Abstract
Background:
SB939 is an orally available, competitive histone deacetylase (HDAC) inhibitor selective for class I, II and IV histone deacetylases. Preclinical evaluation of SB939 revealed a profile suggesting improved efficacy compared to other HDAC inhibitors. This phase I study was carried out to determine the safety, dose-limiting toxicity, recommended phase II dose (RPTD), as well as pharmacokinetic (PK) and pharmacodynamic (PD) profiles of SB939 in a daily × 5 schedule in advanced solid tumours.
Methods:
Sequential dose-escalating cohorts of patients were enrolled into 8 dose levels. At dose level 1, SB939 was taken on days 1–3 and 15–17 every 4 weeks, then on days 1–5 and 15–19 for other dose levels. Detailed PK sampling was performed in cycle 1, days 1 and 5. Peripheral blood mononuclear cells (PBMCs) were collected on cycle 1 at various time points for determination of acetylated histone H3 (AcH3) levels.
Results:
In total, 38 patients received a total of 96 cycles of treatment. The maximal administered dose was 90 mg and the RPTD was 60 mg given 5 consecutive days every 2 weeks. The most frequent non-hematologic adverse events (AEs) of at least possible attribution to SB939 were fatigue, nausea, vomiting, anorexia and diarrhoea. Pharmacokinetic analysis showed dose-proportional increases in AUC across the doses evaluated. Elimination half-life was 5.6–8.9 h. There was no clear relationship between AcH3 changes and dose level or anti-tumour response.
Conclusions:
SB939 is well tolerated in patients with advanced solid tumours. The RPTD of this drug is 60 mg on a schedule of 5 consecutive days every 2 weeks. The toxicities of SB939 are consistent with other HDAC inhibitors.
Keywords: histone deacetylase, phase I, toxicity, pharmacokinetic, pharmacodynamic
Epigenetic changes that occur through chromatin modulation regulate the accessibility of gene promoters to the transcription and replication machinery (Strahl and Allis, 2000; Johnstone and Licht, 2003). Chromatin modulation and its associated effects on gene expression are controlled by opposing effects of two families of enzymes: histone acetylase transferases (HATs) and histone deacetylases, known as HDACs (Chan et al, 2001; Carey and La Thangue, 2006). Histone deacetylation is an important epigenetic event implicated in the development and progression of cancer and provides an attractive anti-tumour therapeutic platform (Pandolfi, 2001; Timmermann et al, 2001; Chambers et al, 2003). The HDAC family is divided into zinc-dependent (class I, II and IV) and zinc-independent, nicotinamide-adenine dinucleotide-dependent (class III) categories (Stimson et al, 2009). At present, most HDAC inhibitors being developed as anti-cancer agents target class I, II and IV enzymes and there is increasing interest in the class III family. The accumulation of acetylated proteins through HDAC inhibition results in a variety of cell type-dependent responses, such as differentiation, induction of cell cycle arrest, apoptosis, as well as altered patterns of gene expression.
SB939 (3-[2-butyl-1-(2-diethylaminoethyl)-1H-benzoimidazol-5-yl]-N-hydroxyacrylami-hydrochloride) is an orally available, competitive inhibitor of HDAC. In vitro studies showed that SB939 has >1000-fold selectivity for class I, II and IV HDACs compared with class III HDACs with no effects on other zinc-binding enzymes (Novotny-Diermayr et al, 2010). The in vitro evaluation of SB939 also showed significant anti-proliferative activities against a wide variety of cell lines. Immunoblotting techniques showed that SB939 treatment of cancer cells results in the accumulation of acetylated histone H3 (AcH3) and acetylated α-tubulin, as well as increased expression levels of the cyclin dependant kinase inhibitor p21 (Novotny-Diermayr et al, 2010).
SB939 has favourable pharmacokinetic (PK) properties in animal pre-clinical models, with >4-fold increased bioavailability and 3.3-fold longer half-life compared with suberoylanilide hydroxamic acid (vorinostat), another HDAC inhibitor active in human malignancies. SB939 also demonstrated prolonged drug accumulation and more sustained inhibition of histone deacetylation in tumour tissues compared with vorinostat in preclinical experiments. In HCT-116 cells, SB939 was more effective than vorinostat. These excellent PK and pharmacodynamic (PD) properties translated into a dose-dependent anti-tumour efficacy in several experimental solid tumours models, including xenograft models of colon (HCT-116), ovarian (A2780) and prostate (PC3, DU145) carcinomas, as well as murine models of acute myeloid leukaemia (MV4-11, HL-60) and B cell lymphoma (Novotny-Diermayr et al, 2010).
Based on the relevance of HDACs as cancer therapeutic targets, and the encouraging preclinical profile of SB939, the NCIC CTG conducted a phase I trial in patients with advanced solid tumours. The primary objective was to determine the RPTD, safety and tolerability of SB939 given on a daily × 5 every other week schedule. Secondary objectives included the assessments of PK and PD changes in peripheral blood mononuclear cells (PBMCs) and preliminary anti-tumour efficacy. The choice for the human starting dose of 10 mg was based on 1/10th of the no observed adverse effect level of the dog species at 60 mg m−2 per day (3 days per week in a 28-day cycle), rounded to the nearest available fixed dose capsule size. In this study, the every other week schedule was evaluated because trials of other HDAC inhibitors suggest fatigue is a common AE that can prohibit continuous dosing. Therefore, a schedule of continuous 5-day dosing on alternate weeks was deemed appropriate to try to avoid dosing interruptions. In addition to this NCIC CTG study, another phase I trial evaluating three times weekly dosing 3 out of 4 weeks was undertaken by investigators in Singapore (Yong et al, 2008, 2009).
Methods
Patient eligibility
Patients were eligible if they had histologically or cytologically documented advanced solid malignancy, refractory to standard therapy or for which conventional therapy was not effective. Other key eligibility criteria included: age ⩾18 years; Eastern Cooperative Oncology Group performance status 0–2; adequate haematologic, hepatic and renal functions (absolute neutrophil count ⩾1.5 × 109 l−1, platelets ⩾100 × 109 l−1, bilirubin ⩽upper limit of normal, aspartate transaminase (AST)/alanine transaminase (ALT) ⩽2.5 times upper limit of normal or <5 times upper limit of normal if documented liver metastases, creatinine ⩽1.2 times upper limit of normal (or calculated creatinine clearance of ⩾60 ml s−1 via Cockroft and Gault formula); adequate cardiac function (LVEF of ⩾50%, Troponin I or T ⩽upper limits of normal and a corrected QT (QTc) interval of ⩽450 ms). Prior therapy, except for HDAC inhibitors, was allowed, but there must be a 4-week interval between previous systemic/radiotherapy and initiation of study drug. Patients with documented central nervous system metastasis, pathologic arrhythmia requiring active treatment within the past 12 months or with significant co-morbidities were excluded from the study. As well, patients on agents with a known risk of Torsades de Pointes were excluded. The institutional review board of both participating centres approved the study, which was conducted in accordance with the federal and institutional guidelines.
Study design and patient evaluation
This was a two-centre, single-agent, open-label dose-escalation phase I study in which SB939 was administered orally in an empty stomach (⩾1 h before a meal or 2 h after). SB939 was administered in an empty stomach as there were no data available on the effect of food intake on drug absorption at the time the trial was initiated. Study drug was administered at a starting dose of 10 mg on days 1–3 and 15–17 for the first dose level while for subsequent dose levels, treatment was administered on days 1–5 and 15–19, within a 28-day cycle. No intra-patient dose escalation was allowed. Dose escalation followed the standard 3+3 rule, but the protocol did allow up to four patients to be entered in the first stage of accrual at each dose level. The maximum administered dose was defined as the dose level at which ⩾2/3 or ⩾2/6 patients experienced dose-limiting toxicity (DLT). The next lower dose would normally be declared the RPTD for expansion to confirm the tolerability and PK and/or PD relationships at this dose level.
In cycle 1, patients were seen on days 1, 5, 8, 15, 19, 22 and weekly in subsequent cycles for laboratory studies and assessment of toxicity. As HDAC inhibitors have been associated with cardiac effects, electrocardiographs (ECGs) were undertaken pre- and post-dose in cycle 1 on days 1, 5, 15, 19 and on day 1 of subsequent cycles. In addition, troponin levels were obtained on days 1, 5, 15 in cycle 1 and on day 1 of subsequent cycles. Toxicity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_30). Dose-limiting toxicities were defined as AEs occurring during the first cycle of SB939 administration and fulfilling one of the following criteria: grade 4 neutropenia lasting ⩾5 days or associated with fever or infection; thrombocytopenia ⩾grade 3 or thrombocytopenic bleeding ⩾grade 3; nausea/vomiting ⩾grade 3 despite anti-emetic therapy; other major organ toxicity of ⩾grade 3 and delay in starting cycle 2 of >2 weeks or missing two or more consecutive doses within a cycle due to treatment-related AEs of laboratory abnormalities.
For patients with measurable disease at baseline, response was assessed using the Response and Evaluation Criteria in Solid Tumours version 1.0 (Therasse et al, 2000).
Dose modification
Patients who experienced any DLT were delayed by 1-week intervals until recovery and could continue on study drug with a reduction of one dose level. If no recovery occurred after a delay of 2 weeks from the next scheduled 5-day treatment, patients were discontinued from protocol treatment. In addition, patients must have an absolute neutrophil count of ⩾1.5 × 109 l−1 and a platelet level of ⩾100 × 109 l−1 before day 1 and 15 dosing. SB939 was held if grade 2 QTc interval prolongation occurred, and resumed upon recovery to baseline/grade 1, while a grade 3/4 prolongation of QTc interval would mandate drug discontinuation.
Duration of study treatment
Patients could receive repeated cycles of treatment until disease progression, unacceptable AE(s), patient's decision to withdraw from the study, or changes in the patient's condition including intercurrent illness rendering the continuation of study treatment unacceptable.
Pharmacokinetic analysis
Blood samples for evaluation of SB939 PK were collected in cycle 1, days 1 and 5 (days 1 and 3 for dose level 1) before dosing, then 0.5, 1, 1.5, 2, 3, 4, 6, 8 and 24–30 h after dosing. Pre-dose PK assessment was also performed on day 15 of treatment.
Whole blood was collected into a 3-ml blood tubes containing K2EDTA anti-coagulant and centrifuged at 1000 g for 10 min at 4°C. Plasma was kept as an aliquot and frozen at −80°C. Levels of SB939 in plasma were determined using a validated LC–MS/MS method (Novotny-Diermayr et al, 2010). The samples were centrifuged at 1200 g for 10 min at 4°C, and the supernatant was stored at −80°C until analysis at MPI Research, Mattawan, MI, USA. Pharmacokinetic parameters were calculated by a non-compartmental method using WinNonlin 4.0 software (Pharsight Corporation, Mountain View, CA, USA). Peak plasma concentration (Cmax) and time to peak concentration (Tmax) were estimated from the plasma drug concentration–time curve. The elimination half-life (T1/2) during the log-linear terminal phase was calculated from the elimination rate constant determined by a linear regression analysis. The area under the concentration time curve extrapolated to infinity (AUC0−∞) was calculated by the log-linear trapezoidal method for the observed values, with extrapolation to infinity including at least three points on the terminal phase.
Pharmacodynamic analysis
Based on the preclinical data showing parallel changes in AcH3 in PBMCs and tumour tissue, PBMCs were selected for study in the phase I trial (Novotny-Diermayr et al, 2008; Yong et al, 2009).
Whole blood samples for PD evaluation were taken in cycle 1, days 1 and 15 at pre-dose followed by 3 and 24 h post-dose. For each sample, blood was collected at room temperature in an 8-ml BD Vacutainer CPT tube (Becton, Dickson and Company, Franklin Lakes, NJ, USA). Blood samples were processed in a refrigerated centrifuge within 30 min of collection to preserve histone acetylation status. PBMCs were extracted from blood, processed to pellet form and stored at −80°C. Levels of AcH3 in PBMCs were determined using a validated western blot technique (Novotny-Diermayr et al, 2008).
Results
Patient demographics, dose escalation and recommended phase II dose
Thirty-nine patients were registered to this study but one patient cancelled registration before treatment. The 38 treated patients (Table 1) completed a total of 96 cycles of SB939 (median, 2; range, 1–12). Eight dose levels ranging from 10 to 90 mg were evaluated (Table 2, dose levels detailed in a chronological order). At the time of this report, all patients are off study treatment.
Table 1. Patient characteristics.
| Characteristics | No. | %a |
|---|---|---|
| No. of treated patients | 38 | |
| Median age, years | 62 | |
| Range | 20–88 | |
| Sex | ||
| Male | 23 | 61 |
| Female | 15 | 39 |
| ECOG PS | ||
| 0 | 7 | 18 |
| 1 | 26 | 68 |
| 2 | 5 | 13 |
| Primary tumour site | ||
| Colon | 15 | 39 |
| Rectal | 3 | 8 |
| Neuroendocrine | 3 | 8 |
| Gastric | 2 | 5 |
| Head and neck | 2 | 5 |
| Non small cell lung cancer | 2 | 5 |
| Small bowel/appendix | 2 | 5 |
| Cervix | 1 | 3 |
| Renal | 1 | 3 |
| Melanoma | 1 | 3 |
| Ovary | 1 | 3 |
| Prostate | 1 | 3 |
| Salivary gland | 1 | 3 |
| Thyroid | 1 | 3 |
| Unknown primary/others | 2 | 5 |
| Measurable disease | ||
| Yes | 36 | 95 |
| No | 2 | 5 |
| Prior therapy | ||
| Chemotherapy | 33 | 87 |
| Hormone therapy | 1 | 3 |
| Immunotherapy | 1 | 3 |
| Radiotherapy | 17 | 45 |
| Other therapy | 16 | 42 |
| No. of prior chemotherapy regimens | ||
| 0 | 5 | 13 |
| 1 | 1 | 3 |
| 2 | 10 | 26 |
| 3 | 14 | 37 |
| 4 | 5 | 13 |
| 5 | 2 | 5 |
| 7 | 1 | 3 |
Abbreviations: ECOG=Eastern Cooperative Oncology Group; PS=performance status.
Percentage values rounded to the nearest zero decimal point, therefore not all values add up to a 100%.
Table 2. Dose level evaluated and DLT encountered.
| Dose level | Dose | Schedule | Total no. of cycles | No. of patients with DLT/total no. of patients treated | DLT |
|---|---|---|---|---|---|
| 1 | 10 | 3 days every 2 weeks | 6 | 0/3 | |
| 2 | 10 | 5 days every 2 weeks | 16 | 1/6a | |
| 3 | 20 | 5 days every 2 weeks | 20 | 1/6 | Grade 3 myositis |
| 4 | 30 | 5 days every 2 weeks | 19 | 0/4 | |
| 5 | 50 | 5 days every 2 weeks | 6 | 0/4 | |
| 6 | 70 | 5 days every 2 weeks | 5 | 0/3 | |
| 7 | 90 | 5 days every 2 weeks | 3 | 1/2b | Grade 3 fatigue Grade 3 vomiting |
| 6 | 70 | 5 days every 2 weeks | 4 | 3/3 | Grade 4 platelets Grade 3 fatigue |
| 5.5 | 60 | 5 days every 2 weeks | 17 | 1/7c | Grade 3 ALTd |
Abbreviations: DLT=dose-limiting toxicity; ALT=alanine transaminase.
Grade 3 bilirubin subsequently declared not drug related.
Neither patient completed a full cycle.
One patient had intolerable G2 nausea vomiting resulting in failure to receive further dose. Although this is not DLT per se according to protocol definition, we have classified this event as a DLT due to the inability to resume treatment.
One patient did not complete cycle 1 because of ALT rise, however, it was not clear if this was entirely related to drug since patient had elevated transaminases with prior treatment.
At the 10 mg dose level (5 days every 2 weeks), a patient was observed to have a dose-limiting grade 3 bilirubin elevation, and this event was initially attributed to drug. Thus, this dose level was expanded by a further three patients, with no further DLTs observed. Although the rise of bilirubin was temporally related to SB939 administration, it was later deemed unrelated to treatment and found to be related to a blocked stent.
At the 20 mg dose level, one patient experienced grade 3 myositis, so again the dose level was expanded with no further DLTs observed. No DLTs were seen at the 30, 50 and 70 mg dose levels during the escalation phase of the trial. At the 90 mg dose level however, two patients were treated and experienced significant toxicity during cycle 1. The first patient had grade 3 fatigue and vomiting, while the dose for the second patient had to be reduced to 70 mg on days 15–19 due to intolerable grade 2 nausea, vomiting and fatigue. It was felt that further dosing of patients at this level was inappropriate and 90 mg was deemed the MAD. As per protocol, the dose level of 70 mg was then re-opened for expansion. At this dose level, the first patient had grade 4 thrombocytopenia with a delay of day 15 dosing, while the second patient had grade 3 fatigue. The third patient in the expansion cohort had grade 2 nausea and vomiting and was unable to complete the first week of treatment. These events suggested that 70 mg was poorly tolerated so an intermediate dose level of 60 mg was open for evaluation. Seven patients were entered for evaluation. One patient came off study after 1 week due to grade 3 ALT rise. This patient, tested negative for viral hepatitis serology and autoimmune workup, had a prior history of transaminitis with other drugs but a relationship with SB939 could not be excluded. Aside from the patient with elevated LFTs, no other DLTs were seen. This dose level (60 mg) was thus concluded to be the RPTD.
Safety and compliance
All 38 treated patients were evaluable for non-haematologic, haematologic and biochemical toxicities. The most frequently reported AEs of all grades and those grades 3 or higher, separated by dose levels and of at least possible relationship to SB939, are described in Table 3 for non-haematologic events. The most frequently reported, related non-haematologic AEs were fatigue (53%), nausea (39%), vomiting (29%), anorexia (29%) and diarrhoea (18%). The majority of these were grade 1 or 2 events, however 4 patients experienced grade 3 fatigue, one patient had grade 3 diarrhea while one patient had grade 3 vomiting. Other grade 3 related events included myositis and syncope (one patient each).
Table 3. Related haematological and biochemical adverse events of all grades and those grade 3 or highera.
| Dose level |
1
|
2
|
3
|
4
|
5
|
5.5
|
6
|
7
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients |
3
|
6
|
6
|
4
|
4
|
7
|
6
|
2
|
||||||||
| Dose |
10 mgb
|
10 mg
|
20 mg
|
30 mg
|
50 mg
|
60 mg
|
70 mg
|
90 mg
|
||||||||
| Grades | All grades | Grades 3/4 | All grade | Grades 3/4 | All grades | Grades 3/4 | All grades | Grades 3/4 | All grades | Grades 3/4 | All grades | Grades 3/4 | All grades | Grades 3/4 | All grades | Grades 3/4 |
| General | ||||||||||||||||
| Fatigue | 0 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 3 | 0 | 6 | 1 | 4 | 2 | 2c | 1 |
| GI | ||||||||||||||||
| Anorexia | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 4 | 0 | 3 | 0 | 1 | 0 |
| Altered taste | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Mucositis | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Nausea | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 5 | 0 | 4 | 0 | 2 | 0 |
| Vomiting | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 4 | 0 | 2 | 0 | 2c | 1 |
| Diarrhoea | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 0 |
| Constipation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Abdominal pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cardiac | ||||||||||||||||
| QT prolongation | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| Ventricular bigemini | 0 | 0 | 1c | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Ventricular arrythmias (PVCs) | 0 | 0 | 1c | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cardiac dysfunction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Supra ventricular arrythmia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cardiac ischaemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cardiac arrythmia other | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Metabolic | ||||||||||||||||
| Creatinine (raised) | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 0 |
| Alkaline phophatase (raised) | 1 | 0 | 5 | 1 | 4 | 1 | 2 | 0 | 2 | 0 | 6 | 0 | 3 | 0 | 1 | 0 |
| ALT (raised) | 0 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 4 | 2 | 1 | 0 | 2 | 0 |
| AST (raised) | 1 | 0 | 3 | 0 | 3 | 0 | 1 | 0 | 2 | 0 | 5 | 0 | 2 | 0 | 2 | 0 |
| Bilirubin (raised) | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| CPK | NA | NA | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Hematologic | ||||||||||||||||
| Anaemia | 2 | 0 | 6 | 0 | 5 | 1 | 4 | 0 | 4 | 0 | 7 | 0 | 6 | 0 | 2 | 0 |
| Leukopenia | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 5 | 1 | 3 | 1 | 1 | 0 |
| Neutropenia | 1 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 5 | 0 | 3 | 1 | 1 | 0 |
| Lymphopenia | 0 | 0 | 5 | 2 | 2 | 0 | 2 | 0 | 4 | 2 | 6 | 3 | 4 | 3 | 2 | 0 |
| Thrombocytopenia | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 6 | 1 | 2 | 0 |
| Others | ||||||||||||||||
| Myositis | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Syncope | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: ALT=alanine transaminase, AST=aspartate transaminase, CPK=creatine phosphokinase; PVCs=premature ventricular contraction.
Graded according to CTCAE version 3.0.
3 days every 2 weeks.
One or more SAE.
In terms of cardiac AEs, four patients had grade 2 QTc prolongation documented in study, three in patients receiving a dose level of 60 mg or higher. One had a single elevated value on day 1 cycle 1 that was not confirmed and received for the remainder of their 5-day treatment at an unchanged dose. Two patients had elevated QTc documented on day 5 of therapy, so following the scheduled off-therapy week, the second 5-day treatment was given at a reduced dose with no recurrence of QTc prolongation. The fourth patient was complex. On Day 1 cycle 7 post-dose ECG showed frequent premature ventricular contractions and some episodes of bigeminy. This lasted about 48 h and on investigation it appeared that patient had had similar episodes over the past 4 years. No cause was found for it. During periods of arrhythmia, LVEF fell (values 20–39% compared with baseline of 55%). Troponin remained normal but QTc was slightly elevated in some of the ECG tracings (in nine tracings done in the 10 h after day 1 dose, three patient had grade 1 elevation and one patient had grade 2 (maximum QTc 475 ms)). Patient was asymptomatic throughout and was removed from the study for progressive disease at the same time. It was unclear if this episode was study drug related.
One patient experienced paroxysmal atrial tachycardia (grade 3, unlikely related to study drug) 27 days after his last dose of SB939 associated with chest pain and dizziness. The patient had a slight elevation in troponin, normal QTc and converted to sinus rhythm with carotid massage. The relationship to SB939 was uncertain as the patient had a significant history of coronary artery disease and angina.
Thirty-six of 38 patients had documented anaemia on study (grade 1=25 patients, grade 2=10 patients and grade 3=1 patient). The number of patients experiencing other haematologic AEs increased with dose, particularly at ⩾60 mg compared with dose levels ⩽50 mg, respectively: thrombocytopenia 87 vs 22% granulocytopenia 60 vs 26% lymphopenia 80 vs 57%. Biochemical changes have been modest, all were grade 1 in patients with normal baseline chemistries except one patient with myositis who experienced a grade 3 CPK elevation, 3 patients at 60 mg who had grade 2 raised AST and hyperbilirubinemia and two patients at that same dose level who had grade 3 ALT rise. Table 3 depicts haematologic and biochemical AEs of all grades and those grades 3 or higher.
Dose reductions or omissions were seen occasionally in patients treated from 10–60 mg but were more frequently observed at 70 and 90 mg dose levels (reductions in 2 of 6 and 2 of 2 patients, respectively). At the RPTD of 60 mg (n=7), two patients had dose reductions (transaminase rise and QTc prolongation), while two patients had dose omissions (both due to alteration of liver function tests).
Anti-tumour activity
Thirty-six of the 38 treated patients had measurable disease at baseline. Of these, five patients were not assessable for response because they received one cycle or less of treatment and were not re-imaged. No objective tumour responses were observed. Ten patients of the 31 evaluable (32%) had a best response of stable disease (SD) while 21 (68%) patients had disease progression. Patients with SD had a median duration of disease control for a median of 5.7 months (range 1.8–10.8 months). Three patients, all with history of metastatic colorectal carcinoma had disease stabilisation of >6 months. These patients were treated at dose levels 2, 3 and 4, with disease stabilisation achieved for a period of 8.1, 10.8 and 7.4 months, respectively, before stopping treatment due to evidence of disease progression.
Pharmacokinetic analysis
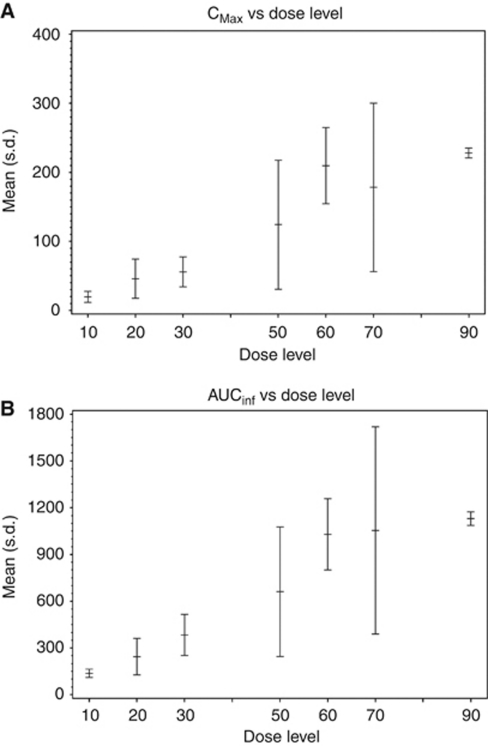
Pharmacokinetic analysis showed SB939 was rapidly absorbed (Tmax range of 1.0–2.5 h) and has bi-exponential disposition. The terminal half-life (T1/2) ranged between 6 and 9 h. There was no accumulation on Day 5 compared with Day 1. Mean AUC increased dose proportionally between 10 and 90 mg. A summary of the Cmax and AUC0−∞ at different dose levels are represented in Figure 1, while the full data on AUC0−∞, T1/2, Cmax and Tmax are detailed in Table 4.
Figure 1.
(A) Mean Cmax and (B) AUC0–∞ of SB939 by dose level.
Table 4. Full data on AUC0−∞, T1/2, Cmax and Tmax for SB939 at different dose levels.
|
Day 1
|
Day 3 or 5
|
|||||
|---|---|---|---|---|---|---|
| Dose level | Dose (mg) | Parameter | Mean | s.d. | Mean | s.d. |
| 1 | 10a | AUC0–∞ (ng h ml-1) | 126.7 | 23.9 | 147.3 | 32.6 |
| T1/2 (h) | 7.1 | 0.2 | 8.3 | 0.7 | ||
| Cmax (ng ml-1) | 14.6 | 6.0 | 18.3 | 6.4 | ||
| Tmax (h) | 2.5 | 0.9 | 1.3 | 0.3 | ||
| 2 | 10 | AUC0–∞ (ng h ml-1) | 137.2 | 26.6 | 187.7 | 32.7 |
| T1/2 (h) | 8.6 | 1.5 | 8.1 | 2.5 | ||
| Cmax (ng ml-1) | 19.5 | 8.1 | 23.3 | 11.4 | ||
| Tmax (h) | 1.1 | 0.6 | 1.3 | 0.6 | ||
| 3 | 20 | AUC0–∞ (ng h ml-1) | 243.7 | 117.1 | 424.0 | 235.4 |
| T1/2 (h) | 5.9 | 2.4 | 7.7 | 0.6 | ||
| Cmax (ng ml-1) | 45.9 | 28.5 | 51.3 | 31.9 | ||
| Tmax (h) | 1.5 | 0.9 | 1.6 | 0.8 | ||
| 4 | 30 | AUC0–∞ (ng h ml-1) | 383.8 | 131.8 | 544.5 | 206.3 |
| T1/2 (h) | 8.6 | 1.7 | 8.8 | 2.1 | ||
| Cmax (ng ml-1) | 55.8 | 21.7 | 74.3 | 28.9 | ||
| Tmax (h) | 1.8 | 0.9 | 1.6 | 0.5 | ||
| 5 | 50 | AUC0–∞ (ng h ml-1) | 661.3 | 415.3 | 793.7 | 11.2 |
| T1/2 (h) | 5.6 | 1.3 | 8.9 | 1.2 | ||
| Cmax (ng ml-1) | 124.1 | 93.5 | 101.3 | 5.6 | ||
| Tmax (h) | 1.6 | 1.0 | 1.7 | 0.3 | ||
| 5.5 | 60 | AUC0–∞ (ng h ml-1) | 1028.7 | 229.4 | 1625.2 | 405.5 |
| T1/2 (h) | 6.7 | 0.4 | 6.9 | 1.6 | ||
| Cmax (ng ml-1) | 209.6 | 55.3 | 262.5 | 65.9 | ||
| Tmax (h) | 1.2 | 0.5 | 1.3 | 0.4 | ||
| 6 | 70 | AUC0–∞ (ng h ml-1) | 1053.8 | 664.9 | 1134.3 | 678.0 |
| T1/2 (h) | 7.0 | 1.7 | 6.4 | 0.7 | ||
| Cmax (ng ml-1) | 178.2 | 122.2 | 159.7 | 118.8 | ||
| Tmax (h) | 1.6 | 0.4 | 1.5 | 0.5 | ||
| 7 | 90 | AUC0–∞ (ng h ml-1) | 1130.0 | 43.8 | 1229.0 | — |
| T1/2 (h) | 6.4 | 0.5 | 6.1 | — | ||
| Cmax (ng ml-1) | 228.9 | 7.1 | 116.0 | — | ||
| Tmax (h) | 1.0 | 0.0 | 1.5 | — | ||
3 days every 2 weeks.
Pharmacodynamic analysis
Increases in AcH3 were seen in patients treated in study with some patients experiencing maximal increase at 3 h post-treatment and others at 24 h post-treatment. There was no clear dose–PD relationship noted. There were several PBMC samples that were unsuitable for analyses in the mid-dose range, resulting in our inability to define a dose–PD correlation. However, two individuals with the largest changes in levels were treated at the 60 and 70 mg dose levels. No relationship between PD and disease stabilisation or time on study could be discerned.
Conclusion
This study demonstrates that SB939 administered orally is tolerable in patients with advanced solid tumours. The RPTD was 60 mg daily utilising a 5-day every 2 weeks schedule. The administration of SB939 results in toxicities that included fatigue, nausea, vomiting, anorexia and diarrhea. These symptoms have been observed previously with this class of agents (Marshall et al, 2002; Sandor et al, 2002; Kelly et al, 2005; Ryan et al, 2005; Giles et al, 2006; Garcia-Manero et al, 2008; Siu et al, 2008). These toxicities, however, were non-life threatening, and were effectively addressed by dosing delay and dose reduction. While QTc elevations were documented, none were grade 3. More data on the frequency of QTc changes will be known following phase II trials where monitoring during cycle 1 will be undertaken.
Although there was no objective tumour response to treatment, 31% of patients observed with SD (median 5.7 months), most of whom had been heavily pre-treated. There were three colorectal cancer patients with durable period of SD, but the numbers were too small for any meaningful conclusion to be made.
When compared to the study by Yong et al, which gave SB939 every other day (three times per week), for 3 out of 4 weeks, our dosing schedule appeared to have somewhat less myelosuppression, but possibly more non-haematological AEs. However, comparison of adverse effects between schedules in a non-randomised setting must be viewed with caution. The schedule selected for phase II evaluation is the every other day schedule, in part because both achieved the same recommended dose and the every other day schedule seemed to have less symptomatic toxicity, but also because more continuous exposure might provide better therapeutic efficacy, based on preclinical schedule data. Furthermore, the every other day schedule explored by Yong et al had demonstrated a dose–PD effect, strengthening the rational for choosing their dose schedule over ours in further development of this drug.
The PK evaluations in this study have demonstrated favourable pharmacologic properties of SB939. The drug was rapidly absorbed within after oral administration, with a median Tmax ranging from 1 to 2.5 h. Importantly, the drug had a long elimination half-life in plasma of up to 9 h, which compares favourably with the shorter, 1–2-h half-lives of other HDAC inhibitors such as vorinostat and belinostat (Kelly et al, 2005; Steele et al, 2008). The PK properties as well as the RPTD of this study were also similar to the trial carried out by our colleagues in Singapore (Yong et al, 2008). In that study, the RPTD of SB939 was also 60 mg in patients with solid tumours and the AUC0−∞, T1/2, Cmax and Tmax in that study were comparable to ours.
The PD activity of HDAC inhibition arising from SB939 administration was explored in this study, utilising a validated western blotting method. From cumulative data thus far, histone acetylation does not show a linear correlation with dose. This may be explained in part by the observation that several of the PBMC samples in our study (in the mid-dose range) were not suitable for PD analyses, resulting in the loss of dose–PD information. Despite this, the highest AcH3 levels in our study were seen in patient cohorts treated with higher doses of SB939, demonstrating the proof of mechanism that this agent indeed leads to histone deacetylation in the clinical setting. Furthermore, our results also parallel the findings of our colleagues in Singapore where a relationship between AcH3 levels and dose was observed (Yong et al, 2009).
In our study, there is also a lack of correlation between AcH3 levels and anti-tumour activity. Although the loss of PD data set point may contribute to this finding, there is increasing evidence of non-histone protein substrates on which HDAC inhibitors exert their anti-cancer effects. These include p53, Ku70, Hsp90, Stat3, NF-κB and tubulin and the modulation of these substrates have a role in regulating cell proliferation and survival (Lin et al, 2006). Furthermore, phase I trial is not the setting in which one expects to observe neither sufficient anti-tumour activities nor clear PD-anti-tumour efficacy correlations.
Despite the promising preclinical activity of HDAC inhibitors, to date these agents have been disappointing in solid tumours clinically. The reasons for this are not completely clear, and may be due to the validity and clinical relevance of HDAC as a therapeutic target. However, other factors may also be important. For example, the level and duration of target inhibition may be suboptimal and the patient populations or tumour types studied may not have been appropriately selected. All of these factors are important to consider when developing drugs in this class, given that it is a crowded field with at least two already approved agents.
With these factors in mind, phase II trials utilising SB939 as monotherapy in translocation-associated sarcoma and prostate cancer patients are underway. The rationale for SB939 treatment in translocation-associated sarcomas is perhaps the best explained in the synovial sarcoma, where fusions of the SS18 and SSX (1, 2 and 4) genes are characteristic. These gene fusions are associated with histone deacetylation and subsequent repression of the tumour suppressor gene EGR1 (Soulez et al, 1999; van der Vlag and Otte, 1999; Yochum and Ayer, 2001; Furuyama et al, 2004; Terry et al, 2007). In vivo studies have shown that the use of HDAC inhibitors leads to the reversal of the above aberrances (i.e., acetylation of histones with restoration of EGR1 expression) and subsequently results in cell death (Lubieniecka et al, 2008). Meanwhile, the TMPRSS2-ERG translocation, which has been found to occur with regular frequency in prostate carcinomas, appears to be associated with HDAC expression and is thus linked with sensitivity to HDAC inhibitors (Tomlins et al, 2005; Iljin et al, 2006; Bjorkman et al, 2008). Additionally, further studies with SB939, especially in combination with other cytotoxic and or targeted therapies, will help define the relevant treatment schedule and best potential anti-tumour activity in specific tumour types.
References
- Bjorkman M, Iljin K, Halonen P, Sara H, Kaivanto E, Nees M, Kallioniemi OP (2008) Defining the molecular action of HDAC inhibitors and synergism with androgen deprivation in ERG-positive prostate cancer. Int J Cancer 123: 2774–2781 [DOI] [PubMed] [Google Scholar]
- Carey N, La Thangue NB (2006) Histone deacetylase inhibitors: gathering pace. Curr Opin Pharmacol 6: 369–375 [DOI] [PubMed] [Google Scholar]
- Chambers AE, Banerjee S, Chaplin T, Dunne J, Debernardi S, Joel SP, Young BD (2003) Histone acetylation-mediated regulation of genes in leukaemic cells. Eur J Cancer 39: 1165–1175 [DOI] [PubMed] [Google Scholar]
- Chan HM, Krstic-Demonacos M, Smith L, Demonacos C, La Thangue NB (2001) Acetylation control of the retinoblastoma tumour-suppressor protein. Nat Cell Biol 3: 667–674 [DOI] [PubMed] [Google Scholar]
- Furuyama T, Banerjee R, Breen TR, Harte PJ (2004) SIR2 is required for polycomb silencing and is associated with an E(Z) histone methyltransferase complex. Curr Biol 14: 1812–1821 [DOI] [PubMed] [Google Scholar]
- Garcia-Manero G, Assouline S, Cortes J, Estrov Z, Kantarjian H, Yang H, Newsome WM, Miller Jr WH, Rousseau C, Kalita A, Bonfils C, Dubay M, Patterson TA, Li Z, Besterman JM, Reid G, Laille E, Martell RE, Minden M (2008) Phase 1 study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood 112: 981–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles F, Fischer T, Cortes J, Garcia-Manero G, Beck J, Ravandi F, Masson E, Rae P, Laird G, Sharma S, Kantarjian H, Dugan M, Albitar M, Bhalla K (2006) A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res 12: 4628–4635 [DOI] [PubMed] [Google Scholar]
- Iljin K, Wolf M, Edgren H, Gupta S, Kilpinen S, Skotheim RI, Peltola M, Smit F, Verhaegh G, Schalken J, Nees M, Kallioniemi O (2006) TMPRSS2 fusions with oncogenic ETS factors in prostate cancer involve unbalanced genomic rearrangements and are associated with HDAC1 and epigenetic reprogramming. Cancer Res 66: 10242–10246 [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Licht JD (2003) Histone deacetylase inhibitors in cancer therapy: is transcription the primary target? Cancer Cell 4: 13–18 [DOI] [PubMed] [Google Scholar]
- Kelly WK, O’Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, MacGregore-Cortelli B, Tong W, Secrist JP, Schwartz L, Richardson S, Chu E, Olgac S, Marks PA, Scher H, Richon VM (2005) Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol 23: 3923–3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Chen CS, Lin SP, Weng JR (2006) Targeting histone deacetylase in cancer therapy. Med Res Rev 26: 397–413 [DOI] [PubMed] [Google Scholar]
- Lubieniecka JM, de Bruijn DR, Su L, van Dijk AH, Subramanian S, van de Rijn M, Poulin N, van Kessel AG, Nielsen TO (2008) Histone deacetylase inhibitors reverse SS18-SSX-mediated polycomb silencing of the tumor suppressor early growth response 1 in synovial sarcoma. Cancer Res 68: 4303–4310 [DOI] [PubMed] [Google Scholar]
- Marshall JL, Rizvi N, Kauh J, Dahut W, Figuera M, Kang MH, Figg WD, Wainer I, Chaissang C, Li MZ, Hawkins MJ (2002) A phase I trial of depsipeptide (FR901228) in patients with advanced cancer. J Exp Ther Oncol 2: 325–332 [DOI] [PubMed] [Google Scholar]
- Novotny-Diermayr V, Nayangam NM, Hentze HH, Liang AL, Loh YK, Sausgruber N, Yeo P, Ethirajulu K, Wood JM (2008) A robust and quantitative biomarker assay for SB939, a potent, orally-active HDAC inhibitor (Abstract). Eur J Cancer (Supplements) 6: 28–29 [Google Scholar]
- Novotny-Diermayr V, Sangthongpitag K, Hu CY, Wu X, Sausgruber N, Yeo P, Greicius G, Pettersson S, Liang AL, Loh YK, Bonday Z, Goh KC, Hentze H, Hart S, Wang H, Ethirajulu K, Wood JM (2010) SB939, a novel potent and orally active histone deacetylase inhibitor with high tumor exposure and efficacy in mouse models of colorectal cancer. Mol Cancer Ther 9: 642–652 [DOI] [PubMed] [Google Scholar]
- Pandolfi PP (2001) Histone deacetylases and transcriptional therapy with their inhibitors. Cancer Chemother Pharmacol 48(Suppl 1): S17–S19 [DOI] [PubMed] [Google Scholar]
- Ryan QC, Headlee D, Acharya M, Sparreboom A, Trepel JB, Ye J, Figg WD, Hwang K, Chung EJ, Murgo A, Melillo G, Elsayed Y, Monga M, Kalnitskiy M, Zwiebel J, Sausville EA (2005) Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J Clin Oncol 23: 3912–3922 [DOI] [PubMed] [Google Scholar]
- Sandor V, Bakke S, Robey RW, Kang MH, Blagosklonny MV, Bender J, Brooks R, Piekarz RL, Tucker E, Figg WD, Chan KK, Goldspiel B, Fojo AT, Balcerzak SP, Bates SE (2002) Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin Cancer Res 8: 718–728 [PubMed] [Google Scholar]
- Siu LL, Pili R, Duran I, Messersmith WA, Chen EX, Sullivan R, MacLean M, King S, Brown S, Reid GK, Li Z, Kalita AM, Laille EJ, Besterman JM, Martell RE, Carducci MA (2008) Phase I study of MGCD0103 given as a three-times-per-week oral dose in patients with advanced solid tumors. J Clin Oncol 26: 1940–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulez M, Saurin AJ, Freemont PS, Knight JC (1999) SSX and the synovial-sarcoma-specific chimaeric protein SYT-SSX co-localize with the human Polycomb group complex. Oncogene 18: 2739–2746 [DOI] [PubMed] [Google Scholar]
- Steele NL, Plumb JA, Vidal L, Tjornelund J, Knoblauch P, Rasmussen A, Ooi CE, Buhl-Jensen P, Brown R, Evans TR, DeBono JS (2008) A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin Cancer Res 14: 804–810 [DOI] [PubMed] [Google Scholar]
- Stimson L, Wood V, Khan O, Fotheringham S, La Thangue NB (2009) HDAC inhibitor-based therapies and haematological malignancy. Ann Oncol 20: 1293–1302 [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD (2000) The language of covalent histone modifications. Nature 403: 41–45 [DOI] [PubMed] [Google Scholar]
- Terry J, Saito T, Subramanian S, Ruttan C, Antonescu CR, Goldblum JR, Downs-Kelly E, Corless CL, Rubin BP, van de Rijn M, Ladanyi M, Nielsen TO (2007) TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol 31: 240–246 [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216 [DOI] [PubMed] [Google Scholar]
- Timmermann S, Lehrmann H, Polesskaya A, Harel-Bellan A (2001) Histone acetylation and disease. Cell Mol Life Sci 58: 728–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM (2005) Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310: 644–648 [DOI] [PubMed] [Google Scholar]
- van der Vlag J, Otte AP (1999) Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation. Nat Genet 23: 474–478 [DOI] [PubMed] [Google Scholar]
- Yochum GS, Ayer DE (2001) Pf1, a novel PHD zinc finger protein that links the TLE corepressor to the mSin3A-histone deacetylase complex. Mol Cell Biol 21: 4110–4118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong W, Goh B, Toh R, Soo R, Diermayr V, Goh A, Ethirajulu K, Lee S, Seah E, Zhu J (2009) Phase I study of SB939 three times weekly for 3 weeks every 4 weeks in patients with advanced solid malignancies (Abstract). J Clin Oncol 27: 2560 [Google Scholar]
- Yong WP, Goh BC, Ethirajulu K, Yeo P, Otheris O, Chao SM, Soo R, Yeo WL, Seah E, Zhu J (2008) A phase I dose escalation study of oral SB939 when administered thrice weekly (every other day) for 3 weeks in a 4-week cycle in patients with advanced solid malignancies (Abstract). Eur J Cancer (Supplements) 6: 130 [Google Scholar]