Abstract
The application of MRI as a non-invasive, quantitative tool for diagnosing lumbar intervertebral disc degeneration is currently an area of active research. The objective of this study was to examine, in vitro, the efficacy of a manganese chloride phantom-based MRI technique for quantitatively assessing lumbar disc composition and degenerative condition. Sixteen human lumbar discs were imaged ex vivo using T2-weighted MRI, and assigned a quantitative grade based on the relative signal intensities of nine phantoms containing serial concentrations of manganese chloride. Discs were then graded macroscopically for degenerative condition, and water and uronic acid (glycosaminoglycan) contents were determined. MRI ranking exhibited significant and strong negative correlation with nucleus pulposus uronic acid content (r = −0.78). MRI grades were significantly higher for degenerate discs. The technique described presents immediate potential for in vitro studies requiring robust, minimally invasive and quantitative determination of lumbar disc composition and condition. Additionally, the technique may have potential as a clinical tool for diagnosing lumbar disc degeneration as it provides a standardised series of reference phantoms facilitating cross-platform consistency, requires short scan times and simple T2-weighted signal intensity measurements.
Keywords: Intervertebral disc, Disc composition, Disc condition, MRI, Manganese chloride
Introduction
Degeneration of the lumbar intervertebral discs is strongly implicated as a cause of low back pain, a condition affecting 70–85% of people at some point in their lives [1]. Early degeneration is characterised biochemically by a decrease in glycosaminoglycan concentration and resulting loss of hydration in the central nucleus pulposus (NP) [2, 3]. Such a loss of hydration reduces the ability of the NP to perform its most critical functional role––the even distribution and transmission of compressive forces between adjacent vertebrae by maintaining a central region of constant hydrostatic pressure [4]. Over time, the resulting alterations in motion segment pressure distribution and mechanical behaviour lead to the formation of artefacts such as radial and concentric fissures in the anulus fibrosus, and in the most severe cases, radial nuclear prolapse [5].
Application of MRI as a non-invasive, quantitative tool for assessing disc condition is currently an area of active research [6, 7]. Relaxation time constants have been demonstrated to be good predictors of disc water and glycosaminoglycan contents [8, 9]; however, calculation of these surrogate magnetic resonance parameters requires comparatively long scan times and intensive post-processing. T2-weighted signal intensity measurements also correlate directly with disc water and glycosaminoglycan contents [10]; their application in a quantitative sense, however, is limited by the intrinsic magnetic field heterogeneities that exist within and between different MRI scanning platforms. These heterogeneities may occur due to poor radio frequency coil uniformity, static field heterogeneity, radio frequency penetration, gradient-driven eddy currents and overall patient anatomy and position, and are in turn associated with inconsistent image signal intensity measurements [11]. The presence of an internal reference system would significantly ameliorate this problem, by limiting the effects of any such heterogeneity to within a single acquisition slice.
Recently, a set of manganese chloride (MnCl2)-based phantoms capable of producing an approximately linear signal intensity response series was proposed as one means of achieving this [12, 13]. The objective of this study was to evaluate, in vitro, the efficacy of a manganese chloride phantom-based MRI technique for determining lumbar intervertebral disc composition and condition. It was hypothesised that MRI grades would correlate significantly with NP water content, glycosaminoglycan content, and accurately predict degenerative grade.
Methods
Specimen preparation
Fourteen human lumbar spines (spine levels T12 to S1) from cases ranging in age from 28 to 87 years were obtained at autopsy within 24 h of death, following research ethics committee and next of kin approvals. All cases were without evidence of musculoskeletal pathology, as identified by clinical history and case notes. Spines were immediately double sealed in plastic and stored at −80°C. Prior to analyses, spines, still sealed in plastic to prevent dehydration, were thawed overnight at 4°C. From these cases, a total of 16 intervertebral discs were randomly selected for analysis (6 × T12-L1, 4 × L2-L3 and 6 × L4-L5). The remaining levels were allocated to unrelated concurrent studies.
Magnetic resonance imaging
Nine cylindrical phantoms were constructed, consisting of 400 mm long, 15 mm diameter polypropylene tubes containing serially graded concentrations of MnCl2 (P1: 0.01000 mM; P2: 0.07125 mM; P3: 0.13250 mM; P4: 0.19375 mM; P5: 0.25500 mM; P6: 0.31625 mM; P7: 0.37750 mM; P8: 0.43875 mM; P9: 0.50000 mM) suspended in gelatin. These MnCl2 concentrations were previously demonstrated to produce an approximately linear series of T2-weighted signal intensities [12]. Phantoms were mounted radially around a magnetic resonance-inert cradle device, consisting of a central, 108 mm internal diameter polyethylene tube, external support ribs and alignment flanges, as illustrated in Fig. 1. Prior to and between imaging sequences, phantoms were stored at 4°C to prevent melting of the gelatin suspension. Each lumbar spine was inserted into the central tube of the cradle device, and both were placed inside a transmit-and-receive knee coil (IGC Medical Advances Inc, WI, USA) for signal enhancement and imaged using a 1.5 T Philips Intera Master horizontal bore magnetic resonance scanner (Philips, Best, The Netherlands). Dimensions of the horizontal bore were 60 cm (diameter) and 157 cm (length). Maximum gradient and slew rate were 30 mT/m and 150 mT/m/s, respectively. T2-weighted spin-echo sequence images were acquired with the following parameters, as described previously [12]: a repetition time of 2,000 ms; an echo time of 50 ms; a slice thickness of 3 mm; a field of view of 160 mm; and an acquisition matrix of 512 × 512. Acquisition time was 4 min 18 s. Intervertebral discs were imaged independently using a seven-slice acquisition block with the central slice orientated through the centre of the disc, transverse to the axis of the spine. A typical image showing a disc and surrounding phantoms is shown in Fig. 2. Images were analysed using EasyVision software (Philips, Best, The Netherlands). The central slice of each disc was identified and the mean T2-weighted signal intensities of the disc and the nine phantoms at that location were calculated. For consistency, the disc signal intensity was measured using one of two fixed region of interest sizes: 469.5 ± 1.0 or 653.5 ± 1.0 mm2, depending on the size of the disc, to encompass the entire nucleus pulposus. For each disc, phantom number plotted against phantom signal intensity was approximately linear; however, the relationship was found to be most accurately approximated by a third-order polynomial (Matlab, Natick, MA, USA). Using this polynomial, the signal intensity of each disc was converted to a quantitative MRI grade in the range one to nine, rounded to two decimal places. Following imaging, each spine was immediately refrozen at −80°C until required for macroscopic disc grading.
Fig. 1.
Custom-made, magnetic resonance-inert imaging cradle, showing the nine radially positioned phantoms, support ribs, alignment flanges and central tube for mounting specimens
Fig. 2.
Typical T2-weighted image, showing an axial cross-section of an intervertebral disc (28 years old, L4-L5) and nine radially positioned manganese chloride phantoms
Macroscopic grading
Macroscopic disc grades were determined using a four-point adaptation of the Thompson scheme [14] modified for transverse sections, where grade one discs were those with no signs of degeneration and grade four discs were those with the most severe signs. Grading was undertaken independently by two investigators.
Biochemical analyses
Following macroscopic grading, inferior and superior hemi-discs were removed from the adjacent vertebral bodies at the end plates using a scalpel, the two halves were combined, and the NP separated from the anulus fibrosus, allowing NP wet weight (Ww) to be measured. Samples were then chopped into small pieces and dried overnight in a 60°C oven to determine dry weight (Wd). NP water content was calculated according to the formula: (Ww–Wd)/Ww. Dried samples were suspended in anhydrous acetone (to prevent non-specific leaching of glycosaminoglycans into solution), homogenised (Ultra-Turrax, Janke and Kunkel, Staufen, Germany) and re-dried overnight under vacuum; 50 mg portions were then completely digested for 48 h at 56°C in 2 ml of proteinase K solution. Aliquots of this digest were then assayed to determine uronic acid content [15] as an estimate of the total glycosaminoglycan content of the tissue. Water and uronic acid contents were reported as mg per mg dry tissue weight.
Statistical analysis
Statistical analyses were performed using GraphPad Prism V5 (GraphPad Software Inc., San Diego, CA, USA). Relationships between MRI grade, water content and uronic acid content were assessed using linear regression analyses, with correlation strength defined as follows: r > 0.70 = strong, 0.50 < r ≤ 0.70 = moderate and r ≤ 0.50 = weak [16]. For comparisons with degenerative condition, discs were classified as either non-degenerate (grade 1 or 2) or degenerate (grade 3 or 4). The influence of disc condition on MRI grade, water content and uronic acid content was assessed using Student’s t tests. Significance was reported for two-tailed p values less than 0.05.
Results
Fitted polynomials were found to approximate the relationship between phantom number (i.e. MnCl2 concentration) and signal intensity with a high degree of accuracy (mean r = 0.99, Fig. 3). Macroscopic grading revealed no grade one discs, seven grade two discs, six grade three discs and three grade four discs. ANOVAs revealed no significant effect of spine level on NP water content (p = 0.57), uronic acid content (p = 0.35), macroscopic grade (p = 0.94) or MRI grade (p = 0.23); specimens from each level were, therefore, pooled. Age was found to exhibit significant, moderate, negative correlation with NP water content (r = −0.70, p < 0.003) and uronic acid content (r = −0.68, p = 0.004), and significant, moderate, positive correlation with MRI grade (r = 0.69, p = 0.003).
Fig. 3.
Representative plot of phantom number (each corresponding to a unique MnCl2 concentration) versus signal intensity, with fitted third-order polynomial (solid line), used to calculate disc MRI grades
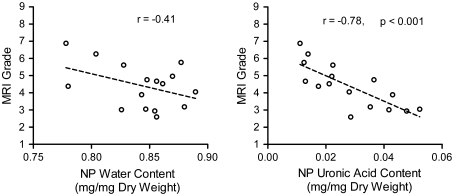
MRI grade demonstrated significant, strong, negative correlation with NP uronic acid (GAG) content (r = −0.78, p < 0.001), while an insignificant trend was observed with respect to NP water content (r = −0.41, p = 0.11, Fig. 4). To assess the capacity of MRI grade to predict disc macroscopic grade, discs were classified as either non-degenerate (macroscopic grade 1 or 2, n = 7) or degenerate (macroscopic grade 3 or 4, n = 9). A Student’s t test revealed that MRI grades were significantly higher for the degenerate group (p = 0.03, Fig. 5a). NP water content was found to be significantly reduced for degenerate specimens compared with non-degenerate specimens (p = 0.03, Fig. 5b), as was uronic acid (GAG) content (p = 0.03, Fig. 5c).
Fig. 4.
Correlations between MRI grade and NP water content (left) and uronic acid content (right)
Fig. 5.
Influence of disc condition on (from left to right) MRI grade, NP water content and NP uronic acid content. *p < 0.05
Discussion
In this study, MRI combined with a set of MnCl2-based phantoms was investigated as a possible standardised, quantitative tool for assessing human lumbar intervertebral disc degeneration. T2-weighted signal intensities of 16 lumbar discs were determined in vitro, and assigned a quantitative grade based on the relative signal intensities of nine radially mounted phantoms containing serially graded concentrations of MnCl2. Following imaging, discs were macroscopically graded, and NP water and glycosaminoglycan contents measured.
In MRI, signal intensity (or image contrast) is dependent upon the selected repetition and echo times (TR and TE, respectively), relative to the T1 and T2 relaxation time constants, which are intrinsic properties of the tissues being examined [17]. In this study, selection of TR (2,000 ms) and TE (50 ms) was initially based upon published approximate T1 and T2 relaxation time constants for the disc in order to optimise T2-weighted contrast [10, 18, 19].
A number of recent studies have focused upon the correlation of surrogate MR parameters, such as relaxation time constants, with disc composition and grade [8, 9, 18, 20–23]. Nucleus pulposus T1-rho-weighted relaxation times, in particular, have been found to correlate well with water and glycosaminoglycan contents, and are able to successfully discriminate between non-degenerate and degenerate discs [8, 9, 16]. A disadvantage of these techniques is the increased scanning time required, which has the potential to decrease the accuracy of results due to patient movement, increase patient discomfort, decrease patient throughput and increase running costs. The technique described here, which utilises T2-weighted signal intensities, involves relatively shorter scan times, and produces equally strong correlation with glycosaminoglycan content.
The technique described in this study focuses on identifying changes to two closely related, biochemical indicators of intervertebral disc degeneration––water and glycosaminoglycan contents. Diagnosis of degeneration using these indicators, however, does not necessarily guarantee progression to painful clinical symptoms, as specific characteristics of degeneration that may directly reflect patient symptoms (low back pain, decreased mobility), such as reduced disc height, nuclear extrusion, nuclear bulging, anular tears or osteophyte formation, are not specifically detected. In a clinical sense, the technique could thus be considered a first-pass tool, capable of identifying potentially painful discs at early and moderate stages of degeneration, and complementing the results of other non-invasive diagnostic examinations, thereby providing the impetus for more radical examination, such as discogram. The technique may also be appropriate for monitoring the progression of degeneration in individuals over time, where increased MRI ranking over time would indicate further deterioration. Conversely, decreased MRI ranking over time would indicate improvement in disc condition in response to therapeutic treatments designed to prevent or ameliorate disc degeneration.
Variations in loading conditions, including those that occur diurnally, have been shown to influence the magnetic resonance response of the disc, most likely as a consequence of fluid redistribution [19, 24]. Performing scans after a prescribed period of inactivity in which the patient remains horizontal may be one possible way to account for such effects.
There are a number of limitations associated with this in vitro study, particularly regarding the challenges to be addressed in translating the technique to the clinic. The phantoms function as an internal reference that allows disc signal intensity to be quantified irrespective of magnetic field heterogeneities which may occur along the long axis of the specimen (i.e. from one disc level to the next), and, in theory, facilitate repeatable measurements to be undertaken on different magnetic resonance imaging platforms. For effective clinical use, T2-weighted signal intensity variations occurring within a single, axial acquisition slice with distance from the coil would need to be addressed; such variations could potentially be corrected computationally [11]. Furthermore, the coil sensitivity profile will not necessarily vary with distance alone and it should be recognised that standardisation of the distance between the coil, spine and phantom components as well as management of issues such as the coil sensitivity profile will be more difficult in the clinical environment. If this technique is to reach clinical application, these limitations will need to be addressed in subsequent studies.
Despite these challenges, the technique described has promise as a clinical tool for the diagnosis of lumbar disc degeneration as it provides a standardised series of reference phantoms facilitating cross-platform consistency, requires short scan times and simple T2-weighted signal intensity measurements, utilises predefined regions-of-interest requiring minimal knowledge of disc anatomy by imaging staff, and facilitates accurate prediction of glycosaminoglycan content and degenerative condition. Future studies should confirm the repeatability of measurements taken of the same disc using several different MRI systems, and address the practical considerations of mounting the phantoms appropriately to facilitate in vivo experiments. Importantly, the technique also presents immediate potential for in vitro studies by facilitating robust, quantitative and technically undemanding determination of lumbar disc composition and condition.
Acknowledgments
This study was funded by a National Health and Medical Research Council Project Grant. The authors acknowledge assistance of radiographers at Flinders Medical Center, mortuary staff at the Royal Adelaide Hospital for assisting with sample collection, and Dr Dawn Elliott for providing insightful comments on the manuscript.
Conflict of interest None.
References
- 1.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Holm S. Nutrition and pathophysiologic aspects of the lumbar intervertebral disc. In: Wiesel SW, Weinstein JN, Herkowitz HN, Dvorak J, Bell G, editors. The lumbar spine. Philadelphia: Saunders; 1996. pp. 285–309. [Google Scholar]
- 4.Adams MA, McNally DS, Dolan P. ‘Stress’ distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br. 1996;78:965–972. doi: 10.1302/0301-620X78B6.1287. [DOI] [PubMed] [Google Scholar]
- 5.Vernon-Roberts B (1988) Disc pathology and disease states. In: Ghosh P (ed) The biology of the intervertebral disc. CRC Press, Boca Raton, pp 73–119
- 6.Wang C, Auerbach JD, Witschey WR, Balderston RA, Reddy R, Borthakur A. Advances in magnetic resonance imaging for the assessment of degenerative disc disease of the lumbar spine. Semin Spine Surg. 2007;19:65–71. doi: 10.1053/j.semss.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majumdar S. Magnetic resonance imaging and spectroscopy of the intervertebral disc. NMR Biomed. 2006;19:894–903. doi: 10.1002/nbm.1106. [DOI] [PubMed] [Google Scholar]
- 8.Auerbach JD, Johannessen W, Borthakur A, Wheaton AJ, Dolinskas CA, Balderston RA, Reddy R, Elliott DM (2006) In vivo quantification of human lumbar disc degeneration using T(1rho)-weighted magnetic resonance imaging. Eur Spine J 15 Suppl 3:S338–344 [DOI] [PMC free article] [PubMed]
- 9.Johannessen W, Auerbach JD, Wheaton AJ, Kurji A, Borthakur A, Reddy R, Elliott DM. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine. 2006;31:1253–1257. doi: 10.1097/01.brs.0000217708.54880.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benneker LM, Heini PF, Anderson SE, Alini M, Ito K. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur Spine J. 2005;14:27–35. doi: 10.1007/s00586-004-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vovk U, Pernus F, Likar B. MRI intensity inhomogeneity correction by combining intensity and spatial information. Phys Med Biol. 2004;49:4119–4133. doi: 10.1088/0031-9155/49/17/020. [DOI] [PubMed] [Google Scholar]
- 12.Kurmis AP, Slavotinek JP, Barber C, Fazzalari NL. Determining disk hydration status with a MnCl2-based MR model. Radiol Technol. 2008;79:507–513. [PubMed] [Google Scholar]
- 13.Kurmis AP, Barber C, Slavotinek JP, Fazzalari NL. A MnCl2-based MR signal intensity linear response phantom. Radiol Technol. 2007;79:119–125. [PubMed] [Google Scholar]
- 14.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen AM, Johannessen W, Yoder JH, Wheaton AJ, Vresilovic EJ, Borthakur A, Elliott DM. Noninvasive quantification of human nucleus pulposus pressure with use of T1rho-weighted magnetic resonance imaging. J Bone Joint Surg Am. 2008;90:796–802. doi: 10.2106/JBJS.G.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McRobbie DW, Moore EA, Graves MJ, Prince MR. MRI from picture to proton. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 18.Perry J, Haughton V, Anderson PA, Wu Y, Fine J, Mistretta C. The value of T2 relaxation times to characterize lumbar intervertebral disks: preliminary results. AJNR Am J Neuroradiol. 2006;27:337–342. [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu EJ, Newitt DC, Segal MR, Hu SS, Lotz JC, Majumdar S. Magnetic resonance imaging measurement of relaxation and water diffusion in the human lumbar intervertebral disc under compression in vitro. Spine. 2001;26:E437–E444. doi: 10.1097/00007632-200110010-00017. [DOI] [PubMed] [Google Scholar]
- 20.Niinimaki J, Ruohonen J, Silfverhuth M, Lappalainen A, Kaapa E, Tervonen O. Quantitative magnetic resonance imaging of experimentally injured porcine intervertebral disc. Acta Radiol. 2007;48:643–649. doi: 10.1080/02841850701326933. [DOI] [PubMed] [Google Scholar]
- 21.Perie D, Iatridis JC, Demers CN, Goswami T, Beaudoin G, Mwale F, Antoniou J. Assessment of compressive modulus, hydraulic permeability and matrix content of trypsin-treated nucleus pulposus using quantitative MRI. J Biomech. 2006;39:1392–1400. doi: 10.1016/j.jbiomech.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Blumenkrantz G, Li X, Han ET, Newitt DC, Crane JC, Link TM, Majumdar S. A feasibility study of in vivo T1rho imaging of the intervertebral disc. Magn Reson Imaging. 2006;24:1001–1007. doi: 10.1016/j.mri.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Antoniou J, Mwale F, Demers CN, Beaudoin G, Goswami T, Aebi M, Alini M. Quantitative magnetic resonance imaging of enzymatically induced degradation of the nucleus pulposus of intervertebral discs. Spine. 2006;31:1547–1554. doi: 10.1097/01.brs.0000221995.77177.9d. [DOI] [PubMed] [Google Scholar]
- 24.Ludescher B, Effelsberg J, Martirosian P, Steidle G, Markert B, Claussen C, Schick F. T2- and diffusion-maps reveal diurnal changes of intervertebral disc composition: an in vivo MRI study at 1.5 Tesla. J Magn Reson Imaging. 2008;28:252–257. doi: 10.1002/jmri.21390. [DOI] [PubMed] [Google Scholar]