Southern blot, using a Kas I/Bam HI fragment from the 5' region of the PPT+/cPPT+ vector's genome as a probe. All vectors were packaged with functional integrase.
Comparison of the reductions in illegitimate integration achieved by two PPT-deleted vectors. Fold change in illegitimate integration between the GFP-expressing, PPT-positive vector (vTK1187) and its PPT-deleted equivalent (vTK1188), as measured by qPCR, is shown on the left. Fold change in illegitimate integration between the luciferase-expressing, PPT-positive vector (vTK993) and its PPT-deleted equivalent (vTK1034), as measured by qPCR, is shown on the right. All vectors shown in this figure were packaged with defective integrase.
Quantification of the effect of the PPT deletion on integration and episomal formation. Quantification of relative amounts of integrated lentiviral genomes and linear, 1-LTR, and 2-LTR episomal genomes produced by PPT-positive (vTK945), PPT-deleted (vTK1179), integrating, and nonintegrating lentivectors in 293T cells. Quantitative analysis of DNA density of the Southern blot presented in Fig. 3d was achieved using ImageJ software.
Titering of vectors packaged with and without functional integrase, with and without the PPT. Titers were determined by scoring GFP expression following serial dilution in 293T cells.