Abstract
Antisense therapy has been successful to skip targeted dystrophin exon with correction of frameshift and nonsense mutations of Duchenne muscular dystrophy (DMD). Systemic production of truncated but functional dystrophin proteins has been achieved in animal models. Furthermore, phase I/II clinical trials in United Kingdom and the Netherlands have demonstrated dystrophin induction by local and systemic administrations of antisense oligomers. However, long-term efficacy and potential toxicity remain to be determined. The present study examined 1-year systemic effect of phosphorodiamidate morpholino oligomers (PMO) treatment targeting mutated dystrophin exon 23 in mdx mice. PMO induced dystrophin expression dose-dependently and significantly improved skeletal muscle pathology and function with reduced creatine kinase (CK) levels by a regimen of 60 mg/kg biweekly administration. This regimen induced <2% dystrophin expression in the heart, but improved cardiac functions demonstrated by hemodynamics analysis. The results suggest that low levels of dystrophin induction may be able to provide detectable benefit to cardiac muscle with limited myopathy. Body weight, serum enzyme tests, and histology analysis showed no sign of toxicity in the mice treated with up to 1.5 g/kg PMO for 6 months. These results indicate that PMO could be used safely as effective drugs for long-term systemic treatment of DMD.
Introduction
Mutations in the dystrophin gene cause two forms of muscular dystrophy, Duchenne and Becker muscular dystrophy (DMD and BMD). DMD is the most common lethal muscle disorder mainly resulting from nonsense and frameshift mutations preventing production of functional dystrophin protein. There is no effective treatment available. BMD is caused by in-frame mutations that typically create shortened, but partially functional dystrophin, leading to variable and often overt symptoms.1,2,3 The variability of disease phenotype among BMD population is the result of multiple factors affecting muscle functions. One of them is the functionality of the truncated dystrophin due to mutations involving different region and the size of deletion. Sufficient preservation of functions by truncated (BMD-like) dystrophin underlies the principle of recombinant adeno-associated virus-mediated mini- and microdystrophin gene therapy.4 The same principle has now been utilized for the antisense oligomer-mediated exon skipping (antisense therapy). In antisense therapy, oligomers are specifically designed to bind target pre-mRNA sequence for exclusion of targeted exon(s). The removal of targeted exon(s) restores dystrophin reading frame, resulting in the expression of BMD-like dystrophins. Fortunately, most DMD mutations occur within the rod domain of the dystrophin gene which spans more than half the length of the protein, but seems to have limited functional importance.5,6 Antisense therapy therefore will be able to remove the mutated or additional exon(s) that disrupt the reading frame, thus restoring the expression of shortened forms of dystrophin protein retaining critical functions in most DMD patients.
Last 10 years have witnessed the significant progress in antisense therapy for DMD.7,8,9,10,11,12,13,14,15,16,17,18,19,20,21 Early studies demonstrated the feasibility of antisense oligonucleotides (AO) to remove targeted dystrophin exon in mouse and human cells.7,8,10 In 2003, AO was for the first time shown to be able to restore functional levels of dystrophin in the dystrophic mdx mice by intramuscular injections.12 Adeno-associated virus-mediated production of AO has been shown to be highly effective for targeted exon skipping both locally and systemically with long-term therapeutic effect.6 This approach thus has a strong advantage over synthetic AOs by eliminating the need for repeated injections. However, establishing the efficacy of adeno-associated virus-mediated gene delivery systemically in human will likely take a prolonged period. In contrast, the effect of synthetic AOs on exon skipping is short-lived and short-term toxicity of most widely used AO chemistries has been established. This provides bases for regulatory authorities to approve synthetic AOs for clinical trials more willingly. As a result, clinical trials targeting human dystrophin exon 51 have been conducted in the Europe to test the principle for treating a subgroup of DMD population.19,20 Since then, AO-mediated exon skipping with synthetic AOs has progressed into one of the most promising experimental therapy for DMD. Systemic efficacy with functional improvement has been demonstrated in both dystrophic mouse and dog models.13,14,18 The use of peptides and cationic polymers for enhanced delivery of phosphorodiamidate morpholino oligomers (PMO) has achieved restoration of dystrophin expression to near normal levels in body-wide skeletal muscles and to a less degree in the cardiac muscle.15,16 High level expression of the restored dystrophin improved functions of both dystrophic skeletal and cardiac muscles significantly. However, increased toxicity and potential immune response to the cationic peptide and polymers demand a more thorough preclinical tests before these modified AOs can be trialed in clinics. Therefore, clinical trials for exon skipping are currently being limited to the use of unmodified PMO and 2'-O-methyl phosphorothioate (2OMePS) chemistries. Completed phase I clinical trials by local muscle injections targeting exon 51 reported the significant amount of targeted exon skipping and dystrophin production in nearly all treated muscles with both 2OMePS and PMO.19,20 More recently, similar to that observed in the animal models, systemic treatment of DMD patients with PMO was reported to induce detectable amount of dystrophin protein in sampled skeletal muscles of all patients, although considerable variation in skipping efficiency was also clearly observed within the trial population.21
AO drugs act on pre-mRNA of dystrophin for the removal of targeted exon. The therapeutic effect is therefore short-lived with single administration. Previous investigations suggested that the effect of dystrophin induction after single intravenous (i.v.) injection of PMO in mdx mice lasted ~2 months.12 Antisense therapy for DMD requires life-long administration and the capacity of the drugs to maintain functional levels of dystrophin with tolerable toxicity. It is therefore important to understand whether therapeutic levels of dystrophin can be maintained by regular injections for a prolonged time. However, nearly all studies of i.v. AO administrations in animal models have so far limited to a short-time period. It is especially important as delivery efficiency of both antisense chemistries, 2OMePS and PMO, is apparently related to the state and degree of muscle degeneration and regeneration, being less effective in normal muscles when compared to degenerative muscles. This raises the concern that regular injections of AO, especially at low dosage might not be able to halt the cycles of degeneration when only a limited amount of dystrophin is produced in only a proportion of muscle fibers. Efficiency of exon skipping and dystrophin production in cardiac muscle with unmodified 2OMePS and PMO is significantly lower than that in skeletal muscles. This also causes concern that restoration of higher levels of dystrophin in skeletal muscles may exacerbate the failure of heart function if dystrophin expression cannot be effectively restored in cardiac muscle.22
In the present study, we investigated a long-term dosage effect of PMO treatment in the dystrophic mdx mice. We demonstrated that PMO was able to induce effective targeted exon skipping and dystrophin expression in all muscles dose-dependently during 1-year treatment although accumulative effect was limited. Higher dosages of PMO were associated with increasing levels of dystrophin expression and improvement of muscle pathology and functions without detectable toxicity.
Results
One-year i.v. administration of 15 mg/kg PMO revealed no significant improvement in muscle pathology
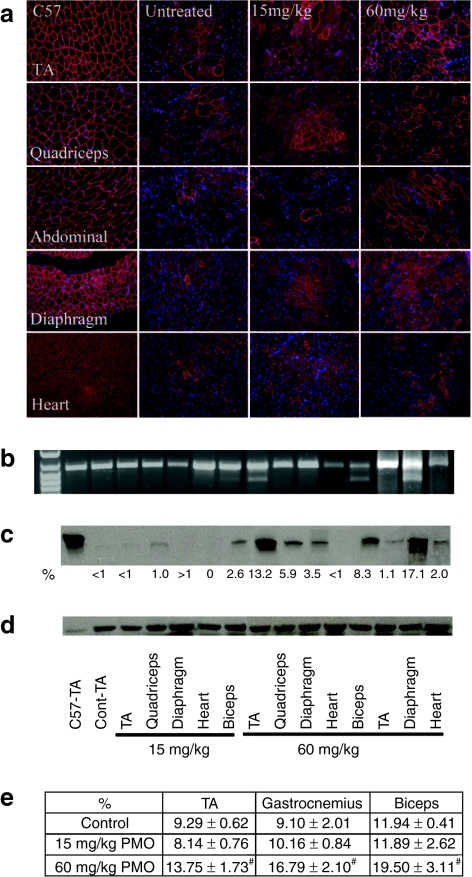
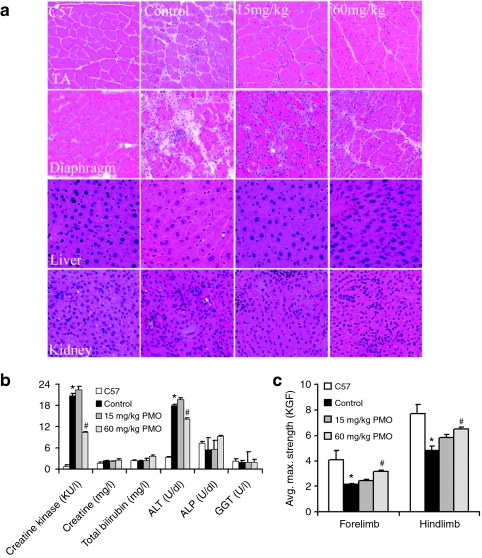
We have earlier shown that a single i.v. injection of 15 mg/kg PMO targeting mouse dystrophin exon 23 was able to induce lower, but detectable levels of dystrophin expression.17 As the half-life of PMO-induced dystrophin expression was reported to be about 2 months, we hypothesized that repeated administration with this dose of PMO biweekly might lead to higher levels of dystrophin induction, sufficient to achieve therapeutic significance. To test this, we treated the mdx mice with 15 mg/kg PMO biweekly for 1 year. Two weeks after the last injections, skeletal and cardiac muscles were examined. The revertant fibers of <1.5% were detected in all muscles from control mdx mice. Dystrophin positive fibers increased to up to 5% in all the skeletal muscles from the PMO-treated mice, although a small number of fibers with weak partial dystrophin positive signal was also observed (Figure 1a). The maximum amount of dystrophin protein examined by western blots accounted for 2.6% of normal level in any skeletal muscles (Figure 1c). This effect was therefore similar to that observed in muscles after single injection of 15 mg/kg PMO, suggesting no significant accumulation in exon-skipping effect by the dosing regimen. No clear difference in pathology and variation in fiber size of skeletal muscle were observed (Figures 1e and 2a). Sirius staining showed no clear reduction in staining intensity for collagens in the treated muscles when compared to the controls (Supplementary Figure S1). Consistently, the function of the skeletal muscles measured by grip-force generation were only marginally improved with no difference in creatine kinase (CK) levels in the treated group when compared to the untreated control mdx mice (Figure 2b,c). No dystrophin expression and specific exon 23 skipping were detected in the cardiac muscle and measurement for cardiac functions revealed no significant difference from the untreated control mdx mice (Figures 1 and 3; Tables 1 and 2). Body weight, serum enzyme tests, and histological examination of lung, kidney, and liver showed no differences between the treated and the untreated control mdx mice (Figure 2).
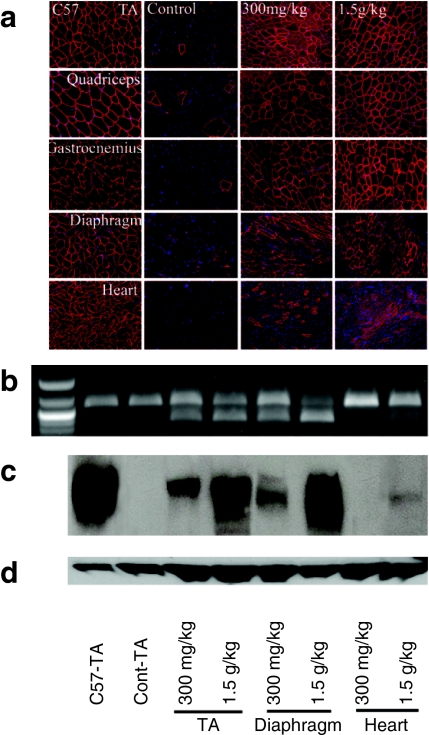
Figure 1.
Restoration of dystrophin expression after 1-year intravenous (i.v.) treatment of 15 or 60 mg/kg phosphorodiamidate morpholino oligomers (PMO). (a) Detection of dystrophin by immunohistochemistry with rabbit polyclonal antibody P7 against dystrophin. Blue nuclear staining with DAPI. (b) Detection of exon 23 skipping in muscles by reverse transcription-PCR (RT-PCR). Lane 1 size marker. (c) Western blot showed levels of dystrophin expression in muscles [10 µg total protein loaded from tibialis anterior (TA) muscle of C57BL/6 mouse; 50 µg total protein loaded from untreated or PMO-treated mdx mice]. C57-TA, TA muscle from normal C57BL/6 mouse; Cont-TA, TA muscle from untreated mdx mouse. 15 or 60 mg/kg, the muscles from 15 or 60 mg/kg PMO-treated mdx mice. The samples from two different mdx mice treated with 60 mg/kg PMO were shown. (d) α-Actin as loading controls. (e) The percentage of non-centranucleated muscle fibers. Control, muscles from untreated mdx mouse. (n = 10; #P ≤ 0.05 compared with untreated mdx mice. Two-tailed t-test).
Figure 2.
Examination of pathology, serum, and skeletal muscle function after 1-year treatment of 15 and 60 mg/kg phosphorodiamidate morpholino oligomers (PMO). (a) Histology [hematoxylin and eosin (H&E) staining] of tibialis anterior (TA), diaphragm, liver, and kidney from the normal C57BL/6 mice, untreated mdx mice (control), 15 or 60 mg/kg PMO-treated mdx mice (15 mg/kg, 60 mg/kg). (b) The levels of serum enzymes. Creatine kinase (KU/l), creatinine (mg/l), urea nitrogen (mg/ml), total bilirubin (mg/l), direct bilirubin (mg/dl), alanine transaminase (ALT) (U/dl), alkaline phosphatase (ALP) (U/dl), and γ-glutamyltransferase (GGT) (U/l). A significant reduction in creatine kinase levels was observed in mdx mice treated with 60 mg/kg PMO when compared with untreated mdx mice. (c) Grip strength measurement. Significant improvement was observed in the mice after treatment with 60 mg/kg PMO. KGF, kilogram force. (n = 10; *P ≤ 0.05 compared with C57BL/6 mice; #P ≤ 0.05 compared with untreated mdx mice. Two-tailed t-test). Final mean body weight: C57BL/6, 31.3 ± 0.8 g; untreated mdx, 34.1 ± 2.0; 15 mg/kg PMO-treated mdx, 31.8 ± 2.4 g; 60 mg/kg PMO-treated mdx, 33.7 ± 1.6 g. PMO, phosphorodiamidate morpholino oligomers.
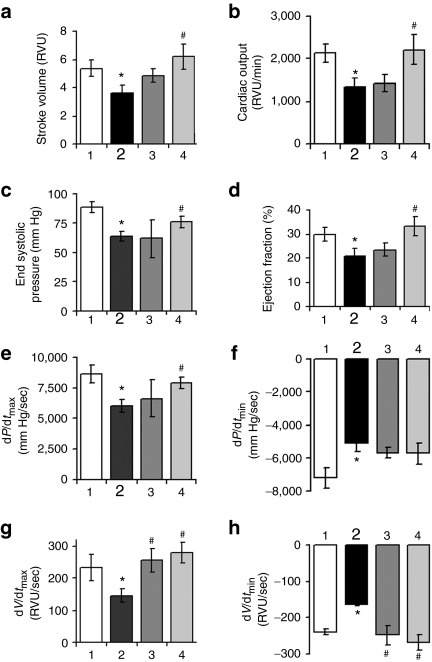
Figure 3.
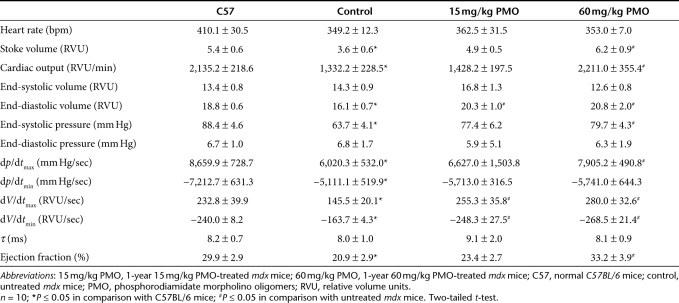
Cardiac functions in the mdx mice treated with 15 or 60 mg/kg phosphorodiamidate morpholino oligomers (PMO) (1 year) by hemodynamics at 15 minutes after dobutamine challenges. Significant improvement was observed in (a) stroke volume, (b) cardiac output, (c) end-systolic pressure, (d) ejection fraction, (e) dp/dtmax, (f) dp/dtmin, (g) dV/dtmax, (h) dV/dtmin. (1) Age-matched normal C57BL/6 mice; (2) age-matched untreated mdx mice; (3) 1-year 15 mg/kg PMO-treated mdx mice; (4) 1-year 60 mg/kg PMO-treated mdx mice. (n = 10; *P ≤ 0.05 in comparison with C57BL/6 mice; #P ≤ 0.05 in comparison with untreated mdx mice. Two-tailed t-test).
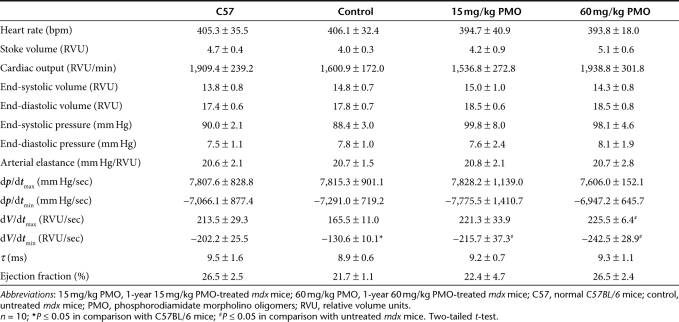
Table 1. Baseline hemodynamic data.
Table 2. Hemodynamic parameters 15 minutes after dobutamine infusion.
One-year i.v. administration of 60 mg/kg PMO significantly improved skeletal muscle pathology
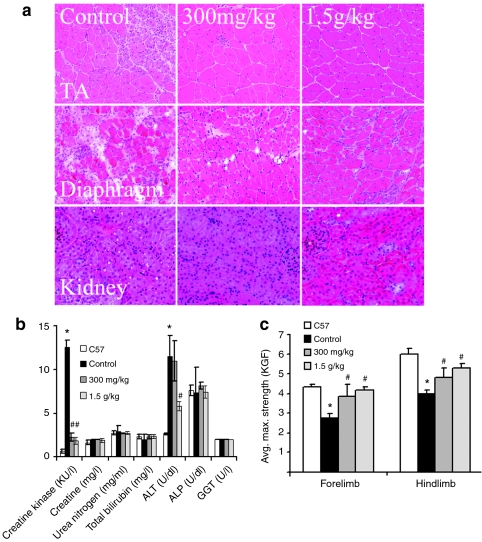
Earlier study showed that single i.v. injection of 2 mg PMO per adult mdx mice (~60–80 mg/kg) was able to induce dystrophin expression in body-wide skeletal muscles of mdx mice.14 We therefore examined long-term effect of 60 mg/kg PMO by regular biweekly i.v. injections. Skeletal muscles after 1-year treatment saw significant induction of targeted exon 23 skipping and dystrophin expression. The levels of exon 23 skipping by reverse transcription-PCR (RT-PCR) ranged from barely detectable to 40% in skeletal muscles (Figure 1b). Dystrophin positive fibers, with highly variable intensity, were counted from 10 to 50% in skeletal muscles examined (Figure 1a). Such variation was seen between different muscles as well as within the same type of muscles from different mdx mice. Variation in exon-skipping efficiency was also demonstrated by western blots with the levels of dystrophin protein up to 17.1% (Figure 1c). The treated mice showed clearly detectable improvement in skeletal muscle pathology with reduced central nucleation (Figure 1e) and a more homogenous population of fibers in size (Figure 2a). Sirius staining showed a clear reduction in the intensity for collagens in the treated skeletal muscles when compared to the controls (Supplementary Figure S1). CK levels in the treated mice were significantly reduced when compared to the untreated control mdx mice, but remained higher than that of normal C57BL/6 mice (Figure 2b). Significant improvement was also detected in muscle functions with grip-force generation (Figure 2c). The results suggest that this dosage of PMO treatment can achieve measurable therapeutic outcome in skeletal muscles without detectable toxicity.
Improvement in cardiac functions after 1-year treatment of 60 mg/kg PMO
The efficiency of exon skipping in the cardiac muscle was considerably lower than that in skeletal muscles after the 1-year PMO treatment. Less than 5% dystrophin positive fibers, most of them with discontinuous weak signals at the cell membrane, were detected in the cardiac muscle (Figure 1a). The levels of dystrophin protein were only up to 2% by western blot in all heart of treated mice (Figure 1c). To determine the potential effect of the treatment with such limited protein induction on cardiac functions, we examined the cardiac muscles first by ultra-high frequency echocardiography. No significant difference in histology and ventricular function of the heart was identified between the treated group and the untreated control mice. We further examined the heart functions of the PMO-treated mdx mice by hemodynamics analysis under dobutamine stress in comparison with the untreated control mdx mice and normal C57BL/6 mice. This analysis has been reported to be highly sensitive to distinguish the degree of underline functional defects and to demonstrate potential therapeutic effect in the cardiac muscle of the mdx mice.15 The untreated control mdx mice showed clear deficits of cardiac functions with reduction in the stoke volume, cardiac output, maximum upstroke velocity (dV/dtmax), ventricular isovolumic relaxation time constant (τ), ejection fraction, and increase of minimum upstroke velocity (dV/dtmin), when compared to the normal C57BL/6 mice (Table 1). One-year treatment of 60 mg/kg PMO slightly improved the stoke volume, cardiac output, ventricular isovolumic relaxation time constant (τ), and ejection fraction, but the changes were not statistically significant. Significant improvement was however, revealed in the maximum and minimum upstroke velocity (dV/dtmax and dV/dtmin) (Table 1). Under dobutamine stress, the untreated control mdx mice showed significant cardiac dysfunctions, including decreases in stoke volume, cardiac output, end-diastolic volume, end-systolic pressure, dp/dtmax, dV/dtmax, ejection fraction, and an increase in dp/dtmin, dV/dtmin (Figure 3 and Table 2). Under the same stress condition, the 60 mg/kg PMO-treated mdx mice showed improved cardiac functions, especially in stoke volume, cardiac output, end-diastolic volume, end-systolic pressure, dp/dtmax, dV/dtmax, dV/dtmin, and ejection fraction (Figure 3 and Table 2).
Repeated high-dose treatments further enhanced the exon-skipping efficiency and skeletal muscle function
The dose-dependent short- and long-term exon-skipping effect and functional improvement prompted us to examine the potential of PMO treatment with two higher doses of 300 mg/kg and 1.5 g/kg. Six months of biweekly treatment with PMO at 300 mg/kg were able to achieve dystrophin expression in >50% fibers in body-wide skeletal muscles (Figure 4a). The levels of truncated dystrophin mRNA ranged from 25 to 40 % (Figure 4b). Western blot detected >15% of normal levels of dystrophin protein in all skeletal muscles (Figure 4c). Muscle pathology was clearly improved with none to only a few individual degenerating fibers detected in any skeletal muscles examined (Figure 5a). The fibers within the same muscle became highly uniform and with no clear inflammatory cell infiltrations. Sirius staining showed a very clear reduction in the intensity for collagens in the treated skeletal muscles when compared to the controls and the lower dose-treated mdx mice (Supplementary Figure S1). Consistently, the CK levels were significantly reduced when compared to those untreated control mice and the mice with lower doses of PMO treatment (Figures 2b and 5b). Muscle functions as measured by grip-force generation were significantly improved (Figure 5c). Dystrophin expression with strong signal was also detected in a small proportion of cardiac muscle fibers and the amount of dystrophin protein became detectable although still <4% in all heart of the treated mice (Figure 4c).
Figure 4.
Restoration of dystrophin expression in the mdx mice after 6 months intravenous (i.v.) treatment with phosphorodiamidate morpholino oligomers (PMO) (300 mg/kg or 1.5 g/kg biweekly). (a) Detection of dystrophin by immunohistochemistry with rabbit polyclonal antibody P7. Blue nuclear staining with DAPI. (b) Detection of exon 23 skipping in muscles by reverse transcription-PCR (RT-PCR). Lane 1, size marker. (c) Western blot for dystrophin expression [25 µg total protein loaded from tibialis anterior (TA) muscle of C57BL/6 mouse; 50 µg total protein loaded from untreated or treated mdx mice]. C57-TA, TA muscle from normal C57BL/6 mouse; Cont-TA, TA muscle from untreated mdx mouse. 300 mg/kg, 300 mg/kg PMO-treated mdx mice. 1.5 g/kg PMO, 1.5 g/kg PMO-treated mdx mice. (d) α-Actin as loading controls.
Figure 5.
Examination of pathology, serum, and skeletal muscle function after 6 months treatment with 300 mg/kg and 1.5 g/kg phosphorodiamidate morpholino oligomers (PMO). (a) Histology [hematoxylin and eosin (H&E) staining] of tibialis anterior (TA), diaphragm, and kidney from untreated (control), 300 mg/kg and 1.5 g/kg PMO-treated mdx mice (300 mg/kg, 1.5 g/kg). (b) The levels of serum enzymes. Creatine kinase (KU/l), creatinine (mg/l), urea nitrogen (mg/ml), total bilirubin (mg/l), direct bilirubin (mg/dl), alanine transaminase (ALT) (U/dl), alkaline phosphatase (ALP) (U/dl), and γ-glutamyltransferase (GGT) (U/l). (c) Grip strength tests. Significant improvement was observed in the mdx mice treated with 300 mg/kg and 1.5 g/kg PMO. KGF, kilogram force. (n = 10; *P ≤ 0.05 in comparison with C57BL/6 mice; #P ≤ 0.05 in comparison with untreated mdx mice. Two-tailed t-test).
Increasing the dose of PMO to 1.5 g/kg saw further improvement of dystrophin induction in all muscles. Nearly 100% fibers in all skeletal muscles expressed dystrophin by immunohistochemistry with the levels of the protein reaching 25–50% (Figure 4a,c). This level of dystrophin expression was associated with significant improvement in muscle function with the grip force reaching the strength near to the normal C57BL/6 mice (Figure 5c). CK levels reduced dramatically compared to those untreated control mice (Figure 5b). All skeletal muscle fibers became highly uniform in size, and degenerating fibers and inflammatory infiltrates were absent in all muscles (Figure 5a). More than 40% of cardiac muscle fibers expressed dystrophin although most of them had weak signal for the protein. During the 6-month treatment with these two high dosages, the mice showed no sign of abnormal behavior and change in body weight. No pathological change of the liver, kidney, and lung was observed by hematoxylin and eosin staining and serum tests for urea nitrogen, total bilirubin, direct bilirubin, alkaline phosphatase, and γ-glutamyltransferase. (Figure 5a,b).
Discussion
Since the first demonstration of therapeutic effect in vivo in DMD animal model of mdx mice, AO-mediated exon skipping has progressed into one of the most promising experimental therapies. Short-term systemic administration of antisense PMO is able to achieve functional levels of dystrophin expression in both mouse and dog models of DMD.14,18 Data from the ongoing clinical trial of PMO targeting exon 51 has confirmed systemically induced dystrophin expression in muscles of DMD boys after weekly treatment.21 However, effective exon skipping for DMD requires life-long administration and the capacity of antisense drugs to maintain functional levels of dystrophin with tolerable toxicity. In this study, we demonstrated that systemically administrated AO as PMO was indeed able to induce effective targeted exon skipping and dystrophin expression in all muscles in a dose-dependent manner for 1 year in mdx mice. Bodyweight, appearance, and behavior of the treated animal with all dosages were not significantly different from the control groups during the period of 1-year treatment. Serum tests and histology analysis showed no pathological changes in the liver, kidney and lung. Lack of toxicity was further demonstrated in the mice treated with up to 1.5 g/kg PMO for 6 months. These results together suggest that PMO treatment can achieve long-term therapeutic effects and is well tolerated.
Appropriate dosage of AO drug given systemically is critical for achieving long-term therapeutic efficacy to DMD. Part of the present study aimed to assess the potential therapeutic dosage and accumulative effect with long-term regular administration of PMO. Our results showed that targeted exon skipping and expression of dystrophin can be detected at the lowest dose of 15 mg/kg with biweekly i.v. injections although this dosage is apparently insufficient to achieve significant levels of dystrophin induction and therapeutic effect. When 60 mg/kg PMO was given biweekly, exon 23 skipping and dystrophin expression were clearly demonstrated in all muscles. Significant improvement in muscle pathology was indicated by reductions in muscle damage, central-nucleated fibers, collagen deposit, and serum CK levels. Furthermore, improvement in muscle function was recorded by grip-force measurement. This result suggests that 60 mg/kg PMO was able to achieve clearly detectable therapeutic effect with measurable improvement in muscle functions in the dystrophic mdx mouse. The result is consistent to our early observation that a similar dose of PMO with 7 weekly treatments is able to achieve clearly detectable improvement in muscle functions.14 Therefore, this dosage could be considered as a useful reference to a treatment regimen aiming to achieve efficacy with PMO-mediated exon skipping systemically.
However, caution needs to be exercised when the data from this study are extrapolated for the purpose of the treatment of DMD. Efficacy of antisense therapy to treat individual DMD patient, depends on many other factors, especially the efficiency of individual antisense drug and the functionality of the truncated dystrophin determined mainly by the location of mutations and size of deletions. Both determinants are difficult or yet to be defined for targeting most human dystrophin exons. However, the following two factors are likely to affect our consideration for the reference value of effective dosage observed in the mdx mice. First, it is most probable that a truncated dystrophin lacking only exon 23 produced in the mdx mice muscles will retain better function than most truncated dystrophins expected from DMD patients after exon skipping. Second, the PMOE23+7-18 used in the present study has been optimized and tested extensively with high degree efficiency in both cell culture and in vivo. This is in contrast to the lack of appropriate system to verify exon-skipping efficiency in vivo for AO drugs targeting human dystrophin exon. It is therefore likely that a considerable higher dosage may be required to achieve clearly measurable therapeutic outcome with most AO drugs targeting human dystrophin exons in clinic providing that dose effect is similar between mouse and human on weight/weight basis. When the outcome of the ongoing clinical trials becomes available, it might be possible to establish a relative efficiency of new AO drugs targeting different exons with the AO on trial as the control. This can be achieved by testing two AOs together in the same cell culture and/or in the same animal model of humanized mdx mice. This together with our understanding of functionality of individual truncated dystrophin would provide a more accurate assessment for a dosing to achieve efficacy in clinics.
DMD mutations affect the expression of dystrophin in all muscles including cardiac muscle. While defects in cardiac functions as a result of dystrophin deficiency is not prominent in the early stage of disease manifestation, failures of cardiac function, and the respiratory system are the leading causes of lethality in later stage of disease progression. Thus, restoration of functional levels of dystrophin to prevent deterioration in cardiac functions constitutes one of the critical aims for the exon skipping to be effective therapy. Furthermore, as DMD patients live longer due to improved multidisciplinary patient care, rescuing dystrophin expression in cardiac muscle becomes more critical for their longevity and quality of life.23,24,25,26,27 Early studies however, revealed that the efficiency of antisense oligomer-induced exon skipping was significantly (several folds) lower in cardiac muscle than in skeletal muscles. Systemic PMO treatment at the doses <300 mg/kg produced only barely detectable (<5% normal levels) dystrophin in the cardiac muscle.14,17 This lower efficiency of exon skipping becomes one of the major concerns for the therapy to be effective to DMD patients. The results from the present study confirmed that the cardiac muscle is much more resistant to PMO-mediated exon skipping. Only 2% or less normal levels of dystrophin expression were detected in the heart after 1-year i.v. injections of 60 mg/kg PMO. The result also indicates a limited accumulative exon-skipping effect in the cardiac muscle with the biweekly treatment regimen. Nevertheless, functional improvement, although limited, was clearly demonstrated by the hemodynamics analysis. This result suggests that low levels of dystrophin induction may be able to provide benefit to cardiac functions although other factors such as improved functions in skeletal muscle especially the diaphragm could also be involved.22 However, it is also possible that such low levels of dystrophin induction, being sufficient to provide improvement to the mildly affected cardiac muscle of the mdx mice, may not be sufficient to improve functions of severely affected cardiac muscles in DMD patients.
The half-life of AO-induced dystrophin protein has been estimated to be about 2 months although it is not clear whether this is the result of protein stability or the sustained effect of AO within the fibers. Previous studies observed significant accumulation in dystrophin protein by short-term weekly or daily injections.14 Therefore, we expected that the levels of dystrophin after 1-year biweekly injections would be significantly higher than that after single injection when the same dose of PMO is used. Unexpectedly, the results showed that the long-term accumulative effect of PMO was limited. The mechanism(s) is not understood, but the following factors may be involved. Firstly, a biweekly regimen will certainly have less accumulative effect than a weekly regimen. Perhaps more importantly, the dependence of unmodified PMO on the increased membrane permeability for effective delivery may constitute another possible explanation. Since the efficiency of PMO relies heavily on permeability of muscle fibers and dystrophic muscles undergo cycles of degeneration and regeneration, the initial a few doses of PMO would achieve higher efficiency through the fraction of muscle fibers with higher permeability. However, such fiber population decreases gradually and most muscle fibers become less permeable to PMO eventually, leading to a diminishing delivery efficiency, thus accumulative effect. This hypothesis is consistent with the fact that normal muscles are resistant to PMO delivery.14 While the limited accumulative effect may indicate the improvement in muscle membrane permeability after the treatment, it also suggests that long-term efficiency in exon skipping with PMO and 2OMePS may be difficult to exceed that achieved by a single dose of AO.
Materials and Methods
Animals, oligonucleotides, and in vivo delivery methods. Ten mdx mice and C57BL/6 mice aged 4–5 weeks were used in each group. Experiments were approved by IACUC Carolinas Medical Center (protocol # 01-08-06A for breeding of mdx mice; protocol # 01-08-07A for PMO oligonucleotide therapy for DMD). The PMO (+07-18) (5′-GGCCAAACCTCG GCTTACCTGAAAT-3′) against the boundary sequences of exon and intron 23 of dystrophin gene (PMO) were used (Gene Tools, Philomath, OR). For i.v. administration, PMO was used in 100-µl saline by retro-orbital injections. Same volume of saline was used in control mice. Mice were killed at desired time points, and muscles were snap-frozen in liquid nitrogen-cooled isopentane and stored at −80 °C.
Antibodies and immunohistochemistry. Sections of 6 µm were cut from at least two-thirds of muscle length of tibialis anterior, quadriceps, biceps, and gastrocnemius at 100 µm intervals and at least 10 levels from all other muscles including heart, diaphragm, intercostals, and abdominal muscles at 100 µm intervals. The intervening muscle sections were collected either for western blot and RT-PCR analysis. The serial sections were stained with rabbit polyclonal antibody P7 against dystrophin sequences from amino acid 2847 to amino acid 2990.12,13,14 The primary antibody was detected by goat-anti-rabbit immunoglobulin Gs Alexa 594 (Invitrogen, Eugene, OR). Sections were also stained with hematoxylin and eosin or picrosirius red solution for histological and collagen assessment.
Protein extraction and western blot. The collected sections were ground into powder and lysed with 200-µl protein extraction buffer as described previously.15,16 The protein concentration was quantified by Protein Assay Kit (Bio-Rad, Hercules, CA). Proteins were loaded onto a 4–15% Tris–HCL gradient gel. Samples were electrophoresed overnight at 10 mA at 4 °C and blotted onto nitrocellulose membrane overnight at 50 V. The membrane was then washed and blocked with 5% skimmed milk and probed with monoclonal antibody NCL-DYS1 against dystrophin rod domain (Vector Labs, Burlingame, CA) overnight. The bound primary antibody was detected by horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G and ECL Western Blotting Analysis System (Perkin Elmer, Waltham, MA). The intensity of the bands obtained from the oligomer-treated mice muscles was measured and compared with that from normal muscles of C57BL/6 mice (NIH ImageJ 1.42 software).
RNA extraction and RT-PCR. The collected sections were homogenized in TRIzol (Invitrogen) by using an Ultra-Turrax homogenizer (Janke and Kunkel, Staufen, Germany). Total RNA was then extracted and 100 ng of RNA template was used for a 50-ml RT-PCR with RT-PCR Master Mix (USB, Cleveland,OH). The primer sequences for the RT-PCR were Ex20Fo 5′-AGAATTCTGCCAATTGCTGAG-3′ and Ex26Ro 5′-TTCTTCAGCT TGTGTCATCC-3′ for amplification of mRNA from exons 20 to 26. A total of 40 cycles were carried out for the RT-PCR. Bands with the expected size for the transcript with exon 23 deleted were extracted and sequenced. The intensity of the bands was measured with the NIH ImageJ 1.42 and percentage of exon skipping was calculated with the intensity of the two bands representing both exon 23 unskipped and skipped as 100%.
Cardiac imaging. Mice were placed in an induction chamber with 4% isoflurane mixed with 100% oxygen. The mouse was monitored for heart rate (350–550 bpm) and rectal temperature (36.5–37.5 °C) throughout the procedure. Two dimensional, M-mode, and spectral Doppler Imaging was performed using the VisualSonics Vevo 770 ultrasound system with the RMV 707 transducer (VisualSonics, Toronto, Ontario, Canada). At the completion of the scanning, the mouse was removed from anesthesia and allowed to wake up prior to returning to the colony.
Grip strength test. Grip strength was assessed using grip strength meter consisting of horizontal forelimb mesh and an angled hind limb mesh (Columbus Instruments, Columbus OH). Five successful hindlimb and forelimb strength measurements within 2 minutes were recorded, and data was normalized to body weight and expressed as kilogram force.
Measurement of serum CK and other components. Mouse blood was taken immediately after cervical dislocation and centrifuged at 1,500 r.p.m. for 3 minutes. Serum was separated and stored at −80 °C. The level of serum components was determined by Charles Riverside Laboratories (Columbia, MO).
In vivo cardiac hemodynamics. Hemodynamics analysis on the treated and untreated mdx as well as the C57BL/6 mice were performed using conductance micromanometry according to previously published methods.28,29,30 Briefly, the mice were anesthetized by inhalation of 1% isoflourane and 99% oxygen. The mice were then ventilated at 120 strokes/minute with a rodent ventilator (Harvard Instruments, Holliston, MA) and kept on a warm pat to stabilize the body temperature at 37° C. A 1.4 F high-fidelity micromanometer catheter (Millar Instruments, Houston, TX) was inserted into the left ventricle through carotid artery under continuous hemodynamic monitoring. Following collection of baseline hemodynamic data, dobutamine was infused at a rate of 6.5 µg/g/min for 3 minutes through the jugular vein. Pressure-volume loops data were collected online at 500 Hz. Hemodynamic parameters were analyzed with PVAN3.3 software (Millar Instruments).
SUPPLEMENTARY MATERIAL Figure S1. Examination of collagen deposition in muscles by picrosirius staining. Diaphragm and quadriceps from normal C57Bl/6 mice (C57Bl/6), untreated mdx mice (mdx control), one year 15 mg/kg PMO-treated mdx mice (mdx+PMO15 mg/kg), one year 60 mg/kg PMO-treated mdx mice (mdx+PMO 60 mg/kg), and 6 months 300 mg/kg PMO-treated mdx mice (mdx+PMO 300 mg/kg).
Acknowledgments
This work was supported by the Carolinas Muscular Dystrophy Research Endowment at the Carolinas HealthCare Foundation and Carolinas Medical Center (Charlotte, NC); US Army Medical Research, Department of Defense (W81XWH-05-1-0616). The authors declared no conflict of interest.
Supplementary Material
Examination of collagen deposition in muscles by picrosirius staining. Diaphragm and quadriceps from normal C57Bl/6 mice (C57Bl/6), untreated mdx mice (mdx control), one year 15 mg/kg PMO-treated mdx mice (mdx+PMO15 mg/kg), one year 60 mg/kg PMO-treated mdx mice (mdx+PMO 60 mg/kg), and 6 months 300 mg/kg PMO-treated mdx mice (mdx+PMO 300 mg/kg).
REFERENCES
- Hoffman EP, Brown RH., Jr, and, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Laing NG. Molecular and Cell Biology of Muscular Dystrophy. In: Partridge, TA (ed.). Chapman: London; 1993. pp. 37–77.. [Google Scholar]
- Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H., and, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2:90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- Athanasopoulos T, Graham IR, Foster H., and, Dickson G. Recombinant adeno-associated viral (rAAV) vectors as therapeutic tools for Duchenne muscular dystrophy (DMD) Gene Ther. 2004;11 Suppl 1:S109–S121. doi: 10.1038/sj.gt.3302379. [DOI] [PubMed] [Google Scholar]
- England SB, Nicholson LV, Johnson MA, Forrest SM, Love DR, Zubrzycka-Gaarn EE, et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343:180–182. doi: 10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- Tang Y, Cummins J, Huard J., and, Wang B. AAV-directed muscular dystrophy gene therapy. Expert Opin Biol Ther. 2010;10:395–408. doi: 10.1517/14712591003604690. [DOI] [PubMed] [Google Scholar]
- Sherratt TG, Vulliamy T, Dubowitz V, Sewry CA., and, Strong PN. Exon skipping and translation in patients with frameshift deletions in the dystrophin gene. Am J Hum Genet. 1993;53:1007–1015. [PMC free article] [PubMed] [Google Scholar]
- Dunckley MG, Manoharan M, Villiet P, Eperon IC., and, Dickson G. Modification of splicing in the dystrophin gene in cultured Mdx muscle cells by antisense oligoribonucleotides. Hum Mol Genet. 1998;7:1083–1090. doi: 10.1093/hmg/7.7.1083. [DOI] [PubMed] [Google Scholar]
- Mann CJ, Honeyman K, Cheng AJ, Ly T, Lloyd F, Fletcher S, et al. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc Natl Acad Sci USA. 2001;98:42–47. doi: 10.1073/pnas.011408598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson G, Hill V., and, Graham IR. Screening for antisense modulation of dystrophin pre-mRNA splicing. Neuromuscul Disord. 2002;12 Suppl 1:S67–S70. doi: 10.1016/s0960-8966(02)00085-8. [DOI] [PubMed] [Google Scholar]
- Aartsma-Rus A, Bremmer-Bout M, Janson AA, den Dunnen JT, van Ommen GJ., and, van Deutekom JC. Targeted exon skipping as a potential gene correction therapy for Duchenne muscular dystrophy. Neuromuscul Disord. 2002;12 Suppl 1:S71–S77. doi: 10.1016/s0960-8966(02)00086-x. [DOI] [PubMed] [Google Scholar]
- Lu QL, Mann CJ, Lou F, Bou-Gharios G, Morris GE, Xue SA, et al. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat Med. 2003;9:1009–1014. doi: 10.1038/nm897. [DOI] [PubMed] [Google Scholar]
- Lu QL, Rabinowitz A, Chen YC, Yokota T, Yin H, Alter J, et al. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci USA. 2005;102:198–203. doi: 10.1073/pnas.0406700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD, et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med. 2006;12:175–177. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- Wu B, Moulton HM, Iversen PL, Jiang J, Li J, Li J, et al. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc Natl Acad Sci USA. 2008;105:14814–14819. doi: 10.1073/pnas.0805676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Li Y, Morcos PA, Doran TJ, Lu P., and, Lu QL. Octa-guanidine morpholino restores dystrophin expression in cardiac and skeletal muscles and ameliorates pathology in dystrophic mdx mice. Mol Ther. 2009;17:864–871. doi: 10.1038/mt.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Lu P, Benrashid E, Malik S, Ashar J, Doran TJ, et al. Dose-dependent restoration of dystrophin expression in cardiac muscle of dystrophic mice by systemically delivered morpholino. Gene Ther. 2010;17:132–140. doi: 10.1038/gt.2009.120. [DOI] [PubMed] [Google Scholar]
- Yokota T, Lu QL, Partridge T, Kobayashi M, Nakamura A, Takeda S, et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65:667–676. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, Adkin C, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- < http://www.avibio.com/news_detail.php?newsId=0085 >
- Townsend D, Yasuda S, Li S, Chamberlain JS., and, Metzger JM. Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Mol Ther. 2008;16:832–835. doi: 10.1038/mt.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K, Foster H., and, Dickson JG. Gene therapy progress and prospects: Duchenne muscular dystrophy. Gene Ther. 2006;13:1677–1685. doi: 10.1038/sj.gt.3302877. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Lechtzin N., and, Judge DP. Current treatment of adult Duchenne muscular dystrophy. Biochim Biophys Acta. 2007;1772:229–237. doi: 10.1016/j.bbadis.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Matsuda R, Nishikawa A., and, Tanaka H. Visualization of dystrophic muscle fibers in mdx mouse by vital staining with Evans blue: evidence of apoptosis in dystrophin-deficient muscle. J Biochem. 1995;118:959–964. doi: 10.1093/jb/118.5.959. [DOI] [PubMed] [Google Scholar]
- Silver MM, Banerjee D., and, Hudson AJ. Segmental myofiber necrosis in myotonic dystrophy - An immunoperoxidase study of immunoglobulins in skeletal muscle. Am J Pathol. 1983;112:294–301. [PMC free article] [PubMed] [Google Scholar]
- Bostick B, Yue Y, Long C., and, Duan D. Prevention of dystrophin-deficient cardiomyopathy in twenty-one-month-old carrier mice by mosaic dystrophin expression or complementary dystrophin/utrophin expression. Circ Res. 2008;102:121–130. doi: 10.1161/CIRCRESAHA.107.162982. [DOI] [PubMed] [Google Scholar]
- Burger AJ, Notarianni MP., and, Aronson D. Safety and efficacy of an accelerated dobutamine stress echocardiography protocol in the evaluation of coronary artery disease. Am J Cardiol. 2000;86:825–829. doi: 10.1016/s0002-9149(00)01100-0. [DOI] [PubMed] [Google Scholar]
- Townsend D, Blankinship MJ, Allen JM, Gregorevic P, Chamberlain JS., and, Metzger JM. Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol Ther. 2007;15:1086–1092. doi: 10.1038/sj.mt.6300144. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Townsend D, Michele DE, Favre EG, Day SM., and, Metzger JM. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature. 2005;436:1025–1029. doi: 10.1038/nature03844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Examination of collagen deposition in muscles by picrosirius staining. Diaphragm and quadriceps from normal C57Bl/6 mice (C57Bl/6), untreated mdx mice (mdx control), one year 15 mg/kg PMO-treated mdx mice (mdx+PMO15 mg/kg), one year 60 mg/kg PMO-treated mdx mice (mdx+PMO 60 mg/kg), and 6 months 300 mg/kg PMO-treated mdx mice (mdx+PMO 300 mg/kg).