Abstract
 Childhood spinal tuberculosis, especially when associated with severe vertebral destruction of more than two vertebral bodies can end up in severe deformity. These children show progressive deformity throughout the period of growth and can develop severe kyphosis of >100°. Such kyphosis is severely disabling with significant risk of neurological deficit and respiratory compromise. Surgical correction of these deformities by both anterior and posterior approaches has been described but each have serious limitations of approach, correctability and safety. We describe here a technique of posterior closing–anterior opening osteotomy, which allowed us to correct a rigid post-tubercular deformity of 118° in a 13-year-old boy with neglected spinal tuberculosis. The patient was a 13-year-old boy, who had contracted spinal tuberculosis at the age of 6 years. Although the disease was cured by anti-tubercular chemotherapy, he continued to deteriorate in deformity and presented to us with severe thoracolumbar kyphosis (118°). He was neurologically intact but was beginning to show shortness of breath on exertion. Patient also had fore shortening of the trunk with impingement of the rib cage on the iliac crest. Radiographs revealed complete destruction of T12, L1 and L2 vertebral bodies with the T11 vertebra fusing with L3 anteriorly. CT scans and MRI revealed severe collapse of the vertebral column and the spinal cord being stretched over the ‘internal gibbus’, which was formed by the remnants of the destroyed vertebrae. A single stage closing–opening osteotomy was done by a midline posterior approach with continuous intraoperative spinal cord monitoring. The procedure involved extensive laminectomy of T11–L2, pedicle screw fixation of three levels above and three levels below the apex, a wedge osteotomy at the apex of the deformity from both sides, anterior column reconstruction by appropriate-sized titanium cage and gradual correction of deformity by closing the posterior column using the cage as a fulcrum. This allowed us to achieve a correction to 38° (68% correction). There was no intraoperative or perioperative adverse event and patient had good functional and radiological outcome at 1-year follow-up. In this Grand Rounds case presentation, we have also discussed the aetiology and evolution of severe post-tubercular kyphosis, which is the most common cause of spinal deformity in the developing world. Early identification of children at risk for severe deformity, the time and ideal methods of prevention of such deformities are discussed. The pros and cons of the available options of surgical correction of established deformity and the merits of our surgical technique are discussed.
Childhood spinal tuberculosis, especially when associated with severe vertebral destruction of more than two vertebral bodies can end up in severe deformity. These children show progressive deformity throughout the period of growth and can develop severe kyphosis of >100°. Such kyphosis is severely disabling with significant risk of neurological deficit and respiratory compromise. Surgical correction of these deformities by both anterior and posterior approaches has been described but each have serious limitations of approach, correctability and safety. We describe here a technique of posterior closing–anterior opening osteotomy, which allowed us to correct a rigid post-tubercular deformity of 118° in a 13-year-old boy with neglected spinal tuberculosis. The patient was a 13-year-old boy, who had contracted spinal tuberculosis at the age of 6 years. Although the disease was cured by anti-tubercular chemotherapy, he continued to deteriorate in deformity and presented to us with severe thoracolumbar kyphosis (118°). He was neurologically intact but was beginning to show shortness of breath on exertion. Patient also had fore shortening of the trunk with impingement of the rib cage on the iliac crest. Radiographs revealed complete destruction of T12, L1 and L2 vertebral bodies with the T11 vertebra fusing with L3 anteriorly. CT scans and MRI revealed severe collapse of the vertebral column and the spinal cord being stretched over the ‘internal gibbus’, which was formed by the remnants of the destroyed vertebrae. A single stage closing–opening osteotomy was done by a midline posterior approach with continuous intraoperative spinal cord monitoring. The procedure involved extensive laminectomy of T11–L2, pedicle screw fixation of three levels above and three levels below the apex, a wedge osteotomy at the apex of the deformity from both sides, anterior column reconstruction by appropriate-sized titanium cage and gradual correction of deformity by closing the posterior column using the cage as a fulcrum. This allowed us to achieve a correction to 38° (68% correction). There was no intraoperative or perioperative adverse event and patient had good functional and radiological outcome at 1-year follow-up. In this Grand Rounds case presentation, we have also discussed the aetiology and evolution of severe post-tubercular kyphosis, which is the most common cause of spinal deformity in the developing world. Early identification of children at risk for severe deformity, the time and ideal methods of prevention of such deformities are discussed. The pros and cons of the available options of surgical correction of established deformity and the merits of our surgical technique are discussed.
Electronic supplementary material
The online version of this article (doi:10.1007/s00586-010-1526-3) contains supplementary material, which is available to authorized users.
Keywords: Post-tubercular, Kyphosis, Closing–opening wedge, Osteotomy
Case presentation
Our patient is a 13-year-old adolescent male who presented with a severe thoracolumbar kyphotic deformity as a late complication of conservative treatment of childhood spinal tuberculosis. The patient had contracted spinal tuberculosis at the age of 6 years for which he was treated with oral antituberculous drugs and a thoracolumbosacral brace for nearly 12 months. He had only a minimal gibbus deformity at that time and this remained non-progressive till the age of 9 years following which he had observed rapid worsening of the deformity. Although neurologically intact, his mobility was becoming restricted due to increasing shortness of breath on exertion for the past few months. He also noticed that he was becoming shorter with increasing collapse of the spine and was also developing pain at the apex of the deformity on prolonged sitting and on the sides due to costocrestal impingement. The patient has also been unable to lie supine for the last few years.
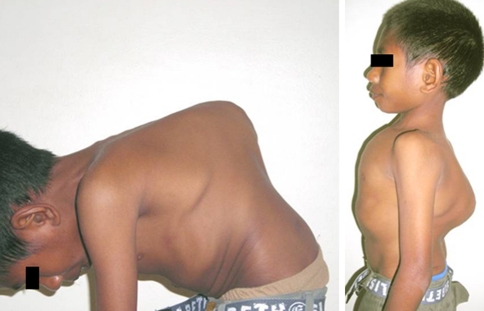
Clinical examination revealed a thin made short statured 13-year-old boy with a severe thoracolumbar kyphosis and a callosity at the apex of the deformity (Fig. 1). There was a prominent thoracolumbar kyphosis which was clinically rigid with no appreciable improvement during a prone hyper extension manoeuver. Forward bending was pain-free and he was neurologically intact. Radiographs showed a thoracolumbar kyphosis between T9 and L3 with gross destruction of T12, L1 and L2 with only partial remnants of the body being spared (Figs. 2, 3). There was a complete toppling over of T11 vertebra with its anterior surface stabilizing over the superior surface of L3. Sagittal Cobb angle measured between T9 and L3 was 118°. MRI showed a prominent ‘internal gibbus’ due to retropulsion of the fusion mass formed by the remnants of the diseased vertebrae and the spinal cord being stretched over it at the apex. There was no radiological evidence of active tuberculosis.
Fig. 1.
Pre-operative clinical photographs of the patient. The standing lateral view shows a severe, rigid kyphotic deformity at the thoraco-lumbar region. The photograph taken in flexion shows the severity of deformity
Fig. 2.
Pre-operative lateral radiograph (a), sagittal (b) and reconstructed (c) CT sections and sagittal (d) MR images of the patient shows a kyphotic deformity of 118° at the thoracolumbar junction between T9 and L3 vertebrae. The destroyed anterior elements of T12, L1 and L2 are fused into a single fusion mass forming the apex. The spinal cord is stretched over the ‘internal’ gibbus
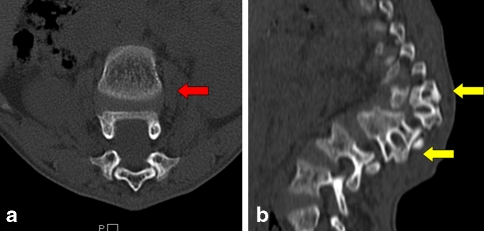
Fig. 3.
CT sections taken through the apex of the deformity. a Two vertebrae in one axial section due to the severe kyphosis (red arrow). b The presence of two pedicles attached to a single vertebral body (yellow arrows). This is due to the extensive destruction of vertebral bodies by the tubercular disease resulting in fusion of remaining anterior vertebral elements with the posterior elements remaining undamaged thus resulting in severe kyphosis
Diagnostic imaging section (Figs. 1, 2, 3)
Description about the condition, epidemiology, diagnosis, pathology, differential diagnosis
The high success of disease cure achieved with the powerful anti-tuberculous drugs has made uncomplicated spinal tuberculosis a ‘medical disease’ [6–9]. Although chemotherapy is effective in inactivating the disease, the vertebrae collapse till the healthy vertebral bodies in the region of the kyphosis meet anteriorly and consolidate. In general, more than 80% of patients with spinal tuberculosis have a certain degree of detectable kyphosis at the time of presentation [10]. With conservative treatment, these lesions heal but there is an average increase of 15% in deformity [11]. When the disease is severe with extensive vertebral destruction, due to increasing kyphosis, the superior healthy vertebra rotates forward till it comes into contact with the inferior vertebra. Such patients (3–5%) end up with a deformity greater than 60° [19]. In certain cases, the kyphotic deformity can further increase gradually with time. The final severity of deformity would then depend on the extent of initial vertebral destruction, the patient’s age and the level of lesion [12].
A deformity of greater than 90° is usually a result of childhood spinal tuberculosis. Due to the higher flexibility of the paediatric spine and increased susceptibility to destruction, children have a higher deformity at presentation and a greater tendency for collapse during the process of healing [12]. More importantly, they continue to show a progress in deformity even after the complete cure of the disease till the skeletal growth is complete. In a 15-year follow-up of paediatric spinal tuberculosis treated conservatively, one-third of children were found to have a greater progress in deformity after the cure of the disease than during the active phase [13]. The patient presented in this Grand Rounds also falls into this category as the majority of the progress in deformity occurred after the cure of the disease. The present case is another perfect example for the principle that children with spinal tuberculosis must be followed up regularly till the growth is complete as the progress in deformity may occur even many years after disease cure is achieved.
Several risk factors that portend a severe deformity have been identified [12–14]. An age of 10 or less at the time of active disease, a vertebral body loss of more than 1.5 before start of treatment, thoracolumbar involvement and a pretreatment kyphotic deformity exceeding 40° have all been noted as risk factors. Rajasekaran has described radiological ‘Spine at risk’ signs which occurred early in the course of the disease and thereby help to identify children who are prone for a severe increase in deformity over the years [13]. These four signs were easily identifiable in plain radiographs, being separation of the facet joints, posterior retropulsion of the diseased vertebral segments, lateral translation of the vertebral column, and the toppling sign. It is notable that this patient had all the above-mentioned risk factors. Recognition of these factors would have helped to advocate a spinal fusion even during the active stage when the deformity was much smaller. This would have made the surgical exercise much simpler as correction of established deformities is not only difficult but is also associated with more complications. Another reason for early intervention in such children is to prevent the phenomenon of ‘Buckling Collapse’ when the kyphosis progresses above 90°. Here, multiple facet joints at the level of deformity dislocate leading to global instability and a severe kyphosis with the entire spine being converted to two large compensatory curves [15].
Rationale for treatment and evidence-based literature
Severe kyphosis is a major cause for cosmetic and psychological disturbance in a growing child. It also affects the child functionally due to costopelvic impingement, respiratory impairment and late-onset paraplegia [19, 20]. Patients with kyphosis having significant pain, neurological deficit, impending buckling, functional or cosmetic disability will require surgical correction of the deformity. When compared with kyphosis due to other aetiologies, post-infective kyphosis correction is more demanding because of grossly distorted anatomical land marks, ventral tethering and adhesions around the dura and multiple involvement of vertebral bodies in the apical fusion mass.
Correction of severe kyphotic deformities can be performed either by an anterior instrumented fusion [2], a posterior corrective osteotomy [1] or a combined anterior–posterior procedure [3], each having its own pros and cons. Anterior only procedures are associated with difficulties in approaching the concavity of the kyphosis in such severe deformities [5]. In post-tubercular patients, the approach is quite complicated by problems of fibrosis, adhesions and requires meticulous debridement of the tissues all around the spinal cord before osteotomy. The surgeon should also be careful about the presence of side-slip deformity at the apex of the curve. In severe childhood disease, 2–3 vertebral bodies are frequently destroyed causing a severe shortening of the anterior column but the posterior column height is maintained. Correction by purely opening up the anterior column can cause severe stretching of the spinal cord with risks of neurological deficits. Posterior closing osteotomy (which shortens the posterior column) has been used effectively for kyphosis due to other causes like trauma, osteoporosis and ankylosing spondylitis [16, 18]. However, in tuberculosis, the destruction of the anterior column can be extensive to involve even two to three complete vertebral bodies. In such instances, a pure closing wedge osteotomy can result in severe kinking of the cord and potential neurological compromise. Several authors have also reported the need for anterior strut graft arthrodesis as a second stage procedure due to either persistent deformity or inadequate deformity correction. Combined anterior and posterior procedures are major surgical procedures especially in the paediatric age group, irrespective of whether it is performed as a single or two-staged procedure [17]. Sequential procedures have also been advocated which involves the stages of fitting of the halo-pelvic distraction apparatus, anterior spinal osteotomy and decompression of the spinal cord, slow and gradual spinal distraction, posterior osteotomy and fusion and then anterior spine fusion after maximum correction [21]. Even with this technique of staged procedures, there was a 10% mortality rate and the correction obtained was only 28%.
An osteotomy that achieves correction without altering the length of the cord, done as a single stage procedure without the need for violating the chest cavity and without much morbidity would be the ideal procedure. A procedure in which the posterior column is closed and anterior column appropriately opened such that the length and function of the spinal cord is not jeopardised has been described by Kawahara et al. [4]. They concluded that this technique offered good correction without jeopardising the integrity of the spinal cord. Hence, we performed a closing–opening osteotomy that shortened the posterior column and opened the anterior column appropriately to correct the deformity without compromising the spinal cord integrity. The challenges of performing a posterior vertebral column resection procedure in post-tubercular kyphosis would be the disparity between anterior and posterior column heights, multiple vertebral involvement and presence of significant fibrosis, adhesions and tethering of the dura and anterior structures to the spinal column.
Procedure
The patient was placed prone over padded bolsters to avoid pressure over the abdomen and all bony points and superficial nerves were protected. Spinal cord monitoring probes (Medtronics NIM Eclipse™) were fixed and checked. A straight vertical midline incision was made over the spinous processes and was extended three or four vertebrae above and below the involved segment. The paraspinal muscles were dissected subperiosteally from the spinous processes and the laminae and then retracted laterally. The operative field was exposed wide enough on both sides to allow dissection around the transverse processes completely from the base to the tip, especially around the apex. The posterior vertebral structures over the planned wedge of resection were identified and an extended laminectomy was performed at the involved vertebral levels from T11 to L2. Pedicle screws were then inserted into T9, T10, T11, L2 and L3 vertebrae. A contoured rod was temporarily fixed on the right side to maintain spinal stability. Dissection was extended far laterally over the ribs and the 10th, 11th and 12th ribs were transected 3–4 cm lateral to the costotransverse joint. The pleura was bluntly separated from the vertebrae. The rib heads along with the transverse processes were then carefully excised, remaining strictly extrapleural. Sometimes the rib heads at the lower adjacent level may also need to be excised, especially in the upper and middle thoracic level where the operative area is small. The T11 and T12 nerve roots were dissected from the vertebral body and gently retracted to make the room for the paravertebral dissection. The nerve roots were then doubly ligated and cut and held with the long ends of the ligature. At the beginning of this step, the segmental arteries were identified bilaterally, ligated and divided. Blunt dissection was performed anteriorly on both sides through the plane between the pleura and the vertebral body. The aorta was carefully dissected anteriorly from the anterior aspect of the vertebral body and held retracted with a spatula.
The apical vertebral fusion mass was carefully removed using an osteotome, curette, rongeur and high-speed drill in a wedge shape approximating the angular deformity, keeping the posterior vertebral cortex intact. The disc was thoroughly removed using a curette, rongeur or drill. The posterior longitudinal ligament was left intact as it is not always necessary to remove it due to the tethering and adhesions to the dura mater. Care must be taken to avoid traction injuries to the spinal cord which can occur if the preserved spinal nerve roots are accidentally pulled. The surgeon should always take utmost care to protect the fragile spinal cord. Once the dissection has been performed on one side, the temporary rod was shifted to the other side and similar steps performed. The wedge to be resected at the apex was marked and verified in the image intensifier (Fig. 4). The resection was performed with a high-speed burr, curettes and osteotomes. A thin shell of the posterior vertebral cortex was left intact till the very end to avoid troublesome bleeding from the epidural veins. The thin cortex also acts as a natural retractor holding the dura protecting it during the wedge resection. Once the apex of the wedge has been resected from either side, the posterior vertebral cortex was finally drilled out using a diamond burr from the lateral direction. It is recommended not to put any instrument between the posterior vertebral cortex and the dura mater while removing the posterior vertebral cortex as it may increase the pressure on the spinal cord. During all these steps, it is important to stabilize the spine with a rod at least on one side to prevent sudden translational movements of the spine.
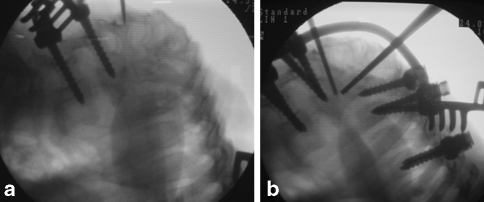
Fig. 4.
Intraoperative fluoroscopy images. a Two pedicle screws on the lower vertebra; the proximal vertebrae are situated almost at right angles to the distal vertebra. b All the pedicle screws inserted and a temporary rod placed to stabilise the spine. Two radio-opaque rods are placed near the apex along the borders of the planned wedge to decide the extent of wedge resection
Now an appropriate-sized Harms’ titanium cage was packed with rib grafts and placed in the anterior defect. Two short rods (correction rod) were positioned vertically and fixed to the pedicle screws cranial and caudal to the osteotomy site on the contralateral side of the temporary rod. Each correction rod was securely held with a rod holder, and correction of spinal deformity was meticulously performed using lever arm force under direct vision of the spinal cord, while loosening the connection of screws of the temporary rod. Use of two rods and connecting them with a Domino will help to achieve better correction in a controlled manner. During this procedure, the initial correction is achieved by the closing wedge technique with the anterior longitudinal ligament as the hinge and the remaining correction is achieved by opening the wedge anteriorly using the cage as a fulcrum. Thus, good correction could be achieved without any neurological compromise (Fig. 5).
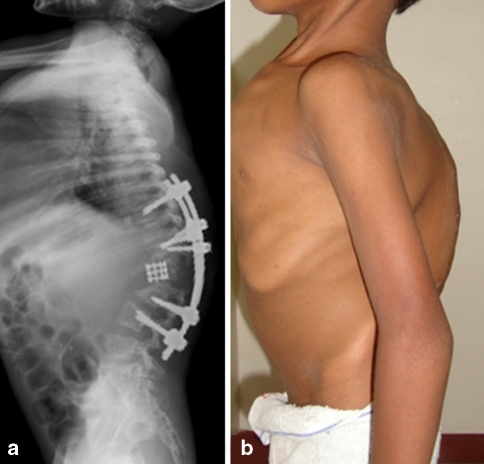
Fig. 5.
Post-operative clinical photograph and lateral radiograph shows good correction of the deformity with pedicle screw instrumentation placed at least three levels proximal and distal to the apex. The ‘opened’ anterior wedge has been reconstructed with a titanium mesh cage
During the correction procedure, the spinal cord-evoked potential monitoring should be performed. It is most important during this step, for the surgeon to constantly visualise the circumferentially decompressed spinal cord. Extending the laminectomy is advised if there is any pressure on the spinal cord at the margins of the laminectomy or reduction in the dural pulsations. Once the correction was assessed in the lateral fluoroscopy view, the temporary rods were replaced in sequence by adequately contoured permanent rods. The posterolateral structures were decorticated and bone grafting was performed.
Procedure imaging section (Figs. 4, 5, 6)
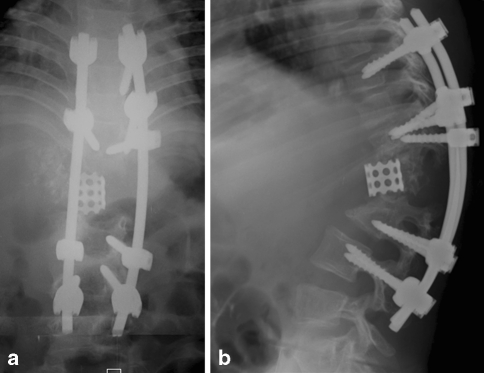
Fig. 6.
Follow-up antero-posterior and lateral radiographs taken at 1 year after surgery show no loss of correction or implant loosening. The anteriorly placed cage remains in position
Outcome with documentation
Suction drains were instituted for 3 days after surgery. There were no post-operative complications. The patient was allowed to start walking from the fourth day after the surgery. The patient wore a body-moulded thoracolumbosacral orthosis for 3–4 months until the bony union was attained. At 1-year follow-up, the deformity correction was maintained and the patient had good functional results (Fig. 6).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Contributor Information
S. Rajasekaran, Phone: +91-9843022325, Email: rajasekaran.orth@gmail.com, Email: sr@gangahospital.com, http://www.gangahospital.com
P. Rishi Mugesh Kanna, Phone: +91-9486600798, Email: rishiortho2003@yahoo.co.in
Ajoy Prasad Shetty, Phone: +91-9344833797, Email: ajoyshetty@gmail.com.
References
- 1.Altman GT, Altman DT, Frankovitch KF. Anterior and posterior fusion for children with tuberculosis of the spine. Clin Orthop. 1996;325:225–231. doi: 10.1097/00003086-199604000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Benli IT, Acaroglu E, Akalin S, et al. Anterior radical debridement and anterior instrumentation in tuberculosis spondylitis. Eur Spine J. 2003;12:224–234. doi: 10.1007/s00586-002-0403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bezer M, Kucukdurmaz F, et al. Transpedicular decancellation osteotomy in the treatment of posttuberculous kyphosis. J Spinal Disord Tech. 2007;20:209–215. doi: 10.1097/01.bsd.0000211271.89485.f1. [DOI] [PubMed] [Google Scholar]
- 4.Kawahara N, Tomita K, Hisatoshi B, et al. Closing–opening wedge osteotomy to correct angular kyphotic deformity by a single posterior approach. Spine. 2001;26:391–402. doi: 10.1097/00007632-200102150-00016. [DOI] [PubMed] [Google Scholar]
- 5.Lonstein JE, et al. Cord compression. In: Bradford DS, Lonstein JE, Ogilvie JW, et al., editors. Moe’s textbook of scoliosis and other spinal deformities. 2. Philadelphia: WB Saunders; 1987. pp. 540–547. [Google Scholar]
- 6.Medical Research Council A 15-year assessment of controlled trials of the management of tuberculosis of the spine in Korea and Hong Kong: thirteenth report. J Bone Joint Surg Br. 1998;80B:456–462. doi: 10.1302/0301-620x.80b3.8544. [DOI] [PubMed] [Google Scholar]
- 7.Moon MS, Woo YK, Lee KS, et al. Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine. 1995;20:1910–1916. doi: 10.1097/00007632-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Moon MS. Tuberculosis of the spine, controversies and a new challenge. Spine. 1997;22:1791–1797. doi: 10.1097/00007632-199708010-00022. [DOI] [PubMed] [Google Scholar]
- 9.Moon MS, Kim I, Woo YK, Park YO. Conservative treatment of tuberculosis of the thoracic and lumbar spine in adults and children. Int Orthop. 1987;11:315–322. doi: 10.1007/BF00271307. [DOI] [PubMed] [Google Scholar]
- 10.Moon MS, Lee MK. The changes of the kyphosis of the tuberculous spine in children following ambulant treatment. Korean Orthop Assoc. 1971;6:203–208. [Google Scholar]
- 11.Parathasarathy R, Sriram K, Satha T, et al. Short course chemotherapy for tuberculosis of the spine: a comparison between ambulant treatment and radical surgery—10 year report. J Bone Joint Surg. 1999;81B:464–471. doi: 10.1302/0301-620X.81B3.9043. [DOI] [PubMed] [Google Scholar]
- 12.Rajasekaran S. The problem of deformity in spinal tuberculosis. Clin Orthop. 2002;398:85–92. doi: 10.1097/00003086-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Rajasekaran S. The natural history of childhood spinal tuberculosis: a 15-year prospective study. J Bone Joint Surg Br. 2001;83B:954–962. doi: 10.1302/0301-620X.83B7.12170. [DOI] [PubMed] [Google Scholar]
- 14.Rajasekaran S, Shanmugasundaram TK. Prediction of the angle of gibbus deformity in tuberculosis of the spine. J Bone Joint Surg Am. 1987;69-A(4):503–509. [PubMed] [Google Scholar]
- 15.Rajasekaran S. Buckling collapse of the spine in childhood spinal tuberculosis. Clin Orthop. 2007;460:86–92. doi: 10.1097/BLO.0b013e31806a9172. [DOI] [PubMed] [Google Scholar]
- 16.Reyes-Sanchez A, Rosales LM, Miramontes VP, et al. Treatment of thoracolumbar fractures by vertebral shortening. Eur Spine J. 2002;11:8–12. doi: 10.1007/s005860000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suk SI, Kim JH, Lee SM, et al. Anterior-posterior surgery versus posterior closing wedge osteotomy in posttraumatic kyphosis with neurologic compromised osteoporotic fracture. Spine. 2003;28:2170–2175. doi: 10.1097/01.BRS.0000090889.45158.5A. [DOI] [PubMed] [Google Scholar]
- 18.Siata K, Hoshini Y, Kikkawa I, et al. Posterior spinal shortening for paraplegia after vertebral collapse caused by osteoporosis. Spine. 2000;25:2832–2835. doi: 10.1097/00007632-200011010-00018. [DOI] [PubMed] [Google Scholar]
- 19.Tuli SM. Severe kyphotic deformity in tuberculosis of the spine. Int Orthop. 1995;19:327–331. doi: 10.1007/BF00181121. [DOI] [PubMed] [Google Scholar]
- 20.Tuli SM. Tuberculosis of the skeletal system. 3. New Delhi: Jaypee Brothers; 1991. [Google Scholar]
- 21.Yau ACMC, Hsu LCS, O’Brien JP, Hodgson AR. Tuberculosis kyphosis-correction with spinal osteotomy, halo-pelvic distraction and anterior and posterior fusion. J Bone Joint Surg. 1974;56A:1419–1434. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.