Abstract
Background:
Little evidence is available on the relation of physical activity with colon adenomas, a colon cancer precursor.
Methods:
We conducted a systematic literature review and meta-analysis of published studies (in English) through April 2010, examining physical activity or exercise and risk or prevalence of colon adenoma or polyp. Random effects models were used to estimate relative risks (RRs) and corresponding confidence intervals (CIs). A total of 20 studies were identified that examined the association and provided RRs and corresponding 95% CIs.
Results:
A significant inverse association between physical activity and colon adenomas was found with an overall RR of 0.84 (CI: 0.77–0.92). The association was similar in men (RR=0.81, CI: 0.67–0.98) and women (RR=0.87, CI: 0.74–1.02). The association appeared slightly stronger in large/advanced polyps (RR=0.70, CI: 0.56–0.88).
Conclusion:
This study confirms previous reports of a significant inverse association of physical activity and colon adenoma, and suggests that physical activity can have an important role in colon cancer prevention.
Keywords: physical activity, colon adenoma, colon polyp
Convincing evidence exists for a causal, inverse association between physical activity and risk of colon cancer (International Agency for Research on Cancer WHO, 2002; World Cancer Research Fund/American Institute for Cancer Research, 2007b; Wolin et al, 2009). This association is plausibly supported by several biological mechanisms, including decreased inflammation, decreased insulin-like growth factor levels, reduced hyperinsulinemia and modulated immune function (Wolin et al, 2009). Fewer data are available with respect to physical activity and colon adenomas, the precursor lesion detected and removed during sigmoidoscopy or colonoscopy. Although numerous studies have examined this association, no comprehensive meta-analysis is available. A limited meta-analysis by the World Cancer Research Fund included three studies, and estimated physical activity was associated with a statistically significant 15% reduction in colon polyp risk (World Cancer Research Fund/American Institute for Cancer Research, 2007a). Estimation of the risk reduction associated with the physical activity is important for public health because lifestyle is associated with a decreased risk of colon cancer, even among those who have undergone colon cancer screening (Wei et al, 2009). Furthermore, evidence on smoking has suggested that risk may vary for colon polyps versus colon cancer (Botteri et al, 2008). We therefore conducted a meta-analysis to estimate the summary relative risk (RR) of colon polyps associated with physical activity.
Materials and methods
We searched the literature using PubMed, CINAHL and Scopus for all studies on physical activity or exercise and colon polyps through April 2010. We employed the terms exercise and physical activity in combination with colon polyps using the terms colon polyp, colon adenoma, colorectal polyp, colorectal adenoma and adenomatous polyps. We also utilised a previous review of the data (Samad et al, 2005; Lee and Oguma, 2006; World Cancer Research Fund/American Institute for Cancer Research, 2007a) and manual searches of the reference lists of identified manuscripts. We included recurrent, incident and prevalent cases of colon polyps. We did not limit studies by type of physical activity or study sample demographics.
Our search yielded 89 potential articles. We excluded reviews, non-human studies, editorials/comments/letters to the editor, studies without colon polyps as an outcome, studies where physical activity was only included as a covariate (and thus no measure of association was presented), and where no metric for effect estimate precision (P-value, s.e., confidence interval (CI)) was provided. Combined with searches from the reference sections of manuscripts and previous reviews, this yielded 20 manuscripts. From each manuscript, we abstracted the sample size (including number of cases), gender, years of follow-up or type of control sample, case definition, physical activity domain, adenoma detection method, sample definition criteria and results. We also abstracted the variables that each study used in its most adjusted analysis. Data extraction was performed by a single investigator (KYW). Where studies included more than one type of physical activity without a summary measure, we included only leisure time physical activity, which is the major modifiable component of energy.
Previous meta-analyses have suggested that results for adenomatous polyps need to be presented separately from hyperplastic or malignant polyps. (Botteri et al, 2008) Although we did not restrict our analysis to studies where data was limited to adenomatous polyps, we did consider those results separately. Specifically, we excluded results for hyperplastic polyps where feasible. We also identified studies considered to be the ‘best approach’ using criteria similar to those used in a previous meta-analysis (Botteri et al, 2008), namely, studies that met all of the following: (1) limited the outcome to only adenomatous polyps; (2) all individuals received a full colonoscopy; and (3) the study population excluded anyone with inherited colorectal cancer syndromes, inflammatory bowel disease, a history of colon polyps or cancer, or a previous colon resection.
Data analysis
Meta-analysis of random effects was used to allow for the heterogeneity of results across studies. (Mosteller and Colditz, 1996) Data were processed in SAS, and the analyses were performed using R-package ‘meta’ (SAS Institute Inc., Cary, NC, USA). A summary forest plot was generated in Stata (StataCorp LP, College Station, TX, USA). As most studies reported RRs or odds ratios (ORs) and their associated 95 percent CIs, we used these data as summary statistics for each study. First, we derived the s.e. of log (RR or OR) using the 95 percent CI, with the expression: (log (upper limit) – log (lower limit))/2*1.96. These s.es were used as weights for summary effect estimates in the meta-analysis. We visually examined publication bias using Funnel plots, and employed the rank correlation method to formally test for bias. (Begg and Mazumdar, 1994) Where studies reported results separately for men and women, we included both estimates when reporting the overall association. To evaluate the potential effects of limiting results to only adenomatous polyps, we conducted exploratory analysis in the subset of those studies. We also included results separately for large/advanced adenoma, if the data were presented as such in the original manuscript. We also conducted exploratory analyses limited to those studies defined as the ‘best approach’. To test sub-analysis differences (large vs all adenomas; best approach vs all studies), we partitioned ‘total heterogeneity’ into between-group and within-group heterogeneity, and used the ‘between-group’ heterogeneity index as the test statistic against χ2 distribution with 1 degree of freedom. (Cooper and Hedges, 1994).
Results
We identified 20 studies of physical activity and colon adenomas (Table 1). (Kono et al, 1991, 1999; Little et al, 1993; Shinchi et al, 1994; Giovannucci et al, 1995, 1996; Sandler et al, 1995; Neugut et al, 1996; Enger et al, 1997; Lubin et al, 1997; Kahn et al, 1998; Boutron-Ruault et al, 2001; Colbert et al, 2002; Lieberman et al, 2003; Tiemersma et al, 2003; Hauret et al, 2004; Wallace et al, 2005; Larsen et al, 2006; Rosenberg et al, 2006; Hermann et al, 2009) Most collected physical activity information via questionnaire, with nine studies only collecting information on leisure activity. Studies often did not specify or query the reasons participants underwent colonoscopy or sigmoidoscopy, thus, cases included are both symptomatic and screening. Only two studies (Colbert et al, 2002; Wallace et al, 2005) included procedures for the study, both were in studies of polyp recurrence. All but two studies (Kahn et al, 1998; Rosenberg et al, 2006) reported results for adenomas separately from all polyps or limited results to adenomas. A total of 10 studies (Shinchi et al, 1994; Giovannucci et al, 1995, 1996; Lubin et al, 1997; Kono et al, 1999; Boutron-Ruault et al, 2001; Colbert et al, 2002; Lieberman et al, 2003; Wallace et al, 2005; Larsen et al, 2006) reported results separately for large or advanced adenomas.
Table 1. Studies include in meta-analysis of physical activity and colon polyps.
| Author and Year | Gender | Number of study subjects | Number of Cases | Relative Risk | Lower Confidence Interval | Upper Confidence Interval | Type of Physical Activity | Case definition | Non-case/ comparison definition |
|---|---|---|---|---|---|---|---|---|---|
| Kono et al, 1991 | Both | 1148 | 80 | 0.44 | 0.22 | 0.87 | Leisure | Adenoma | None |
| Little et al, 1993 | Both | 300 | 147 | 0.46 | 0.17 | 1.29 | Leisure | Adenoma | FOBT negative |
| Shinchi et al, 1994 | Both | 1712 | 228 | 1.2 | 0.8 | 2 | Leisure | Adenoma | None |
| Giovannucci et al, 1995 | Men | 12 879 | 455 | 0.79 | 0.57 | 1.09 | Leisure | Distal Adenoma | No polyp |
| Sandler, 1995 | Men | 234 | 86 | 0.92 | 0.36 | 2.31 | Leisure | Adenoma | Hyperplastic/none |
| Sandler, 1995 | Women | 350 | 114 | 0.64 | 0.35 | 1.19 | Leisure | Adenoma | Hyperplastic/none |
| Giovannucci et al, 1996 | Women | 13 057 | 330 | 0.58 | 0.4 | 0.86 | Leisure | Distal Adenoma | None |
| Neugut, 1996 | Men | 400 | 225 | 0.6 | 0.4 | 1 | Total | Adenoma | None |
| Neugut, 1996 | Women | 411 | 283 | 1.3 | 0.7 | 2.3 | Total | Adenoma | None |
| Enger et al, 1997 | Both | 920 | 460 | 1 | 0.7 | 1.5 | Total | Adenoma | No polyp |
| Lubin et al, 1997 | Both | 392 | 196 | 0.6 | 0.3 | 0.9 | Total | Large/advanced Adenoma | Hyperplastic/None |
| Kahn et al, 1998 | Men | 72 868 | 7504 | 0.83 | 0.76 | 0.91 | Total | All polyps | None |
| Kahn et al, 1998 | Women | 81 356 | 5111 | 0.9 | 0.78 | 1.03 | Total | All polyps | None |
| Kono et al, 1999 | Both | 415 | 189 | 0.6 | 0.3 | 1.3 | Leisure | Adenoma | Normal |
| Boutron-Ruault et al, 2001 | Both | 581 | 154 | 1.3 | 0.7 | 2.5 | Total | Small adenoma | None |
| Boutron-Ruault et al, 2001 | Both | 635 | 208 | 0.8 | 0.4 | 1.5 | Total | Large/advanced adenoma | None |
| Colbert et al, 2002 | Both | 1839 | 733 | 1.2 | 0.9 | 1.6 | Total | Adenoma or cancer | None |
| Lieberman et al, 2003 | Both | 2082 | 312 | 0.94 | 0.86 | 1.02 | Total | Large/advanced adenoma | None |
| Tiemersma et al, 2003 | Women | 471 | 196 | 1.05 | 0.72 | 1.54 | Not specified | Adenoma | None |
| Tiemersma et al, 2003 | Men | 398 | 237 | 0.69 | 0.43 | 1.1 | Not specified | Adenoma | None |
| Hauret et al, 2004 | Both | 405 | 177 | 0.63 | 0.34 | 1.17 | Total | Adenoma | Hyperplastic/None |
| Wallace et al, 2005 | Men | 787 | 539 | 0.35 | 0.17 | 0.72 | Total | Large/advanced adenoma | None |
| Wallace et al, 2005 | Women | 787 | 205 | 1.21 | 0.36 | 4.03 | Total | Large/advanced adenoma | None |
| Larsen et al, 2006 | Both | 3696 | 426 | 0.96 | 0.74 | 1.25 | Total | Low risk adenoma | None |
| Larsen et al, 2006 | Both | 3376 | 106 | 0.56 | 0.34 | 0.92 | Total | Large/advanced adenoma or cancer | None |
| Rosenberg et al, 2006 | Women | 45 400 | 1390 | 0.72 | 0.57 | 0.91 | Leisure | All polyps | None |
| Hermann et al, 2009 | Both | 4510 | 527 | 1.02 | 0.74 | 1.42 | Total | Adenoma | None |
Abbreviation: FOBT=Fecal occult blood test.
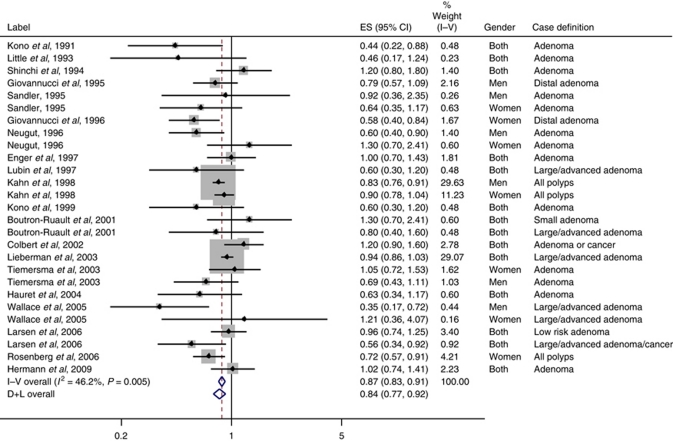
We found significant heterogeneity in the results (P<0.01) and thus, focus our report on the random effects analysis (Figure 1). Overall, there was a significant inverse association between physical activity and colon polyps (fixed effect RR=0.87, 95% CI: 0.83–0.91; random effects RR=0.84, 95% CI: 0.77–0.92) when comparing the most to least active individuals in each study. The summary RR was significant and similar in men (RR=0.81, 95% CI: 0.67–0.98) and women (RR=0.87, 95% CI: 0.74–1.02).
Figure 1.
Meta-analysis of physical activity and colon adenoma. Study physical activity level comparisons are as follows: Kono et al, 1991: ⩾120 vs 0 min per week; Little et al, 1993: ⩾30 min vs none; Shinchi et al, 1994: daily vs none; Giovannucci et al, 1995: highest vs lowest quintile; Sandler, 1995: highest vs lowest quartile; Giovannucci et al, 1996: highest vs lowest quintile; Neugut et al, 1996: any vs none; Lubin et al, 1997: >5.5 h per day vs <4 h per day; Enger et al, 1997: highest vs lowest quartile; Kahn et al, 1998: high vs low; Kono et al, 1999: ⩾36 MET h per wk vs <4 MET h per wk; Boutron-Ruault et al, 2001: high vs low; Colbert et al, 2002: high vs low quartile; Lieberman et al, 2003: per 5 unit change in physical activity index; Tiemersma et al, 2003: not specified; Hauret et al, 2004: >40 MET h per wk vs <17.1 MET h per wk; Wallace et al, 2005: high vs low tertile; Larsen et al, 2006: high vs low quartile; Rosenberg et al, 2006: ⩾40 MET h per wk vs none; Hermann et al, 2009: active vs inactive. ES=effect size; MET=metabolic equivalent.
There was a tendency for the effect of physical activity to be restricted to large or advanced adenomas and not low grade ones. Similarly, physical activity was associated with large (>1 cm) (RR=0.63, 95% CI: 0.36–1.10), but not with small adenomas in a sample of US male health professionals (Giovannucci et al, 1995). In a cohort of US female nurses, a significant overall risk reduction (RR=0.58, 95% CI: 0.40–0.86) was reported, which was also stronger for larger than smaller adenomas (Giovannucci et al, 1996). Our meta-analysis found the effect was stronger, though not significantly so (P=0.16), for large or advanced (RR=0.70, 95% CI: 0.56–0.88) adenomas than for the overall effect. In analyses limited to the 18 studies where results for adenomatous polyps were separated from all polyps (i.e., hyperplastic, malignant polyps), the meta-analysis estimate for the association between physical activity and risk of polyps was largely unchanged (RR=0.83, 95% CI: 0.73–0.93). In analysis limited to the six studies (Kono et al, 1991; Sandler et al, 1995; Colbert et al, 2002; Lieberman et al, 2003; Tiemersma et al, 2003; Hauret et al, 2004) defined as the ‘best approach,’ the effect estimate was similar to that for all studies (RR=0.87, 95% CI: 0.73–1.05), though not statistically significant.
Discussion
Previous, though limited, reviews have indicated physical activity is associated with a significant reduction in colon polyp risk. (World Cancer Research Fund/American Institute for Cancer Research, 2007a) Our comprehensive meta-analysis supports this conclusion, showing a significant 16% risk reduction when comparing the most to the least active. Risk reductions were similar for men and women, and held when limited to studies designated as the best approach. We found the association was notably stronger when analyses were limited to advanced or large polyps, with a risk reduction of 35%.
These results support the previously documented role of physical activity in colon cancer prevention (International Agency for Research on Cancer WHO, 2002; World Cancer Research Fund/American Institute for Cancer Research, 2007a and 2007b; Wolin et al, 2009). Earlier reports that failed to find an association between physical activity and colon polyps had suggested that physical activity may be more important in the adenoma to carcinoma sequence than in adenoma development (Colbert et al, 2002). Our meta-analysis, combined with the above-mentioned data demonstrating physical activity's role in colon cancer prevention, suggests that physical activity has a role across the carcinogenic process. Several mechanisms have been proposed for such effects, including enhanced immune function, decreased inflammation, reduced insulin levels and insulin resistance, and higher vitamin D levels (Wolin et al, 2009). Hyperinsulinemia has also been directly related to colon polyp risk (Wei et al, 2006).
This comprehensive meta-analysis provides support for an inverse association between physical activity and colon polyps, and also for the role of physical activity in colon cancer carcinogenesis. Physical activity may reduce the risk of colon polyps by 15% and may provide a substantially larger reduction in risk of large and advanced polyps.
Acknowledgments
GAC is supported by an American Cancer Society Clinical Research Professorship. KYW, GAC and YY are supported by CA091842.
References
- Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101 [PubMed] [Google Scholar]
- Botteri E, Iodice S, Raimondi S, Maisonneuve P, Lowenfels AB (2008) Cigarette smoking and adenomatous polyps: a meta-analysis. Gastroenterology 134: 388–395 [DOI] [PubMed] [Google Scholar]
- Boutron-Ruault MC, Senesse P, Meance S, Belghiti C, Faivre J (2001) Energy intake, body mass index, physical activity, and the colorectal adenoma-carcinoma sequence. Nutr Cancer 39: 50–57 [DOI] [PubMed] [Google Scholar]
- Colbert LH, Lanza E, Ballard-Barbash R, Slattery ML, Tangrea JA, Caan B, Paskett ED, Iber F, Kikendall W, Lance P, Shike M, Schoen RE, Daston C, Schatzkin A (2002) Adenomatous polyp recurrence and physical activity in the Polyp Prevention Trial (United States). Cancer Causes Control 13: 445–453 [DOI] [PubMed] [Google Scholar]
- Cooper H, Hedges LV (1994) The Handbook of Research Synthesis: Part VI: Statistically Analyzing Effect Size. Newbury Park, CA: Russell Sage Foundation [Google Scholar]
- Enger SM, Longnecker MP, Lee ER, Frankl HD, Haile RW (1997) Recent and past physical activity and prevalence of colorectal adenomas. Br J Cancer 75: 740–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC (1995) Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 122: 327–334 [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Colditz GA, Stampfer MJ, Willett WC (1996) Physical activity, obesity, and risk of colorectal adenoma in women (United States). Cancer Causes Control 7: 253–263 [DOI] [PubMed] [Google Scholar]
- Hauret KG, Bostick RM, Matthews CE, Hussey JR, Fina MF, Geisinger KR, Roufail WM (2004) Physical activity and reduced risk of incident sporadic colorectal adenomas: observational support for mechanisms involving energy balance and inflammation modulation. Am J Epidemiol 159: 983–992 [DOI] [PubMed] [Google Scholar]
- Hermann S, Rohrmann S, Linseisen J (2009) Lifestyle factors, obesity and the risk of colorectal adenomas in EPIC-Heidelberg. Cancer Causes Control 20: 1397–1408 [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer WHO (2002) IARC Handbooks of Cancer Prevention: Weight Control and Physical Activity, Volume 6. International Agency for Research on Cancer: Lyon, France [Google Scholar]
- Kahn HS, Tatham LM, Thun MJ, Heath Jr CW (1998) Risk factors for self-reported colon polyps. J Gen Intern Med 13: 303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono S, Handa K, Hayabuchi H, Kiyohara C, Inoue H, Marugame T, Shinomiya S, Hamada H, Onuma K, Koga H (1999) Obesity, weight gain and risk of colon adenomas in Japanese men. Jpn J Cancer Res 90: 805–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono S, Shinchi K, Ikeda N, Yanai F, Imanishi K (1991) Physical activity, dietary habits and adenomatous polyps of the sigmoid colon: a study of self-defense officials in Japan. J Clin Epidemiol 44: 1255–1261 [DOI] [PubMed] [Google Scholar]
- Larsen IK, Grotmol T, Almendingen K, Hoff G (2006) Lifestyle as a predictor for colonic neoplasia in asymptomatic individuals. BMC Gastroenterol 6: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IM, Oguma Y (2006) Physical activity. In Cancer Epidemiology and Prevention, Third Edition, Schottenfeld D, Fraumeni JF, Jr (eds). pp 449–467. Oxford University Press: New York [Google Scholar]
- Lieberman DA, Prindiville S, Weiss DG, Willett W (2003) Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 290: 2959–2967 [DOI] [PubMed] [Google Scholar]
- Little J, Logan RF, Hawtin PG, Hardcastle JD, Turner ID (1993) Colorectal adenomas and energy intake, body size and physical activity: a case-control study of subjects participating in the Nottingham faecal occult blood screening programme. Br J Cancer 67: 172–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin F, Rozen P, Arieli B, Farbstein M, Knaani Y, Bat L, Farbstein H (1997) Nutritional and lifestyle habits and water-fiber interaction in colorectal adenoma etiology. Cancer Epidemiol Biomarkers Prev 6: 79–85 [PubMed] [Google Scholar]
- Mosteller F, Colditz GA (1996) Understanding research synthesis (meta-analysis). Annu Rev Public Health 17: 1–23 [DOI] [PubMed] [Google Scholar]
- Neugut AI, Terry MB, Hocking G, Mosca L, Garbowski GC, Forde KA, Treat MR, Waye J (1996) Leisure and occupational physical activity and risk of colorectal adenomatous polyps. Int J Canc 68: 744–748 [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Boggs D, Wise LA, Palmer JR, Roltsch MH, Makambi KH, Adams-Campbell LL (2006) A follow-up study of physical activity and incidence of colorectal polyps in African-American women. Cancer Epidemiol Biomarkers Prev 15: 1438–1442 [DOI] [PubMed] [Google Scholar]
- Samad AK, Taylor RS, Marshall T, Chapman MA (2005) A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis 7: 204–213 [DOI] [PubMed] [Google Scholar]
- Sandler RS, Pritchard ML, Bangdiwala SI (1995) Physical activity and the risk of colorectal adenomas. Epidemiology 6: 602–606 [DOI] [PubMed] [Google Scholar]
- Shinchi K, Kono S, Honjo S, Todoroki I, Sakurai Y, Imanishi K, Nishikawa H, Ogawa S, Katsurada M, Hirohata T (1994) Obesity and adenomatous polyps of the sigmoid colon. Jpn J Cancer Res 85: 479–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemersma EW, Wark PA, Ocke MC, Bunschoten A, Otten MH, Kok FJ, Kampman E (2003) Alcohol consumption, alcohol dehydrogenase 3 polymorphism, and colorectal adenomas. Cancer Epidemiol Biomarkers Prev 12: 419–425 [PubMed] [Google Scholar]
- Wallace K, Baron JA, Karagas MR, Cole BF, Byers T, Beach MA, Pearson LH, Burke CA, Silverman WB, Sandler RS (2005) The association of physical activity and body mass index with the risk of large bowel polyps. Cancer Epidemiol Biomarkers Prev 14: 2082–2086 [DOI] [PubMed] [Google Scholar]
- Wei EK, Colditz GA, Giovannucci EL, Fuchs CS, Rosner BA (2009) Cumulative risk of colon cancer up to age 70 years by risk factor status using data from the Nurses’ Health Study. Am J Epidemiol 170: 863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei EK, Ma J, Pollak MN, Rifai N, Fuchs CS, Hankinson SE, Giovannucci E (2006) C-peptide, insulin-like growth factor binding protein-1, glycosylated hemoglobin, and the risk of distal colorectal adenoma in women. Cancer Epidemiol Biomarkers Prev 15: 750–755 [DOI] [PubMed] [Google Scholar]
- Wolin KY, Yan Y, Colditz GA, Lee IM (2009) Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer 100: 611–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research (2007a) The associations between food, nutrition and physical activity and the risk of colorectal polyps and underlying mechanisms. In Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective- Systematic Literature Review – Support Resource, World Cancer Research Fund/American Institute for Cancer Research (ed). AICR: Washington, DC [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research (2007b) Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. AICR: Washington DC [Google Scholar]