Abstract
A major hurdle for harnessing small interfering RNA (siRNA) for therapeutic application is an effective and safe delivery of siRNA to target tissues and cells via systemic administration. While lipid nanoparticles (LNPs) composed of a cationic lipid, poly-(ethylene glycol) lipid and cholesterol, are effective in delivering siRNA to hepatocytes via systemic administration, they may induce multi-faceted toxicities in a dose-dependent manner, independently of target silencing. To understand the underlying mechanism of toxicities, pharmacological probes including anti-inflammation drugs and specific inhibitors blocking different pathways of innate immunity were evaluated for their abilities to mitigate LNP-siRNA-induced toxicities in rodents. Three categories of rescue effects were observed: (i) pretreatment with a Janus kinase (Jak) inhibitor or dexamethasone abrogated LNP-siRNA-mediated lethality and toxicities including cytokine induction, organ impairments, thrombocytopenia and coagulopathy without affecting siRNA-mediated gene silencing; (ii) inhibitors of PI3K, mammalian target of rapamycin (mTOR), p38 and IκB kinase (IKK)1/2 exhibited a partial alleviative effect; (iii) FK506 and etoricoxib displayed no protection. Furthermore, knockout of Jak3, tumor necrosis factor receptors (Tnfr)p55/p75, interleukin 6 (IL-6) or interferon (IFN)-γ alone was insufficient to alleviate LNP-siRNA-associated toxicities in mice. These indicate that activation of innate immune response is a primary trigger of systemic toxicities and that multiple innate immune pathways and cytokines can mediate toxic responses. Jak inhibitors are effective in mitigating LNP-siRNA-induced toxicities.
Introduction
Small interfering RNAs (siRNAs) hold a great promise to become a new therapeutic entity as they are able to silence gene expression specifically by triggering RNA interference, an evolutionarily conserved cellular process for repressing gene expression.1 Since naked siRNAs, even with selected sequences and chemical modifications, lack drug-like pharmacokinetic properties, tissue bioavailability and the ability of entering cells, a major hurdle for harnessing siRNA for broad therapeutic use is an effective and safe delivery of siRNA to diseased tissues and cells via systemic administration.2,3 Many platforms, such as liposomes, lipoplexes, cationic polymers, and antibody-, peptide- or cholesterol-conjugates, have been developed for systemic delivery of siRNA.2,4 Among these, cationic lipid-based vehicles are the most widely validated means for liver delivery and have shown superior activities in delivering siRNA to hepatocytes in rodents and nonhuman primates, resulting in a robust target knockdown and mechanism-based pharmacological sequela.5,6,7 Recently several lipid-assembled siRNA reagents entered clinical trials for an evaluation of pharmacokinetic and pharmacodynamic properties and safety profiles.
One major concern about using cationic lipid-based carriers for systemic delivery of siRNA is the potential to trigger an inflammation-like response, anaphylactic reaction and organ damages,3,8,9 as cationic lipid-assembled DNA constructs or antisense oligonucleotides elicit such toxicities.10,11 It has been shown that intravenous (IV) administration of some lipid-encapsulated siRNA nanoparticles can cause induction of proinflammatory cytokines and elevation of serum transaminases in mice and nonhuman primates at high doses.5,9,12,13 This resembles the toxicity induced by liposomal DNA assemblies.10 While the scope and magnitude of toxic responses may vary depending on lipid nanoparticle (LNP) compositions, the nature of payloads, and doses, cytokine induction and hepatotoxicity are commonly seen among lipid-siRNA nanoparticle-triggered reactions.3,8,9,14 Recently, significant progress has been made in enhancing target-silencing potency of LNP-siRNA assemblies through empirical screening of LNPs,15,16 which might increase the therapeutic index. However, the mechanism underlying LNP-siRNA-associated toxicities remains unclear, which hinders the rational development of lipid-based vehicles with improved safety profiles, including the identification of biomarkers and the design of assays for screening LNP formulations, as well as the development of strategies to ameliorate LNP-siRNA toxicities. LNP-siRNA assemblies might over-stimulate the innate immune system, thereby causing organ damages and systemic toxicities. Alternatively, cationic lipid-mediated cellular interactions and cytotoxicity may directly inflict cells, resulting in a secondary inflammation.
The innate immune system consists of membrane-associated toll-like receptors (TLRs), cytoplasmic immunoreceptors and receptor-linked intracellular signaling pathways.17,18,19 While TLRs located at the plasma membrane, such as TLRs-2,4, recognize lipid components of pathogen membranes, TLRs residing at endosomal membrane including TLRs-3, −7/8, and −9 as well as cytoplasmic immunoreceptors, such as retinoid inducible gene-1, are responsible for detecting foreign nucleic acids with specific molecular patterns. Ligand-stimulated TLRs and cytoplasmic sensors elicit cytokine expression via activating IκB kinase (IKK)/NFkB, p38/AP1, interferon regulatory factor3/5/7, and PI3K pathways.17,20,21,22 Induced cytokines further stimulate the production of cytokines/chemokines and drive inflammation/immune response by engaging the Janus kinase (Jak)/signal transducers and activators and NFkB pathways.20,23,24 The Jak/signal transducers and activators pathway associated with receptors of multiple cytokines is essential for executing inflammation/immune responses.23,25 Overstimulation of innate immune system is pathologic.8,26,27 Lipid-formulated siRNA has the potential to stimulate both lipid- and RNA-sensing TLRs and cytoplasmic immunoreceptors, whereas sequence selection and chemical modifications of siRNA can greatly reduce siRNA-mediated immunostimulatory activity.8,13,28,29
A LNP (LNP05), composed of cholesterol-linolyl dimethyl amine, cholesterol, and polyethylene glycol-dimethylglycerol has been developed for systemic delivery of siRNA to hepatocytes. While LNP05-formulated siRNA nanoparticles exhibited robust efficacy in silencing multiple liver targets, including apolipoprotein B (ApoB) and La antigen (SSB), a ubiquitously expressed gene involved in tRNA maturation,30 they triggered multi-systemic toxicities and lethality in a dose-dependent manner. This is despite the fact that these siRNA payloads are sequence-optimized and chemically-modified for minimizing siRNA-dependent immunostimulation as described before.9,28 Using LNP05-encapsulated SSB siRNA (LNP05-SSB) or ApoB siRNA (LNP05-ApoB), we investigated the etiology of LNP05-siRNA-triggered pathologies by determining the activity of three classes of pharmacological probes in mitigating LNP05-siRNA-induced lethality and toxicities in rodents: (i) antagonists of Jak, p38, IKK1/2, PI3K and mammalian target of rapamycin (mTOR) which block different pathways of innate immune response, respectively, (ii) dexamethasone, a multifunctional suppressor of inflammation/immune response,31,32 and FK506, an immunosuppressant inhibiting the nuclear factor of activated T cells-mediated response33 and (iii) Etoricoxib, an inhibitor of cyclooxygenase-2.34 Moreover, we evaluated LNP05-siRNA toxicities in Jak3−/−, tumor necrosis factor receptor (Tnfr)p55/p75−/−, interleukin 6 (IL-6−/−) and interferon (IFN)-γ−/− mice to further probe the role of these molecules in mediating LNP05-siRNA toxicities.
Results
LNP05-siRNA nanoparticles are efficacious but toxic in rodents
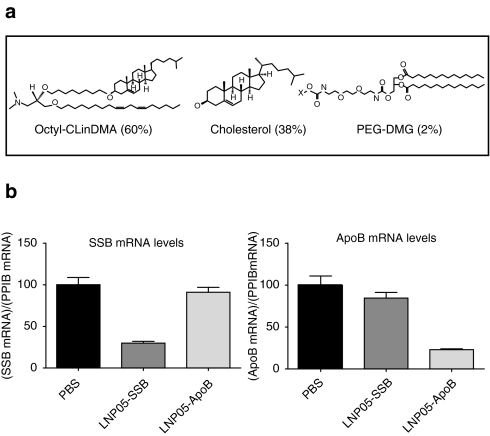
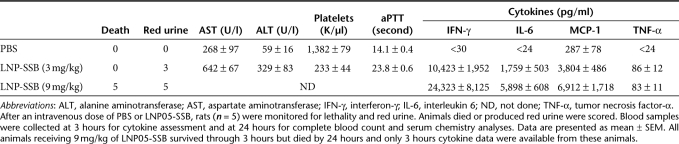
LNP05 consists of cholesterol-linolyl dimethyl amine, a cationic lipid (pKa = 8.9), cholesterol and polyethylene glycol-dimethylglycerol at a molar ratio of 60:38:2 (Figure 1a). When assembled with either SSB or ApoB siRNA, the mean nanoparticle size was 110 ± 1.8 nm in diameter with +16 mV surface charge and siRNA encapsulation efficiency was 91–93% with total lipid:siRNA mass ratio = 12:1. Both SSB and ApoB siRNAs were sequence-selected and chemically modified as previously described to increase stability and to mitigate immunostimulatory activity.9,28 All lipid-siRNA nanoparticles were made from endotoxin-free material and all final products were examined for potential endotoxin contamination using a US Food and Drug Administration-approved method to ensure that the endotoxin levels, if any, were well below the endotoxin release limit defined for humans by US Food and Drug Administration as described in Materials and Methods. Both LNP05-SSB and LNP05-ApoB were potent in silencing target expression, with IC50 values of 0.52 nmol/l and 0.76 nmol/l toward SSB and ApoB respectively in cultured HepG2 cells. In rats, a single IV dose of LNP05-SSB or LNP05-ApoB at 1 mg/kg (siRNA dose) caused >70% reduction in liver SSB or ApoB mRNA levels specifically (Figure 1b), without inducing overt toxicities. To characterize LNP05-siRNA-linked toxicities, rats were IV dosed with 3 or 9 mg/kg of LNP05-SSB or PBS and then monitored for adverse responses, including plasma cytokine levels at 3 hours, an optimal time for monitoring induction of major proinflammatory cytokines identified from former kinetics studies, complete blood counts, serum chemistry and coagulation parameters at 24-hour postdosing. In addition, urine was collected over the course of 24 hours for visual examination of red urine and mortality was monitored as well. Multifaceted toxicities were detected, including (i) lethality (all animals receiving 9 mg/kg of LNP05-SSB died by 24 hours), (ii) elevation of cytokines in plasma, among which IL-6, IFN-γ, tumor necrosis factor (TNF)-α and MCP-1 were consistently and markedly induced, (iii) elevation of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST), (iv) thrombocytopenia, (v) coagulopathy, manifested by an elongation of activated partial thromboplastin time, and (vi) red urine, indicative of hematuria (Table 1). No alterations in erythrocyte counts, hemoglobin levels, hematocrit levels or prothrombin time were detected. LNP05-formulated with an ApoB siRNA (LNP05-ApoB) caused comparable toxic responses (Supplementary Table S1), indicating that the observed toxicities are independent of siRNA sequences or target repression. Similar toxicities with LNP05-SSB were also observed in mice (Supplementary Figure S1).
Figure 1.
Lipid composition and in vivo target silencing activities of LNP05-SSB and LNP05-ApoB nanoparticles. (a) Lipid structures and composition of LNP05.(b) LNP05-SSB and LNP05-ApoB silenced target gene specifically. Rats (four per group) were dosed with vehicle (PBS), LNP05-SSB or LNP05-ApoB at 1 mg/kg via tail vein injection. Twenty four hours post dosing, mRNA levels of SSB, ApoB and Ppib (a housekeeping gene) in liver medial lobe were determined by quantitative reverse transcriptase-polymerase chain reaction. The quantities of SSB and ApoB mRNA relative to Ppib levels are presented. Bars indicate SEM. PEG-DMG, polyethylene glycol-dimethylglycerol.
Table 1. Summary of LNP05-SSB-induced toxicities in rats.
Identification of Jak inhibitors and dexamethasone as suppressors of LNP05-siRNA-induced lethality in rats
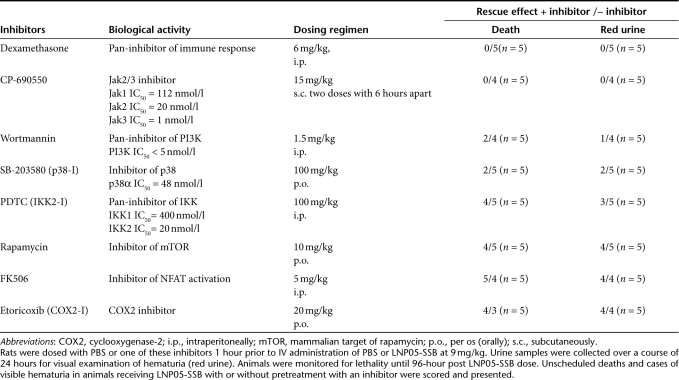
Since LNP05-siRNA nanoparticles provoked lethality and multifactorial toxicities involving different systems in rats, this offers an in vivo model for pharmacologically probing the mechanism underlying LNP05-siRNA toxicities. We first evaluated the suppression of LNP05-SSB-induced lethality and visible hematuria by anti-inflammation drugs and pathway-specific inhibitors of innate immunity as shown in Table 2. The in vivo pharmacological activities of these reagents were reported and their target inhibitory activities and pharmacodynamic-effective dosing regimens identified from former rodent studies were summarized in Table 2.9,33,34,35,36,37,38,39,40,41 Rats were predosed with either PBS or one of these inhibitors using the dosing regimen shown in Table 2, followed by an IV dose of LNP05-SSB at 9 mg/kg 1 hour later. Animals were monitored for lethality for 96 hours postadministration of LNP05-SSB. In addition, urine was collected over a course of 24 hours for visual examination of hematuria. As shown in Table 2, CP-690,550 (CP), a Jak2/3 inhibitor,40,42 and dexamethasone abrogated LNP05-SSB-induced lethality and red urine whereas inhibitors of PI3K, p38, IKK1/2 and mTOR exhibited partial rescue effect by reducing mortality and/or the cases of visible hematuria. On the contrary, FK506 and etoricoxib showed no protection (Table 2). We also evaluated CP for mitigating LNP05-ApoB-induced lethality and hematuria and found that CP abrogated LNP05-ApoB toxicities as well (Supplementary Table S1 and Supplementary Figure S2). This indicates that CP-mediated protection is independent of siRNA sequences. To preclude the possibility that CP-mediated rescue is due to an unknown property of this compound other than Jak2/3 inhibition, we tested another Jak1/2 inhibitor (Jak-IA) and found that Jak-IA also alleviated LNP05-SSB-induced lethality and visible hematuria (Supplementary Table S2), confirming that Jak inhibition mitigated LNP05-SSB-mediated toxicities.
Table 2. Suppression of LNP05-SSB-induced lethality and visible hematuria by different inhibitors.
Jak inhibitors and dexamethasone mitigated cytokine release and multi-systemic toxicities induced by LNP05-siRNA nanoparticles
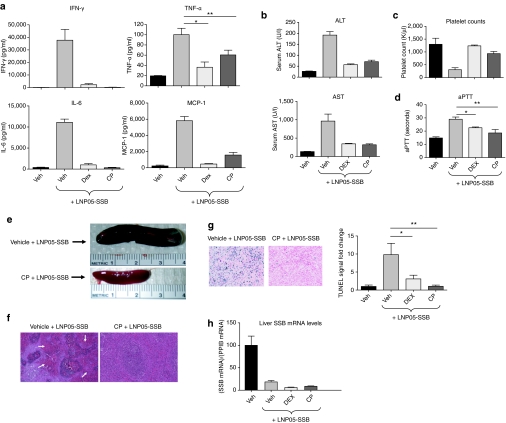
As Jak kinases play a central role in mediating cytokine response,23 the identification of CP as a suppressor of LNP05-siRNA-induced lethality suggests that Jak-mediated cytokine response is an initiator of LNP05-siRNA-associated toxicities. If this is the case, CP should be able to block not only cytokine release but also other toxic responses. To test this possibility, rats were treated with vehicle, CP or dexamethasone 1 hour prior to an IV administration of 3 mg/kg LNP05-SSB. Blood was collected 3-hour postinjection of LNP05-SSB for cytokine assessment and animals were killed at 24 hours for collection of blood and tissues for various analyses as described above. While animals treated with the vehicle followed by LNP05-SSB displayed multiple abnormalities (Figure 2a–g), pretreatment with either CP or dexamethasone suppressed all toxic responses including cytokine induction, ALT and AST elevation, thrombocytopenia, activated partial thromboplastin time elongation, as well as megalosplenia, splenic hemorrhage, and cell death (Figure 2a–g). Importantly, pretreatment with CP or dexamethasone did not affect LNP05-SSB-mediated SSB knockdown (Figure 2h), therefore disconnecting LNP05-SSB efficacy from its toxicities. Like CP, Jak-IA was effective in mitigating LNP05-SSB toxicities (Supplementary Table S2).
Figure 2.
Pretreatment with CP690550 (CP) or dexamethasone abrogates LNP05-SSB-induced toxicities in rats. Rats (n = 5) were dosed with a vehicle (Veh, PEG400 with 5% glucose), CP or dexamethasone (DEX) using the dosing regimens shown in Table 2, 1 hour prior to an intravenous dose of PBS or LNP05-SSB (3 mg/kg). Blood and tissue samples were collected at different times post administration of LNP05-SSB for various analyses as shown. No unscheduled deaths were detected. Bars indicate SEM. (a) Quantification of cytokines in plasma at 3-hour post LNP05-SSB treatment. *P = 0.002 and **P = 0.017. (b) Measurements of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum at 24 hours. (c) Platelet counts at 24 hours. (d) Activated partial thromboplastin time (aPTT) measurements at 24 hours. *P = 0.003 and **P = 0.008. (e) Representative photos of the spleen at 24 hours. (f) Representative images of spleen sections stained with hematoxylin/eosin (H&E). Arrows indicate the hemorrhagic areas in red pulps. (g) TUNEL analysis on spleen tissues. Representative images are shown. Quantification of TUNEL staining was performed using the Ariol System and 9 randomly chosen fields from each animal sample were imaged and analyzed. *P = 0.019 and **P = 0.002. (h) Quantification of SSB mRNA relative to PpiB levels in the medial lobe of rat livers. IFN-γ, interferon-γ IL-6, interleukin 6; TNF-α, tumor necrosis factor-α.
Inhibitors of PI3K, mTOR, p38 and IKK1/2 partially mitigated LNP05-siRNA-induced toxicities
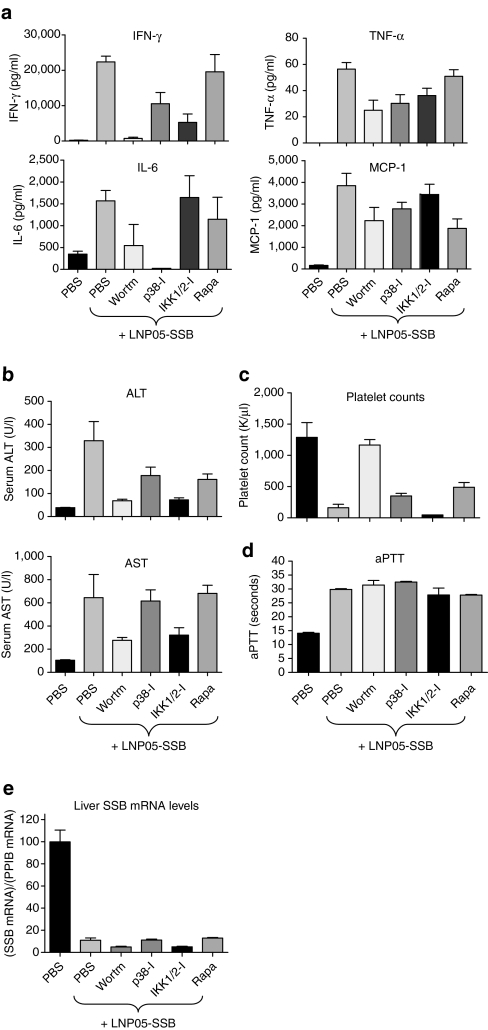
Inhibitors of PI3K, mTOR, p38 and IKK1/2 exhibited partial rescue of LNP05-SSB-induced lethality and visible hematuria (Table 2). It is interesting to examine their abilities in mitigating different toxic responses triggered by LNP05-SSB. As described above, rats were predosed with PBS or one of these inhibitors 1 hour prior to an IV dose of LNP05-SSB at 3 mg/kg, and animals were monitored for various toxic responses. Whereas pretreatment with these inhibitors attenuated cytokine induction and/or ALT/AST elevation to different extents, only wortmannin, an inhibitor of PI3K, mitigated thrombocytopenia (Figure 3a–c) and none of these compounds alleviated the elongation of activated partial thromboplastin time (Figure 3d). Unlike CP, none of these reagents could fully rescue LNP05-SSB-induced toxicities. Additionally, pretreatment with these inhibitors did not interfere with LNP05-SSB-induced SSB knockdown (Figure 3e).
Figure 3.
The alleviative effects on LNP05-SSB-induced toxicities by wortmannin, p38-I (SB-203580), IKK1/2-I (PDTC) and rapamycin (Rapa) in rats. Animals (n = 5) were treated with PBS, wortmannin, p38-I, IKK1/2-I or rapamycin using the regimen shown in Table 2, 1 hour prior to the administration of PBS or LNP05-SSB (3 mg/kg). Blood and tissue samples were collected for various analyses as shown. One out of five animals receiving LNP05-SSB with PBS pretreatment died by 24 hours. No unscheduled deaths were detected in other groups. Bars indicate SEM. (a) Quantification of cytokines in plasma at 3 hours post LNP05-SSB dose. (b) Measurements of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum at 24 hours. (c) Platelet counts at 24 hours. (d) Activated partial thromboplastin time (aPTT) measurements at 24 hours. (e) Quantification of SSB mRNA relative to Ppib levels in the medial lobe of rat livers. IFN-γ, interferon-γ IL-6, interleukin 6; TNF-α, tumor necrosis factor-α.
FK506 and etoricoxib did not rescue LNP05-SSB nanoparticle-mediated toxicities
Although FK506 and etoricoxib were inactive in suppressing LNP05-SSB lethality (Table 2), we further evaluated their activities for mitigating other toxic responses. One hour after treatment with one of these reagents, rats were dosed with LNP05-SSB at 3 mg/kg and the toxicities were monitored as described above. As summarized in Supplementary Table S3, these agents showed no appreciable suppression of cytokine release, elevation of serum transaminases, thrombocytopenia or coagulopathy induced by LNP05-SSB (Supplementary Table S3), consistent with their incompetence in mitigating LNP05-SSB lethality (Table 2).
Genetic ablation of Jak3, Tnfr p55/p75, IL-6 or IFN-γ could not rescue LNP05-SSB-induced toxicities in mice
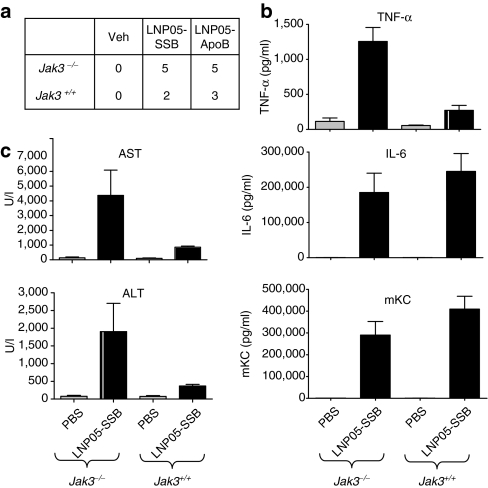
The suppression of multifaceted toxicities by Jak inhibitors indicates that Jak-dependent cytokine response is a primary trigger of toxicities induced by LNP05-siRNA. As CP might inhibit more than one Jak kinase of the Jak family,42 it is unclear whether inhibition of a single Jak is sufficient to rescue LNP05-siRNA toxicities. While Jak1-deficient mice showed perinatal lethality and Jak2-deficient mice were embryonically lethal,23 Jak3-deficient mice were viable and showed profound defects in lymphoid development and function.43 Thus, we examined the role of Jak3 in mediating LNP-siRNA toxicities by comparing LNP05-siRNA-induced toxicities between Jak3-deficient and wild-type mice. As mice are less sensitive to LNP-siRNA-associated toxicities, higher doses (9–18 mg/kg) of LNP05-SSB have been used in mouse toxicology studies. Although Jak3 deficiency caused lymphopenia (0.86 ± 0.12 K/µl in Jak3−/− mice versus 6.14 ± 0.82 K/µl in Jak3+/+ mice) as reported,43 it did not ameliorate LNP05-SSB-induced lethality, serum AST/ALT elevation or induction of TNF-α, IL-6 and mKC (Figure 4a–c), suggesting that Jak3 inhibition alone is insufficient to block LNP05-siRNA toxicities. Rather, a greater elevation of TNF-α and ALT/AST was detected in Jak3−/− mice (Figure 4b,c). This may be related to a negative regulatory function of Jak3 in dendritic-cell survival and cytokine production.44 Consistent with former study results,44 lipopolysaccharide caused greater elevation of IL-12 and IL-10 in Jak3−/− mice than in Jak3+/+ mice (Supplementary Figure S3).
Figure 4.
Comparison of LNP05-siRNA-induced toxicities between Jak3−/− and Jak3+/+ mice. (a) Mice (n = 5) were intravenously (IV) dosed with LNP05-SSB or LNP05-ApoB at 18 mg/kg and then monitored for lethality for 96 hours. Unscheduled deaths were scored and presented, and all unscheduled deaths occurred by 48-hour post dosing. (b,c) Mice (n = 5) were IV dosed with LNP05-SSB at 9 mg/kg. Blood was collected (b) at 3 hours for cytokine assessment and (c) at 24 hours for evaluation of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT). No unscheduled deaths were detected. Bars indicate SEM. IL-6, interleukin 6; TNF-α, tumor necrosis factor-α.
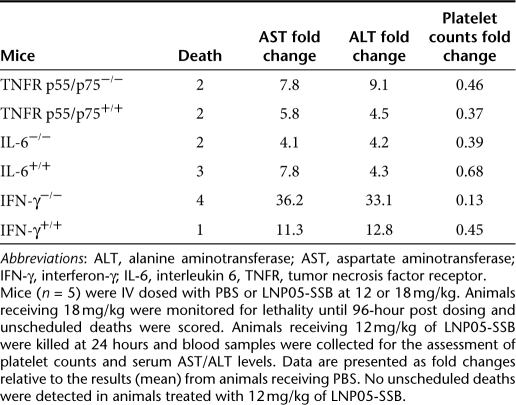
Since proinflammatory cytokines TNF-α, IL-6, and INF-γ were consistently induced at an early time following treatment with LNP05-siRNA (Table 1, Figures 2 and 3), we further evaluated their roles in mediating LNP05-siRNA toxicities by using mouse line deficient in one of these cytokines. Compared to genetically matched wild-type animals, mice deficient in Tnfrp55/p75, IL-6 or IFN-γ45,46,47 displayed no or very mild alleviation in toxic responses (Table 3 and Supplementary Table S4). This suggests that these cytokines can mediate toxicities independently and thus ablation of a single cytokine is not sufficient to effectively mitigate LNP05-siRNA-induced toxicities.
Table 3. Evaluation of LNP05-SSB toxicities in Tnfr p55/p75−/−, IL-6−/−, IFN-γ−/− and wild-type mice.
Discussion
Although lipid-based vehicles are effective in delivering siRNA to hepatocytes,5,6,7,28 the toxicities associated with lipid-siRNA nanoparticles may limit the therapeutic index.3,8,9 Although many former studies focused on understanding the role of siRNA payload in eliciting immunotoxicity and developed strategies to mitigate siRNA-elicited immune response, we show in this report and former studies9 that these cationic LNPs with a positive surface charge at a physiologic pH, even assembled with sequence-optimized and chemically modified siRNAs, can induce multi-systemic toxicities at high doses. Of course, the physicochemical properties of LNPs such as particle size, surface charge and stability might contribute to their toxicological properties. Therefore, an in-depth understanding of the mechanism underlying LNP-siRNA-induced pathologies is useful for designing assays to select for safer formulations and for identifying effective mitigation strategies. Four types of toxicities were observed in rats following systemic administration of LNP05-siRNA nanoparticles, namely (i) induction of multiple proinflammatory cytokines, (ii) hepatic, splenic and renal impairments, manifested by an elevation of serum ALT and AST, splenic cell death and hemorrhage, and visible hematuria, (iii) thrombocytopenia and (iv) coagulopathy (Table 1, Figures 2 and 3, Supplementary Table S1, Supplementary Figure S2). Since LNP05 formulated with distinct siRNA sequences targeting different genes (SSB and ApoB) causes comparable toxic responses (Tables 1 and 2 and Supplementary Table S1), it is unlikely that the observed toxicities are caused by a specific siRNA or due to the repression of a specific gene. Recapitulation of similar toxic responses in mice (Supplementary Figure S1, Figure 4 and Table 3) suggests that LNP05-siRNA-associated toxicities are across species. LNP05-siRNA nanoparticles may induce multi-systemic toxicities in two different modes. First, they may trigger one initial toxic event which in turn elicits subsequent toxicities involving different systems. Second, they may provoke multifaceted toxicities independently by directly afflicting cells of different organs such as immune cells, platelets, endothelial cells, hepatocytes and interacting with plasma proteins, etc. If in the former mode, blocking the initial toxic event can prevent secondary pathological responses and thus identification of the initiating toxic event and the underlying mechanism is crucial. Since activation of innate immune response characterized by robust induction of multiple cytokines is a commonly seen early event among LNP-siRNA-induced toxicities (Table 1, Figures 2a and 3a),9 we determined the activities of both multifunctional and pathway-specific inhibitors of innate immunity and inflammation in suppressing LNP05-siRNA toxicities. Our finding that Jak inhibitors can suppress LNP05-siRNA-induced lethality and multi-systemic toxicities indicate that LNP05-siRNA-induced toxic responses are sequential and interdependent and that activation of innate immunity is a primary trigger (Tables 1 and 2, Figures 1 and 2). This observation is consistent with former demonstrations that overproduction of cytokines could damage multiple organs, disrupt hematopoietic homeostasis and cause coagulation disorders, thereby inducing multi-systemic toxicities.8,10,26,27,48,49
While the suppression of LNP05-siRNA-triggered innate immune response by dexamethasone is not unexpected owing to its ability in inhibiting multiple pathways of innate immune response and inflammatory reactions,31,32 differential activities in mitigating cytokine response and multi-systemic toxicities by pathway-specific inhibitors are intriguing and shed light on the pathways responsible for mediating LNP05-siRNA immunotoxicity. Stimulation of TLRs and/or cytoplasmic immunoreceptors, such as retinoid inducible gene-1, can activate multiple downstream pathways including NFĸB, p38/AP1, PI3K and interferon regulatory factor3/5/7, leading to induction of cytokines and chemokines.14,17,19,20,21,22,50 Partial, but not complete, mitigation of cytokine induction and other toxicities by wortmannin, rapamycin and inhibitors of p38 and IKK1/2, which inhibit PI3K, mTOR, p38/AP1 and NFĸB pathways, respectively (Table 2, Figure 3), suggests a functional overlap of these pathways in mediating cytokine induction upon stimulation by LNP05-siRNA. In addition, although wortmannin and p38-I can abrogate the induction of IFN-γ and IL-6 respectively (Figure 3a), neither of these agents is able to fully rescue LNP05-siRNA-induced lethality and toxicities (Table 2 and Figure 3b–d), suggesting that suppression of a single cytokine is not sufficient to block immune response-mediated pathologies. Consistently, knockout of IL-6, IFN-γ or Tnfrp55/p75 alone fails to alleviate LNP05-SSB toxicities (Table 3). In contrast to the role of PI3K, p38 and IKK1/2 in the innate immune system, the Jak family of kinases associate with receptors of multiple cytokines and they are required for mediating the functionality of multiple cytokines in terms of amplifying cytokine production, executing inflammatory responses and stimulating growth and differentiation of immune cells.23 An effective blockade of LNP05-siRNA-triggered lethality and systemic toxicities by CP, which selectively inhibits Jak2/3, suggests that Jak2/3-dependent cytokine response is responsible for inducing secondary toxicities. The finding that Jak3 deficiency fails to rescue LNP05-siRNA toxicities in mice suggests that Jak2 may play a role in mediating LNP05-siRNA-triggered immune response (Figure 4). Consistently, a Jak1/2 inhibitor (Jak-IA) also rescues LNP05-SSB toxicities (Supplementary Table S2). Among the cytokines whose receptors associated with Jak2, IFN-γ and IL-6 belong to the most robustly induced cytokines by LNP05-siRNA. A profound inhibition of IFN-γ, IL-6 and MCP-1 (an IFN-γ-inducible cytokine) and a moderate suppression of TNF-α by CP suggest that these cytokines are key mediators of subsequent toxicities. cyclooxygenase-2 is a downstream effector of an inflammatory response regulated by cytokines,34 and nuclear factor of activated T cells participates in T cell activation.33 The lack of protection by etoricoxib and FK506, which inhibit cyclooxygenase-2 and nuclear factor of activated T cells respectively, reveals that neither of these molecules is essential for executing toxic response triggered by LNP05-siRNA. Taken together, our results suggest that the induction of several cytokines, most likely IFN-γ, IL-6, MCP-1 and TNF-α, is an apical toxic event which is responsible for inducing systemic toxicities. Jak inhibitors can suppress not only the full induction of these cytokines but also the cytokine-mediated immune response, thereby mitigating subsequent toxic responses. How LNP05-siRNA activates innate immunity is under investigation.
This study demonstrates the need to optimize lipid-based delivery vehicles and provides useful guidance to the development of LNP-formulated siRNA for therapeutics. Optimizing lipid formulations to minimize immunostimulatory activity is crucial for improving their safety profiles. Monitoring a panel of cytokines, not a single one, is needed for selecting new formulations and evaluating their toxicities. In clinical studies, premedication to suppress immune response may be a viable strategy to alleviate lipid-siRNA-associated side effects and Jak inhibitors could be used to mitigate lipid-siRNA nanoparticle-induced toxicities.
Materials and Methods
Reagents. CP-690,550 (Axon MedChem, Groningen, Netherland), dexamethasone (American Reagent Inc., Shirley, NY), Wortmannin (EMD Biosciences, Inc., La Jolla, CA), SB203580 (Axon MedChem), PDTC (Sigma, St Louis, MO), rapamycin (EMD Biosciences, Inc.), and FK506 (Sigma) were formulated and administrated according to manufacturers' recommendations or former reporters. Etoricoxib and Jak-IA (Merck & Co., Inc., Boston, MA) were dissolved in dimethylsulfoxide and 10% Tween-80 (vol/vol in PBS) respectively for oral administration. Dosing regiments for these reagents are listed in Table 2. Chemically modified siRNAs including ribo (r), deoxy (d), 2′-Fluro pyrimidine (flu), O-methyl (ome) and inverted abasic end caps (iB) at the passenger strand as described28 were synthesized at Merck & Co., Inc. The SSB siRNA was described before.9 ApoB siRNAs are as follows:
Passenger strand:
5′iB;fluC;fluU;fluU;fluU;dA;dA;fluC;dA;dA;fluU;fluU;fluC;fluC;fluU; dG;dA;dA;dA;fluU;dT;dT;iB3′
Guide strand:
5′rA;rU;rU;fluU;fluC;omeA;omeG;omeG;omeA;omeA;fluU;fluU; omeG;fluU;fluU;omeA;omeA;omeA;omeG;omeU;omeU3′
siRNAs were encapsulated into liposomes to produce LNP05-siRNA nanoparticles by mixing the lipid mixture in an ethanol solution with an aqueous solution of siRNA, followed by stepwise diafiltration as described.9 Particle size was measured by dynamic light scattering using Zetasizer (Malvern Instruments, Westborough, MA) and surface charge was assessed by measuring Zeta potential using ZetaPlus (Brookhaven Instruments, Hotsville, NY). The siRNA encapsulation efficiency was determined using a RiboGreen assay (Invitrogen, Carlsbad, CA). The potential endotoxin contamination was examined using a chromogenic limulus amebocyte lysate assay (Lonza, Basel, Switzerland). In all LNP-siRNA preparations used in our animal studies, the endotoxin levels at the highest dose of LNP05-siRNA were <0.25 EU/kg (body weight), significantly below the endotoxin release limit for humans (5 EU/kg), defined by US Food and Drug Administration and World Health Organization.
Rodent studies. All animal studies were conducted at Association for Assessment and Accreditation of Laboratory Animal Care-accredited Merck Research laboratories' animal facility located at West Point, PA, and all study protocols were approved by Merck West Point Institutional Animal Care and Use Committee. Female Sprague–Dawley rats obtained from Charles River were used in rat studies at an age of 4–6 weeks and with a body weight of 120–160 gm. Anti-inflammation agents were administrated as described in Table 2, 1 hour prior to tail vein injection of liposomal siRNA nanoparticles in PBS in a volume of 0.8 ml under normal pressure. At 3-hour post injection of liposomal siRNA, ~0.5 ml blood was collected by retro-orbital bleed under anesthesia and processed as plasma for the assessment of cytokines. At 24 hours, blood was collected for evaluation of complete blood cell counts, coagulation parameters and serum chemistry by venipuncture under anesthesia followed by exsanguination, and tissues from liver, spleen and kidney were collected for determination of SSB and ApoB mRNA levels and terminal deoxynucleotidyl transferase-mediated dUTP-biotin Nick End Labeling (TUNEL) analysis. For visual examination of urine color, rats were housed in metabolic cages to collect urine over a course of 24 hours after injection of liposomal siRNA nanoparticles. Animals were examined 3 times a day for detection of lethality over a course of 96 hours.
Jak3−/−, IL-6−/−, IFN-γ, and Tnfr p55/p75 double knockout mice as well as genetically matched wild-type mice were purchased from The Jackson Laboratories (Bar Harbor, ME) and were at 6–12 weeks at the time of studies. LNP05-siRNA was dosed via tail vein injection in a volume of 0.2 ml.
Analyses of rodent blood samples. Rat cytokines in plasma were quantified using a microsphere bead-based, multiplexed assay, Milliplex RCYTO-80K-PMX23 (Millipore, St. Charles, MO) which allows simultaneous quantification of 23 rat cytokines including IFN-γ, IL-6, MCP-1 and TNF-α. Mouse plasma cytokines were assessed using a 12-plex Searchlight enzyme-linked immunosorbent assay (Aushon Biosystems, Billerica, MA). All complete blood count, coagulation, and serum chemistry parameters were analyzed by Merck Laboratory of Animal Resources and the Safety Assessment Department. Complete blood count was determined on whole blood placed in an EDTA-treated tube using an Advia 120 Hematology Analyzer (Siemens, Deerfield, IL). Coagulation parameters in citrated plasma were assessed with a Behring Coagulation System (Siemens) and serum chemistry evaluation was conducted with an Advia 1800 Clinical Chemistry Analyzer (Siemens).
TUNEL. TUNEL staining in tissue sections was performed using a “TACS TdT-Blue Label in Situ Apoptosis Detection Kit” (Trevigen, Gaithersburg, MD). Briefly, 5 µm paraffin embedded sections of liver tissue were deparaffinized, fixed, labeled, and counterstained according to the manufacturer's recommendations. In Situ labeling procedure includes extension of 3′ ends with Biotin-dNTP (TdT Labeling Rxn), followed by additions of Strep-horseradish peroxidase and “TACS-Blue” label or 3,3′-diaminobenzidine tetrahydrochloride for color development. TUNEL signal was quantified using the Ariol System (Applied Imaging, San Jose, CA).
Quantification of mRNA. The quantitative reverse transcriptase-polymerase chain reaction assays were used to quantify SSB and ApoB mRNA levels relative to the housekeeping gene Ppib in lysates prepared from tissues using kits from Applied Biosystems (Foster City, CA).
Statistics. Statistical significance was determined by Student's t test (unpaired, one-tailed).
SUPPLEMENTARY MATERIAL Figure S1. LNP05-SSB induces toxicities in mice. C57BL/6 mice were treated with vehicle (PBS) or LNP05-SSB at 9 mg/kg (n=5). Blood was collected at 3 hr for cytokine assessment and at 24 hr for serum chemistry and CBC analyses. Liver and spleen samples were collected for histological analysis. A: serum cytokine data. B: Serum ALT and AST data. Bars indicate SEM. C: Platelet counts. D: Representative images of liver sections stained with H&E. Arrows indicate hemorrhagic areas and necrosis (nuclear condensation or fragmentation) in the liver. Visible hematuria and aPTT were not assessed in mice due to technical limitations. Figure S2. Pre-treatment with CP690,550 mitigates LNP05-ApoB-induced bloody urine. A photo of urine samples collected over 24 hours from the rats receiving 9 mg/kg of LNP05-ApoB with the pretreatment of either the vehicle (PEG400 with 5% glucose) or CP. Figure S3. Induction of IL-12 and IL-10 in plasma by LPS and LNP05-SSB in Jak3–/– and Jak3+/+ mice. Jak3–/– and Jak3+/+ mice (n=5) were treated with PBS, LPS (2 mg/kg, i.p.) or LNP05-SSB (9 mg/kg, i.v.). 3 hr post dosing, blood was collected and levels of cytokines in plasma were determined as described in Materials and Methods. Bars indicate SEM. Table S1. Systemic administration of LNP05-ApoB causes lethality and multifaceted toxicities in rats and pretreatment with CP690,550 (CP) abrogates LNP05-ApoB-induced toxicities. Table S2. Suppression of LNP05-SSB-induced toxicities by Jak-IA in rats. Table S2. Suppression of LNP05-SSB-induced toxicities by Jak-IA in rats. Table S4. Elevation of serum ALT and AST induced by LNP05-SSB in Tnfr p55/p75–/–, IL-6–/–, IFN-γ –/– mice and wild-type mice.
Acknowledgments
We thank the departments of Medicinal Chemistry and Pharmaceutical Research & Development for analyzing and providing LNP05-siRNA nanoparticles, Laboratory of Animal Resources for technical support, and the Safety Assessment Department for coagulation analyses. We thank Drs. Gary Marshall and Jonathan Young for providing Jak-IA. We also thank Vicki South, Andrea Matter, Kathleen Haskell and Dr. Bin Shi for technical assistance. We are very grateful to Drs. John Hunter, Rainer Metternich and Gary Gilliland for helpful discussion and their unending support to innovative research.
Supplementary Material
LNP05-SSB induces toxicities in mice. C57BL/6 mice were treated with vehicle (PBS) or LNP05-SSB at 9 mg/kg (n=5). Blood was collected at 3 hr for cytokine assessment and at 24 hr for serum chemistry and CBC analyses. Liver and spleen samples were collected for histological analysis. A: serum cytokine data. B: Serum ALT and AST data. Bars indicate SEM. C: Platelet counts. D: Representative images of liver sections stained with H&E. Arrows indicate hemorrhagic areas and necrosis (nuclear condensation or fragmentation) in the liver. Visible hematuria and aPTT were not assessed in mice due to technical limitations.
Pre-treatment with CP690,550 mitigates LNP05-ApoB-induced bloody urine. A photo of urine samples collected over 24 hours from the rats receiving 9 mg/kg of LNP05-ApoB with the pretreatment of either the vehicle (PEG400 with 5% glucose) or CP.
Induction of IL-12 and IL-10 in plasma by LPS and LNP05-SSB in Jak3–/– and Jak3+/+ mice. Jak3–/– and Jak3+/+ mice (n=5) were treated with PBS, LPS (2 mg/kg, i.p.) or LNP05-SSB (9 mg/kg, i.v.). 3 hr post dosing, blood was collected and levels of cytokines in plasma were determined as described in Materials and Methods. Bars indicate SEM.
Systemic administration of LNP05-ApoB causes lethality and multifaceted toxicities in rats and pretreatment with CP690,550 (CP) abrogates LNP05-ApoB-induced toxicities.
Suppression of LNP05-SSB-induced toxicities by Jak-IA in rats.
Activities of FK506 and Etoricoxib in suppression of LNP05-SSB-induced toxicities.
Elevation of serum ALT and AST induced by LNP05-SSB in Tnfr p55/p75–/–, IL-6–/–, IFN-γ –/– mice and wild-type mice.
REFERENCES
- Dorsett Y., and, Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev Drug Discov. 2004;3:318–329. doi: 10.1038/nrd1345. [DOI] [PubMed] [Google Scholar]
- de Fougerolles A, Vornlocher HP, Maraganore J., and, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp-Lorenzino L., and, Ruddy M. Challenges and opportunities for local and systemic delivery of siRNA and antisense oligonucleotides. Clin Pharmacol Ther. 2008;84:628–632. doi: 10.1038/clpt.2008.174. [DOI] [PubMed] [Google Scholar]
- Whitehead KA, Langer R., and, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci USA. 2008;105:11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge A., and, MacLachlan I. Overcoming the innate immune response to small interfering RNA. Hum Gene Ther. 2008;19:111–124. doi: 10.1089/hum.2007.179. [DOI] [PubMed] [Google Scholar]
- Abrams MT, Koser ML, Seitzer J, Williams SC, DiPietro MA, Wang W, et al. Evaluation of efficacy, biodistribution, and inflammation for a potent siRNA nanoparticle: effect of dexamethasone co-treatment. Mol Ther. 2010;18:171–180. doi: 10.1038/mt.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousignant JD, Gates AL, Ingram LA, Johnson CL, Nietupski JB, Cheng SH, et al. Comprehensive analysis of the acute toxicities induced by systemic administration of cationic lipid:plasmid DNA complexes in mice. Hum Gene Ther. 2000;11:2493–2513. doi: 10.1089/10430340050207984. [DOI] [PubMed] [Google Scholar]
- Rudin CM, Marshall JL, Huang CH, Kindler HL, Zhang C, Kumar D, et al. Delivery of a liposomal c-raf-1 antisense oligonucleotide by weekly bolus dosing in patients with advanced solid tumors: a phase I study. Clin Cancer Res. 2004;10:7244–7251. doi: 10.1158/1078-0432.CCR-04-0642. [DOI] [PubMed] [Google Scholar]
- Judge AD, Sood V, Shaw JR, Fang D, McClintock K., and, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- Kim JY, Choung S, Lee EJ, Kim YJ., and, Choi YC. Immune activation by siRNA/liposome complexes in mice is sequence- independent: lack of a role for Toll-like receptor 3 signaling. Mol Cells. 2007;24:247–254. [PubMed] [Google Scholar]
- Schlee M, Hornung V., and, Hartmann G. siRNA and isRNA: two edges of one sword. Mol Ther. 2006;14:463–470. doi: 10.1016/j.ymthe.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci USA. 2010;107:1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinc A, Goldberg M, Qin J, Dorkin JR, Gamba-Vitalo C, Maier M, et al. Development of lipidoid-siRNA formulations for systemic delivery to the liver. Mol Ther. 2009;17:872–879. doi: 10.1038/mt.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S., and, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Staros EB. Innate immunity: New approaches to understanding its clinical significance. Am J Clin Pathol. 2005;123:305–312. doi: 10.1309/n0c7-0vcu-3ehl-57wk. [DOI] [PubMed] [Google Scholar]
- Barton GM. A calculated response: control of inflammation by the innate immune system. J Clin Invest. 2008;118:413–420. doi: 10.1172/JCI34431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP., and, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JL, Badger AM, Kumar S., and, Lee JC. p38 MAP kinase: molecular target for the inhibition of pro-inflammatory cytokines. Prog Med Chem. 2001;38:1–60. doi: 10.1016/s0079-6468(08)70091-2. [DOI] [PubMed] [Google Scholar]
- Guiducci C, Ghirelli C, Marloie-Provost MA, Matray T, Coffman RL, Liu YJ, et al. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med. 2008;205:315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada K., and, Leonard WJ. The Jak-STAT pathway. Mol Immunol. 2000;37:1–11. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- Karin M, Yamamoto Y., and, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A., and, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- Judge AD, Bola G, Lee AC., and, MacLachlan I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol Ther. 2006;13:494–505. doi: 10.1016/j.ymthe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Park JM, Kohn MJ, Bruinsma MW, Vech C, Intine RV, Fuhrmann S, et al. The multifunctional RNA-binding protein La is required for mouse development and for the establishment of embryonic stem cells. Mol Cell Biol. 2006;26:1445–1451. doi: 10.1128/MCB.26.4.1445-1451.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynagh PN. Toll-like receptor signalling pathways as key targets for mediating the anti-inflammatory and immunosuppressive effects of glucocorticoids. J Endocrinol. 2003;179:139–144. doi: 10.1677/joe.0.1790139. [DOI] [PubMed] [Google Scholar]
- Morand EF. Effects of glucocorticoids on inflammation and arthritis. Curr Opin Rheumatol. 2007;19:302–307. doi: 10.1097/BOR.0b013e32805e87d0. [DOI] [PubMed] [Google Scholar]
- Magari K, Miyata S, Ohkubo Y, Mutoh S., and, Goto T. Calcineurin inhibitors exert rapid reduction of inflammatory pain in rat adjuvant-induced arthritis. Br J Pharmacol. 2003;139:927–934. doi: 10.1038/sj.bjp.0705310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riendeau D, Percival MD, Brideau C, Charleson S, Dubé D, Ethier D, et al. Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol Exp Ther. 2001;296:558–566. [PubMed] [Google Scholar]
- Kudlacz E, Perry B, Sawyer P, Conklyn M, McCurdy S, Brissette W, et al. The novel JAK-3 inhibitor CP-690550 is a potent immunosuppressive agent in various murine models. Am J Transplant. 2004;4:51–57. doi: 10.1046/j.1600-6143.2003.00281.x. [DOI] [PubMed] [Google Scholar]
- Podolin PL, Callahan JF, Bolognese BJ, Li YH, Carlson K, Davis TG, et al. Attenuation of murine collagen-induced arthritis by a novel, potent, selective small molecule inhibitor of IkappaB Kinase 2, TPCA-1 (2-[(aminocarbonyl)amino]-5- (4-fluorophenyl)-3-thiophenecarboxamide), occurs via reduction of proinflammatory cytokines and antigen-induced T cell Proliferation. J Pharmacol Exp Ther. 2005;312:373–381. doi: 10.1124/jpet.104.074484. [DOI] [PubMed] [Google Scholar]
- Badger AM, Bradbeer JN, Votta B, Lee JC, Adams JL., and, Griswold DE. Pharmacological profile of SB 203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J Pharmacol Exp Ther. 1996;279:1453–1461. [PubMed] [Google Scholar]
- Adams JL, Boehm JC, Gallagher TF, Kassis S, Webb EF, Hall R, et al. Pyrimidinylimidazole inhibitors of p38: cyclic N-1 imidazole substituents enhance p38 kinase inhibition and oral activity. Bioorg Med Chem Lett. 2001;11:2867–2870. doi: 10.1016/s0960-894x(01)00570-4. [DOI] [PubMed] [Google Scholar]
- Yatscoff RW, Wang P, Chan K, Hicks D., and, Zimmerman J. Rapamycin: distribution, pharmacokinetics, and therapeutic range investigations. Ther Drug Monit. 1995;17:666–671. doi: 10.1097/00007691-199512000-00020. [DOI] [PubMed] [Google Scholar]
- Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- Ui M, Okada T, Hazeki K., and, Hazeki O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem Sci. 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- Thomis DC, Gurniak CB, Tivol E, Sharpe AH., and, Berg LJ. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995;270:794–797. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Min B, Zhou YJ, Paul WE., and, O'shea JJ. Jak3 negatively regulates dendritic-cell cytokine production and survival. Blood. 2005;106:3227–3233. doi: 10.1182/blood-2005-02-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- Fattori E, Cappelletti M, Costa P, Sellitto C, Cantoni L, Carelli M, et al. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180:1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A., and, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Joseph L, Fink LM., and, Hauer-Jensen M. Cytokines in coagulation and thrombosis: a preclinical and clinical review. Blood Coagul Fibrinolysis. 2002;13:105–116. doi: 10.1097/00001721-200203000-00005. [DOI] [PubMed] [Google Scholar]
- Furie MB., and, Randolph GJ. Chemokines and tissue injury. Am J Pathol. 1995;146:1287–1301. [PMC free article] [PubMed] [Google Scholar]
- Rommel C, Camps M., and, Ji H. PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond. Nat Rev Immunol. 2007;7:191–201. doi: 10.1038/nri2036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LNP05-SSB induces toxicities in mice. C57BL/6 mice were treated with vehicle (PBS) or LNP05-SSB at 9 mg/kg (n=5). Blood was collected at 3 hr for cytokine assessment and at 24 hr for serum chemistry and CBC analyses. Liver and spleen samples were collected for histological analysis. A: serum cytokine data. B: Serum ALT and AST data. Bars indicate SEM. C: Platelet counts. D: Representative images of liver sections stained with H&E. Arrows indicate hemorrhagic areas and necrosis (nuclear condensation or fragmentation) in the liver. Visible hematuria and aPTT were not assessed in mice due to technical limitations.
Pre-treatment with CP690,550 mitigates LNP05-ApoB-induced bloody urine. A photo of urine samples collected over 24 hours from the rats receiving 9 mg/kg of LNP05-ApoB with the pretreatment of either the vehicle (PEG400 with 5% glucose) or CP.
Induction of IL-12 and IL-10 in plasma by LPS and LNP05-SSB in Jak3–/– and Jak3+/+ mice. Jak3–/– and Jak3+/+ mice (n=5) were treated with PBS, LPS (2 mg/kg, i.p.) or LNP05-SSB (9 mg/kg, i.v.). 3 hr post dosing, blood was collected and levels of cytokines in plasma were determined as described in Materials and Methods. Bars indicate SEM.
Systemic administration of LNP05-ApoB causes lethality and multifaceted toxicities in rats and pretreatment with CP690,550 (CP) abrogates LNP05-ApoB-induced toxicities.
Suppression of LNP05-SSB-induced toxicities by Jak-IA in rats.
Activities of FK506 and Etoricoxib in suppression of LNP05-SSB-induced toxicities.
Elevation of serum ALT and AST induced by LNP05-SSB in Tnfr p55/p75–/–, IL-6–/–, IFN-γ –/– mice and wild-type mice.