Abstract
Although clinical guidelines advocate exercise and activity in the management of non-specific low back pain (NSLBP), the link between levels of physical activity and outcomes is unclear. This systematic review investigated the relationships between free living activity levels after onset of low back pain (LBP) and measures of pain, and disability in patients with NSLBP. Cohort and cross-sectional studies were located using OVID, CINAHL, Medline, AMED, Embase, Biomed, PubMed-National Library of Medicine, Proquest and Cochrane Databases, and hand searches of reference lists. Studies were included if a statistical relationship was investigated between measures of free living physical activity (PA) in subjects with LBP and LBP outcome measures. Twelve studies (seven cohort and five cross-sectional) were included. One prospective study reported a statistically significant relationship between increased leisure time activity and improved LBP outcomes, and one cross-sectional study found that lower levels of sporting activity were associated with higher levels of pain and disability. All other studies (n = 10) found no relationship between measures of activity levels and either pain or disability. Heterogeneity of study designs, particularly in terms of activity measurement, made comparisons between studies difficult. These data suggest that the activity levels of patients with NSLBP are neither associated with, nor predictive of, disability or pain levels. Validated activity measurement in prospective research is required to better evaluate the relationships between PA and LBP.
Keywords: Physical activity, Low back pain, Systematic review, Outcomes, Guidelines
Introduction
The importance of exercise and physical activity (PA) has been recognised as a prime strategy in international guidelines for the primary care management of acute and chronic low back pain (LBP) [3, 54]. Advice to stay active, early and gradual activation, and discouragement of bed rest are all key features of primary care LBP management guidelines [6, 30]. However, effective strategies to manage LBP and prevent recurrence and chronicity are elusive [29, 44], and there is an increasing focus to create strategies for preventing the negative consequences of chronic LBP [35]. A potential role for PA in the prevention of chronic LBP has been proposed [28]. A recent review found evidence supporting the use of advice to remain active as a key element of active self-management in chronic LBP populations [34]. While graded activity programmes have been trialled as a management strategy for acute and chronic LBP populations [19, 50], these studies did not assess activity levels in free living, and thus it is not possible to determine the relationship between any activity change and measures of LBP recovery.
The proposed effect of pain on activity levels of patients with LBP has to a large part been based upon the deconditioning model of LBP [60]. This model is supported by evidence of various changes in physical functioning [11], neuromuscular changes [14], psychological effects [51], decreases in physical fitness [46], alterations in the patterns [55] and levels of activity of patients with LBP [52]. The evidence for deconditioning as a result of LBP has also been challenged [45], and a number of studies report no difference in either fitness levels [43, 45] or activity levels of patients with LBP compared with healthy controls [47]. However, little is known about whether activity levels of patients with LBP are associated with LBP outcomes.
Although a dose–response relationship for PA in the primary and secondary prevention and management of many chronic diseases has now been shown [58], the role of activity in populations with LBP has yet to be determined. The activity level of patients with LBP does not appear to be a significant predictor of prognosis [29, 49] although most prospective studies have failed to specifically examine it as a potential prognostic factor. The primary aim of this systematic review was to explore the relationship(s) between PA levels in patients with LBP and relevant outcome measures that included measures of LBP-related disability and pain. The secondary aims were to explore whether specific activity levels and/or types of activities more strongly related to LBP outcome measures.
Methods
Search strategy
A systematic review of NSLBP observational studies that investigated relationships between PA levels and LBP outcome measures was carried out. Although longitudinal analyses within a randomised control trial design is preferable for seeking predictive relationships [2, 9], we also accepted a cross-sectional design as appropriate for this exploratory investigation of relationships between activity and LBP.
The following databases were searched independently by two reviewers to obtain relevant studies for this review: OVID, CINAHL, Medline, AMED, Embase, Biomed, PubMed-National Library of Medicine, Proquest and Cochrane Database (1990 to January 2009). The search strategy used the following text, keyword and MESH terms in each database: accelerometer, activities of daily living, activity diary, activity level, activity questionnaire, energy expenditure, heart rate monitor, pedometer, physical activities, disuse and LBP (in appropriate combinations). The search was restricted to studies in the English language.
Inclusion criteria
The inclusion criteria are detailed in Table 1. Studies which specifically evaluated exercise therapy for LBP but did not include a measurement of free living activity were not included. Free living activity measurement was defined as a measure of activity undertaken in day-to-day life including occupational, sports and leisure activities [7]. Studies that only measured activity limitation or pain with activity, which measured activities with which the patient was having difficulty rather than their actual level or type of PA, were not included. For the purpose of this review, psychosocial factors (including fear avoidance, locus of control and job satisfaction) were not considered primary outcome measures for LBP, and studies which assessed the relationship of PA exclusively to one of these variables were excluded.
Table 1.
Inclusion criteria
| 1. Design was a randomised controlled trial (RCT), cohort, case–control, or cross-sectional |
| 2. Participants were adults (>18 years) |
| 3. Participants had acute, sub acute or chronic NSLBP, which did not specifically include the following pathologies: infection, tumour, osteoporosis, ankylosing spondylitis, fracture, deformity, inflammatory process, cauda equina syndrome or spinal surgery |
| 4. Free living PA measures included at least one of the following: doubly labelled water (DLW), accelerometers, heart rate monitors, pedometers, global positioning system (GPS), interviews, logs, surveys, questionnaires and activity diaries |
| 5. Validated LBP measures were measured |
| 6. Free living PA and LBP outcomes were evaluated statistically |
Review process
The full text of citations (title and abstract) which made reference to PA measurement in an adult population of patients with NSLBP was retrieved in full and inclusion criteria (Table 1) applied. Reference lists of all retrieved articles were checked for additional relevant studies. Potential studies were then checked independently by two reviewers (PH and BR) for inclusion, and any discrepancies were discussed and resolved by consensus within the team. Experts in the field of LBP and activity and those authors whose studies met the inclusion criteria were also contacted in order to identify additional studies.
Data extraction
Data from included studies were independently extracted by two reviewers (PH and BR). If there was disagreement, consensus was reached after a meeting with a third reviewer (GDB). Data were extracted using a standardised data extraction sheet and tabulated. Data included study design, number of participants, type of control group (if relevant), demographic characteristics including age and gender, type of LBP (acute, sub-acute and chronic), treatment received (if appropriate), details of PA measurement, duration of PA measurement, duration and timing of follow-up, outcome measures employed including means and standard deviations, attrition rates, and the statistical relationship between PA and LBP outcome measure(s).
Methodological quality assessment
The methodological quality of each study was assessed using a modified Downs and Black checklist [12]. The focus of the quality assessment was based on the validity and accuracy of the physical activity measure and adjustment for potential confounding (Table 2). Two reviewers independently assessed the methodological qualities of the scored cohort studies (PH and SM) and cross-sectional studies (PH and LH). Any disagreement between these authors was resolved with a third reviewer (GDB). Articles were not excluded based upon methodological quality.
Table 2.
Modified Downs and Black quality checklist to assess measurement of PA in LBP populations
| Score | |
|---|---|
| Reporting | |
| 1. Is the hypothesis/aim/objective of the study clearly described? | 1 |
| 2. Are the main physical activity outcomes to be measured clearly described in the “Introduction” or “Methods” section? | 1 |
| 3. Are the characteristics of the patients included in the study clearly described? | 1 |
| 4. Are the physical activity measurements of interest clearly described? | 1 |
| 5. Are the distributions of principal confounders in each group of subjects to be compared clearly described? | 1 |
| 6. Are the main physical activity findings of the study clearly described? | 1 |
| 7. Does the study provide estimates of the random variability in the physical activity data for the main LBP outcomes? | 1 |
| 8. Have the characteristics of patients lost to follow-up been described? | 1 |
| 9. Have actual probability values been reported (e.g. 0.035 rather than <0.05) for the main outcomes except where the probability value is less than 0.001? | 1 |
| External validity | |
| 10. Were the subjects asked to participate in the study representative of the entire population from which they were recruited? | 1 |
| 11. Were those subjects who were prepared to participate representative of the entire population from which they were recruited? | 1 |
| Internal validity | |
| 12. If any of the results of the study were based on “data dredging”, was this made clear? | 1 |
| 13. In trials and cohort studies, do the analyses adjust for different lengths of follow-up of patients, or in case–control studies, is the time period between the physical activity measurement and outcome the same for cases and controls? | 1 |
| 14. Were the statistical tests used to assess the relationship between the LBP outcome measure and physical activity outcomes appropriate? | 1 |
| 15. Was compliance with the physical activity measurement/s reliable? | 1 |
| 16. Were the main physical activity outcome measures used accurate (valid and reliable)? | 1 |
| Confounding (selection bias) | |
| 17. Were the patients in different physical activity intervention groups (trials and cohort studies) or were the cases and controls (case–control studies) recruited from the same population? | 1 |
| 18. Were study subjects in different physical activity intervention groups (trials and cohort studies) or were the cases and controls (case–control studies) recruited over the same period of time? | 1 |
| 19. Were study subjects randomised to physical activity intervention groups? | 1 |
| 20. Was the randomised intervention assignment concealed from both patients and health care staff until recruitment was complete and irrevocable? | 1 |
| 21. Was there adequate adjustment for confounding factors between physical activity and LBP in the analyses from which the main findings were drawn? | 1 |
| 22. Were losses of patients to follow-up taken into account? | 1 |
| Power | |
| 23. Did the study have sufficient power to detect a clinically important effect where the probability value for a difference being due to chance is less than 5%? | 5 |
| Total | 27 |
Statistical analyses
For the purposes of this review, the significance level was set at p < 0.05, or alternatively if the 95% confidence interval (CI) about the odds ratio (OR) did not cross 1. If studies assessed the relationship between activity and a validated LBP outcome over time, any significant statistical relationships are presented at these time points. Due to the heterogeneity of the statistical methods employed to evaluate the statistical relationships between activity and LBP, it was decided to only report the univariate analyses [27] unless the multivariate analyses alone were available. Considerable disparity in study methods including differences in PA measures, outcome measures and statistical analyses precluded the use of a meta-analysis.
Results
Study selection
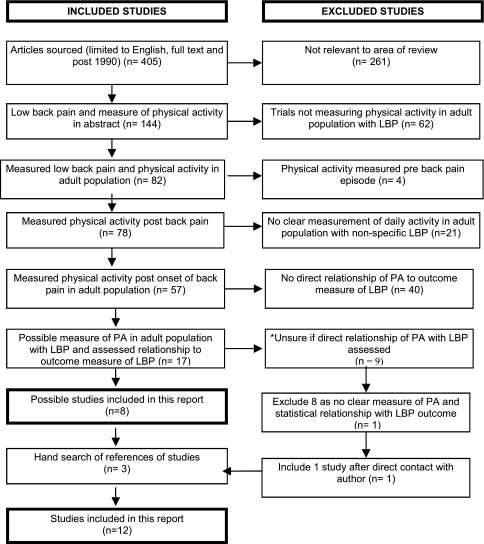
Although 78 studies measured PA in an LBP population, 66 were excluded for the following reasons: post-lumbar surgery, no direct comparison of PA with an LBP outcome measure, measurement of night-time activity only, retrospective measure of PA, mixture of low back pain and other pains, and no clear measure of free living PA. Following further review and author clarification of activity measurement, one further study was included in the analysis [31] (Fig. 1).
Fig. 1.
QUORUM flow chart of search results from systematic
Study characteristics
Twelve articles measured PA in an adult NSLBP population and assessed the relationship with an LBP outcome measure. These included seven cohort studies (Table 3) and five cross-sectional studies (Table 4). Although several studies were randomised controlled trials investigating effectiveness of various interventions prospectively [22, 31, 32], these studies also investigated the relationship between activity and LBP in a longitudinal cohort design when assessing the relationship of activity to the outcome measure.
Table 3.
Characteristics and results of cohort studies
| Author | Study score [25] | Subjects and follow-up | Measurement of physical activity | Classification of physical activity | Duration of PA measure/follow-up | Relevant LBP outcome measure | Main results |
|---|---|---|---|---|---|---|---|
| Bousema et al. [8] | 20 | 124 sub-acute LBP 18–60 years (106 completed study) | RT3 Triaxial accelerometer | Total sum of RT3 counts/day (PAL) | 7 days Data collected twice: at inclusion (PAL-0) and after 1 year (PAL-1) |
PCS, QBPDS, TSK, BDI | No difference in PAL change (PAL-1-PAL-0) in recovered and non-recovered participants (F = 0.31, p = 0.58) |
| Hurwitz et al. [22] | 15 | 610 non-specific LBP 18–70+ years | Self-reported PA questionnaire | Activity classified as weekly MET values 0, 0.1–10.49, 10.5–25.9, ≥26 | Baseline, 6 weeks and 6, 12, 18 months | NRS for pain, RMDQ | Recreational PA inversely associated with NRS (p < 0.05) and RMDQ (p < 0.05) |
| Mortimer et al. [37] | 12 | 459 non-specific LBP 20–59 years | Self-reported PA questionnaire | Low exercise <2 h/week at 4 MET. Medium exercise >3 h/week at 4 MET. High exercise 1 h/week at 5 MET or higher | Data collection on entry and at 5-year follow-up | Van Korff procedure to classify pain and disability | No significant relationship between PA and change in pain from baseline to 5-year follow-up (p = 0.14) and PA and change in disability from baseline to 5-year follow-up (p = 0.20) |
| Oleske et al. [39] | 9 | 352 autoworkers diagnosed with work-related non-specific LBP | PA question: exercise or physical activities outside work (Y/N) | Y/N | Baseline measurement | No. of health care treatments for LBP at 1 year | PA (outside work) was not significant predictor of LBP recurrence (p = 0.064) |
| Leonhardt et al. [32] | 18 | 1,378 patients with non-specific LBP (18–65 years) 1,211 (follow-up) | Freiburger questionnaire on physical activity | Total MET hours/week | Measurements at baseline, 6 and 12 months | Van Korff procedure to classify pain and disability | No influence of the total EE after 6 months on pain chronification (no p value reported) |
| Kuukkanen et al. [31] | 11 | 57 CLBP patients (disabling pain over 3 years); 22–50; 47 at 5 years (follow-up) | MET-based questionnaire for the study of PA | The sum and the highest MET values | Measurements at baseline, 3, 6, 12 months and 5 years | Borg CR-10 scale and the ODI | No significant correlation between the Borg CR-10, the ODI and PA at 3, 6, 12 months and 5 years. (no p value reported) |
| Jacob et al. [24] | 19 | 555 non-specific LBP aged 22–70 years followed. 367 (66%) follow-up | BPAQ (self-administered) | BPAQ score classification into occupational, sports and leisure time activity score | Baseline measurement | Modified RMDQ SFI and SBI | PA was not an independent predictor of RMDQ, SFI or SBI at 2 or 12 months (no p value reported) |
BDI Beck depression inventory, BPAQ Baecke physical activity questionnaire, CLBP chronic low back pain, LBP low back pain, EE energy expenditure, MET metabolic equivalent task, NRS numerical rating scale, PA physical activity, ODI Oswestry disability questionnaire, PAL physical activity level, PAL-0 baseline activity level, PAL-1 physical activity level at 1 year, PCS pain catastrophising scale, QBPDS Quebec back pain disability scale, RMDQ 24-item Roland Morris low back pain disability questionnaire, TSK Tampa scale for kinesiophobia, SBI pain symptoms bothersomeness, SFI pain symptoms frequency
Table 4.
Characteristics and results of cross-sectional studies
| Author | Study score [25] | Subjects | Mode of measurement of physical activity | Classification of physical activity | Duration of measure | Relevant outcome measure | Main results |
|---|---|---|---|---|---|---|---|
| Verbunt et al. [56] | 10 | 13 CLBP 13 controls 18–60 years |
Tracmor accelerometer: counts/min and DLW (PAL = ADMR/RMR) | PAL = ADMR/RMR <1.60, low 1.60 < PAL < 1.85, moderate >1.85, high |
14 days of continual monitoring | RMDQ and VAS | Correlation of PA (Tracmor) and RMDQ: (r = 0.10, p = 0.76) Correlation of PAL and RMDQ: (r = −0.06, p = 0.74) |
| Verbunt et al. [57] | 16 | 123 sub-acute LBP 18–60 years | RT3 triaxial accelerometer Activity log BPAQ |
RT3 counts per day (LBP PAL) |
7 days of continual monitoring | VAS score QBPDS BDI |
PAL did not contribute to the explanation of disability. PAL versus QBPDS (p = −0.16) |
| Cunha et al. [10] | 11 | 51 subjects with non-specific LBP (26–65 years of age) | The self-reported PA rating (scale of 0–8) | Scale rating (0–8) | N/A | RMDQ | Correlation of PA with RMDQ (r = −0.04) |
| Johansson et al. [26] | 14 | 72 participants (18–65 years) with duration of LBP of at least 4 weeks | The general activity scale (self-reported questionnaire) | Scale: 18 items perform activities (0 = never, 6 = very often) | N/A | RMDQ | No significant relationship between PA and RMDQ. Low negative correlation of RM-SW with general activity (r = 0.27, p < 0.05) |
| Jacob et al. [25] | 17 | 555 non-specific LBP aged 22–70 years | BPAQ (self-administered) | BPAQ score classification into occupational, sports and leisure time activity score | N/A | Modified RMDQ SFI and SBI | Significant relationship between SAI and (Beta, 95% CI) RMDQ (−0.09, −0.1 to −0.02) SFI (−0.08, −0.14 to −0.02) SBI (−0.06, −0.1 to −0.005) |
ADMR average daily metabolic rate, BDI Beck depression inventory, BPAQ Baecke physical activity questionnaire, CLBP chronic low back pain, DLW doubly labelled water, LBP low back pain, MET metabolic equivalent task, NRS numerical rating scale, PA physical activity, PAL physical activity level, PE physical exercise, QBPDS Quebec back pain disability scale, RMDQ 24-item Roland Morris low back pain disability questionnaire, RMR resting metabolic rate, SAI sport activity index, SBI pain symptoms bothersomeness, SFI pain symptoms frequency, TSK Tampa scale for kinesiophobia, VAS visual analogue scale
Cohort studies
Only one of seven articles found a significant relationship between activity and LBP, whereby recreational activity (combined sports and leisure activities) was found to be inversely associated with both pain and disability at 18 months [22]. The odds of having a clinically meaningful disability were 30% lower among participants in the upper two quartiles of the PA distribution than among inactive participants. The remaining studies found no significant relationship between activity levels and LBP disability, pain or healthcare utilisation.
Study analysis
Quality rating scores (Table 3) demonstrated no apparent relationship between either the total score or the rating within each of the score domains and the relationship between PA and the LBP outcome measure. Studies were generally of moderate to poor quality with only one of the 12 studies scoring highly on the criteria for external validity [24]. Controlling or adjusting for potential bias and confounding issues were scored from ‘poor’ to ‘moderately good’ for all studies. No studies used power estimations to detect relationship between activity and LBP outcome measure. However, the study numbers were relatively large (ranging from n = 57 to n = 1,378) with five of the included studies having greater than 300 participants.
Types of participants
The majority of studies included participants of a working age population (18–65 years), diagnosed with NSLBP of varying symptom duration [25, 32, 37, 39]. Two studies investigated PA within exclusively sub-acute [7] or chronic LBP populations [31]. The levels of disability and pain at entry into the study were generally moderate [8, 22, 24, 31, 37, 39]; while one study reported mixed disability levels [32], the majority of participants within the three included groups had a low disability rating.
Activity measurement
Each of the studies employed a range of PA measures (Table 3) with only one using an objective measure of PA [8]; all other studies investigated PA with various types of recall questionnaires. Four studies employed self-report questionnaires, classifying various activities into metabolic equivalent of task (MET) energy levels [22, 31, 32, 37], while one study used a questionnaire requiring the participant to state (yes/no) whether they participated in exercise or activity outside work [39]. Only Jacob et al. [24] employed a previously validated LBP recall instrument [23] at 1-year follow-up in an observational study [25]. The reliability and validity of the other PA recall questionnaires had not been previously investigated within an LBP population.
Repeated measures design was used by three studies to investigate the relationship between activity and LBP. A significant relationship between PA and LBP outcome was identified in a study [22] that investigated the number of leisure hours per week spent in walking, light, moderate and strenuous physical activity at each of four measurement points. Another repeated PA measures study identified that the proportion of women reporting previous periods of acute/sub acute NSLBP during the preceding 5 years was highest (66%) in the ‘medium’ exercise group as opposed to the low and high exercise groups [37]. However, Jacob et al. [24]’s repeated measures study found no predictive relationship between PA classified into a work, leisure and occupational score from a single baseline measurement and pain or disability at 1 year.
Follow-up characteristics
The minimum follow-up period was 1 year with the number and scale of follow-up periods ranging from two (at baseline and at 1 year [8]) to five separate measurements over 5 years [31]. Loss of participants to follow-up in each of the studies ranged from 42% over 5 years [37] to 10% over 18 months [22]. Two studies did not report characteristics of those lost to follow-up [22, 39], and a further study did not take into account the potential effects of loss to follow-up [31].
Confounding factors included in the relationship between and activity and LBP
The majority of prospective studies included a range of potential confounders in the multivariate analyses with two studies not assessing and/or adjusting for such factors [31, 37]. The only study to find a significant relationship between activity and LBP in multivariate analyses [22] included a greater number and range of potential confounders, in terms of behavioural, psychosocial and other patient characteristics.
Cross-sectional studies
Only one of five cross-sectional studies reported a significant relationship between PA and measures of LBP [25], observing low sports activity index (SAI) scores contributed to higher scores for most LBP measures.
Types of participants
The five cross-sectional studies included patients of working age (18–65 years) with LBP duration ranging from sub-acute [26, 57] to chronic [56]. Both Jacobs et al. [25] and Cunha et al. [10] also included patients with a range of LBP durations.
The study quality ranged considerably with none specifically powered to investigate relationships between activity and LBP and study numbers varying widely from 13 to 555 participants. Small numbers of participants in most of these studies may have led to a lack of observed relationships. Only the numerically stronger Jacob et al. [25] reported an association between PA and low back pain outcomes (n = 550).
One study included participants both with and without LBP to compare and contrast their activity levels [56] whilst the other studies were performed exclusively on populations with LBP. All studies used validated measures of LBP disability; in some cases, measures of pain, depression and fear avoidance were also employed (Table 4).
Activity measurement
Two studies employed objective measures of PA [56, 57], while three studies made use of various types of PA recall questionnaires. Verbunt et al. [56] utilised doubly labelled water (DLW) [1] and the Tracmor accelerometer [41] to sub-classify activity levels into low, moderate and high. The other study employing an objective measured used the RT3 accelerometer along with an activity log and the Beacke physical activity questionnaire (BPAQ) to examine the contribution of activity on a sub-acute LBP population [57]. Two studies included self-reported activity scales [10, 26] to assess free living activities. However, neither of these self-reporting instruments has been validated as a measure of activity within an LBP population, and little data were available within healthy human populations as a comparative measure. Jacob et al. [25] employed the BPAQ [42] as a validated PA tool to sub-classify participants’ occupational, leisure and sports activities.
Discussion
There is little evidence for a relationship between free living PA and measures of disability, pain or health utilisation in NSLBP populations. Only one cohort study reported a statistically significant relationship between activity and disability [22] whereby lower levels of recreational activity were inversely associated with pain (p < 0.05) and LBP-related disability (p < 0.05). One cross-sectional study [25] reported that lower levels of self-reported sporting activity were associated with higher levels of pain and disability. The remaining studies (n = 10) found no relationship between free living PA and LBP outcome measures either cross-sectionally or longitudinally. These findings call into question the role that activity plays in patients with LBP.
As a cross-sectional design cannot identify cause/effect associations between two factors [9], potential relationships between activity and LBP outcome measures were primarily driven by results from prospective cohort studies. Such observational studies are important for health research, particularly when assessing for prognostic or aetiological factors [20, 36]. The current results provide moderate evidence (level II) [20] that activity, or activity change, in patients with NSLBP is not predictive or associated with LBP outcomes.
The quality of the observational studies was mixed, and there were a number of research design issues affecting the validity of the findings. A variety of PA measures were used, most commonly recall questionnaires, with the majority either untested or non-validated within LBP populations. Activity measures employed by Leonhardt et al. [32] allowed for specific comparisons to non-LBP populations [13], while the BPAQ [24] permits comparison to both LBP and non-LBP populations [23]. The generic MET-based questionnaires have provided more general comparison to activity levels in other populations although different methods of calculation introduces potential for bias or confounding. Validation and between study comparison of activity measurement tools is important as it allows direct and accurate comparisons of PA measurement and the investigation of change in activity.
The PA recall questionnaires used may have affected the reliability and validity of reported results. The ability to accurately assess and determine activity change over time differs between objective and activity recall instruments [5, 13]. As self-report measures employed in a number of the studies did not specify that activities should only be recorded since the current episode of LBP [22, 31, 32, 39], it is possible that activity levels prior to the onset of LBP may have also been reported by participants. If different to activity levels with LBP, these may have clouded the potential affect of PA on recovery.
There were also potential confounders to the relationship between activity and disability [44], as well as factors identified as mediators between PA and disability [38, 40]. A number of behavioural and psychosocial variables were accounted for in the multivariate models; however, no research expressly investigated potential mediating effects of these variables on the relationship between activity and the LBP outcome. Non-associations may also be influenced by other behavioural or psychosocial factors, including fear avoidance [4], and thus confound or moderate the relationship between PA and LBP.
The role of activity and exercise in LBP
Advice to remain active as an adjunct to physiotherapy treatment appears to have favourable results for both acute and chronic LBP populations [34]; however, literature supporting this argument did not include studies which had specifically measured PA in an LBP population [34]. A potential role for PA in the incidence of LBP has also been investigated [15, 16, 33, 48] with no definitive conclusions. A recent review, employing a short recall questionnaire, assessed the retrospective relationship between activity and point prevalence of LBP and reported a U-shaped relationship in that both high and low levels of activity increased the likelihood of a retrospective report of LBP chronicity, particularly in females [18]. Only one longitudinal study specifically stratified activity into low, moderate and high levels at baseline and used self-report measures to explore the relationship with LBP outcomes [37]. This study found no significant relationship between activity levels recorded at baseline and change in pain and disability at 5 years. No other studies specifically assessed whether maintenance of a reasonable level of activity during an episode of LBP, as opposed to low or high activity levels, is related to outcomes. Further longitudinal research into the potential U-shaped effects of too little or excessive activity levels is warranted.
Although the role of exercise in the management of LBP generally shows positive results [17, 53, 59], this review is the first to assess the relationship between activity levels in LBP patients with measures of LBP disability, pain and recovery. When considering the importance placed on activity in the management of LBP, this review illustrates the sparsity of research in this area and the lack of evidence for a positive role for activity in this population.
Research limitations
A number of additional studies may have been inadvertently missed from this review due to the exclusion of grey literature and restriction to English language journals. Although every effort was made to ensure a systematic and rigorous process, multiple study designs meant the search terms for PA were not always listed as keywords or contained within the abstract.
Further research direction
Few studies employed multiple measurement points to assess the relationship between activity and LBP outcomes. One of the major difficulties when assessing the relationship between activity and LBP outcomes within a longitudinal design is the potential for such relationships to be in a state of flux, and thus exploring for potential relationships needs to consider “temporal stability” when describing the observed variance (and co-variance) between activity and LBP outcomes at those discreet points in time [9]. Timing of the assessments to examine the effects of such a relationship can be critical. In order for such potential effects to be explored, regular activity measurement employing both objective and non-objective measures across a range of time periods and within varying LBP populations is required.
It is increasingly recognised that both the types of activities and their variability may be important distinguishing factors for patients with LBP [21, 55]. A recent study found that activity fluctuations rather than the mean activity level over time contributed significantly to levels of disability in a cohort of patients with CLBP [21]. Therefore, further research is required to assess the relationship between these activity fluctuations within populations with LBP and recovery over time.
Although some studies assessed the dimensions of PA by using established cut-points for activity-based MET hours of energy, no studies employed both objective and non-objective measures of activity to assess change in the various dimensions of activity (intensity frequency and duration) over time. Consequently, there is a need to explore the potential predictive relationship between LBP recovery and PA levels in prospective research.
Conclusion
This systematic review evaluated the evidence for the relationship between free living physical activity in patients with NSLBP and outcomes, recovery and reoccurrence. The findings do not support a relationship between activity and NSLBP outcome measures. On the other hand, no evidence was found for detrimental effects from engaging in higher levels of activity in patients with LBP. Considering the known health benefits of increasing activity, current recommendations for patients with LBP to maintain, restore and increase their activity as part of their overall management should probably continue to be made. The results, however, highlight the need for continued research to evaluate and clarify the role of PA for patients with NSLBP in terms of its outcome and prognosis.
References
- 1.Ainslie P, Reilly T, Westerterp K. Estimating human energy expenditure: a review of techniques with particular reference to doubly labelled water. Sports Med. 2003;33:683–698. doi: 10.2165/00007256-200333090-00004. [DOI] [PubMed] [Google Scholar]
- 2.Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, Gotzsche PC, Lang T. The revised CONSORT statement for reporting randomised trials: explanation and elaboration. Ann Intern Med. 2001;134:663–695. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 3.Arnau JM, Vallano A, Lopez A, Pellise F, Delgado MJ, Prat N. A critical review of guidelines for low back pain treatment. Eur Spine J. 2006;15:543–553. doi: 10.1007/s00586-005-1027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basler HD, Luckmann J, Wolf U, Quint S. Fear-avoidance beliefs, physical activity, and disability in elderly individuals with chronic low back pain and healthy controls. Clin J Pain. 2008;24:604–610. doi: 10.1097/AJP.0b013e31816b54f6. [DOI] [PubMed] [Google Scholar]
- 5.Bassett DR, Cureton AL, Ainsworth BE. Measurement of daily walking distance—questionnaire versus pedometer. Med Sci Sports Exerc. 2000;32:1018–1023. doi: 10.1097/00005768-200005000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Bekkering GE, Hendriks HJM, Koes BW, Oostendorp RAB, Ostelo RWJG, Thomassen JMC, Tulder MW. Dutch physiotherapy guidelines for low back pain. Physiotherapy. 2003;89:82–96. doi: 10.1016/S0031-9406(05)60579-2. [DOI] [Google Scholar]
- 7.Bouchard C, Blair SN, Haskell WL. Physical activity and health. Champaign: Human Kinetics; 2007. [Google Scholar]
- 8.Bousema EJ, Verbunt JA, Seelen HAM, Vlaeyen JWS, Andre Knottnerus J. Disuse and physical deconditioning in the first year after the onset of back pain. Pain. 2007;130:279–286. doi: 10.1016/j.pain.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Cole DA, Maxwell SE. Testing mediational models with longitudinal data: questions and tips in the use of structural equation modeling. J Abnorm Psychol. 2003;112:558–577. doi: 10.1037/0021-843X.112.4.558. [DOI] [PubMed] [Google Scholar]
- 10.Cunha IT, Simmonds MJ, Protas EJ, Jones S. Back pain, physical function, and estimates of aerobic capacity: what are the relationships among methods and measures? Am J Phys Med Rehabil. 2002;81:913–920. doi: 10.1097/00002060-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Di Iorio A, Abate M, Guralnik JM, Bandinelli S, Cecchi F, Cherubini A, Corsonello A, Foschini N, Guglielmi M, Lauretani F, Volpato S, Abate G, Ferrucci L. From chronic low back pain to disability, a multifactorial mediated pathway: the InCHIANTI study. Spine. 2007;32:E809–E815. doi: 10.1097/BRS.0b013e31815cd422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frey I, Berg A, Grathwohl DK, Keul J. Freiburger questionnaire on physical activity—design, validation and application. Soz Praventivmed. 1999;44:55–64. doi: 10.1007/BF01667127. [DOI] [PubMed] [Google Scholar]
- 14.Hammill RR, Beazell JR, Hart JM. Neuromuscular consequences of low back pain and core dysfunction. Clin Sports Med. 2008;27:449–462. doi: 10.1016/j.csm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Hartvigsen J, Bakketeig LS, Leboeuf-Yde C, Engberg M, Lauritzen T. The association between physical workload and low back pain clouded by the “healthy worker” effect: population-based cross-sectional and 5-year prospective questionnaire study. Spine. 2001;26:1788–1792. doi: 10.1097/00007632-200108150-00011. [DOI] [PubMed] [Google Scholar]
- 16.Hartvigsen J, Frederiksen H, Christensen K. Physical and mental function and incident low back pain in seniors: a population-based two-year prospective study of 1387 Danish Twins aged 70 to 100 years. Spine. 2006;31:1628–1632. doi: 10.1097/01.brs.0000222021.00531.ea. [DOI] [PubMed] [Google Scholar]
- 17.Hayden JA, Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142:776–785. doi: 10.7326/0003-4819-142-9-200505030-00014. [DOI] [PubMed] [Google Scholar]
- 18.Heneweer H, Vanhees L, Picavet HSJ. Physical activity and low back pain: a U-shaped relation? Pain. 2009;143:21–25. doi: 10.1016/j.pain.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Hlobil H, Staal JB, Twisk J, Koke A, Ariens G, Smid T, Mechelen W. The effects of a graded activity intervention for low back pain in occupational health on sick leave, functional status and pain: 12-month results of a randomized controlled trial. J Occup Rehabil. 2005;15:569–580. doi: 10.1007/s10926-005-8035-y. [DOI] [PubMed] [Google Scholar]
- 20.Hoppe DJ, Schemitsch EH, Morshed S, Tornetta P, Bhandari M. Hierarchy of evidence: where observational studies fit in and why we need them. J Bone Joint Surg Am. 2009;91(Suppl 3):2–9. doi: 10.2106/JBJS.H.01571. [DOI] [PubMed] [Google Scholar]
- 21.Huijnen IPJ, Verbunt JA, Roelofs J, Goossens M, Peters M. The disabling role of fluctuations in physical activity in patients with chronic low back pain. Eur J Pain. 2009;13:1076–1079. doi: 10.1016/j.ejpain.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Hurwitz EL, Morgenstern H, Chiao C. Effects of recreational physical activity and back exercises on low back pain and psychological distress: findings from the UCLA Low Back Pain Study. Am J Public Health. 2005;95:1817–1824. doi: 10.2105/AJPH.2004.052993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacob T, Baras M, Epstein L. Low back pain: reliability of a set of pain measurement tools. Arch Phys Med Rehabil. 2001;82:735–742. doi: 10.1053/apmr.2001.22623. [DOI] [PubMed] [Google Scholar]
- 24.Jacob T, Baras M, Zeev A, Epstein L. A longitudinal, community-based study of low back pain outcomes. Spine. 2004;29:1810–1817. doi: 10.1097/01.BRS.0000135834.87106.19. [DOI] [PubMed] [Google Scholar]
- 25.Jacob T, Baras M, Zeev A, Epstein L. Physical activities and low back pain: a community-based study. Med Sci Sports Exerc. 2004;36:9–15. doi: 10.1249/01.MSS.0000106166.94343.02. [DOI] [PubMed] [Google Scholar]
- 26.Johansson E, Lindberg P. Subacute and chronic low back pain. Reliability and validity of a Swedish version of the Roland Morris Disability Questionnaire. J Rehabil Med. 1998;30:139–140. doi: 10.1080/003655098444066. [DOI] [PubMed] [Google Scholar]
- 27.Kamper SJ, Rebbeck TJ, Maher CG, McAuley JH, Sterling M. Course and prognostic factors of whiplash: a systematic review and meta-analysis. Pain. 2008;138:617–629. doi: 10.1016/j.pain.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Karmisholt K, Gotzsche PC. Physical activity for secondary prevention of disease. Systematic reviews of randomised clinical trials. Dan Med Bull. 2005;52:90–94. [PubMed] [Google Scholar]
- 29.Kent PM, Keating JL. Can we predict poor recovery from recent-onset nonspecific low back pain? A systematic review. Man Ther. 2008;13:12–28. doi: 10.1016/j.math.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Koes BW, Tulder MW, Ostelo R, Kim Burton A, Waddell G. Clinical guidelines for the management of low back pain in primary care: an international comparison. Spine. 2001;26:2504–2513. doi: 10.1097/00007632-200111150-00022. [DOI] [PubMed] [Google Scholar]
- 31.Kuukkanen T, Malkia E, Kautiainen H, Pohjolainen T. Effectiveness of a home exercise programme in low back pain: a randomized five-year follow-up study. Physiother Res Int. 2007;12:213–224. doi: 10.1002/pri.378. [DOI] [PubMed] [Google Scholar]
- 32.Leonhardt C, Keller S, Chenot JF, Luckmann J, Basler HD, Wegscheider K, Baum E, Donner-Banzhoff N, Pfingsten M, Hildebrandt J, Kochen MM, Becker A. TTM-based motivational counselling does not increase physical activity of low back pain patients in a primary care setting—a cluster-randomized controlled trial. Patient Educ Couns. 2008;70:50–60. doi: 10.1016/j.pec.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Levangie PK. Association of low back pain with self-reported risk factors among patients seeking physical therapy services. Phys Ther. 1999;79:757–766. [PubMed] [Google Scholar]
- 34.Liddle SD, Gracey JH, Baxter GD. Advice for the management of low back pain: a systematic review of randomised controlled trials. Man Ther. 2007;12:310–327. doi: 10.1016/j.math.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Majid K, Truumees E. Epidemiology and natural history of low back pain. Semin Spine Surg. 2008;20:87–92. doi: 10.1053/j.semss.2008.02.003. [DOI] [Google Scholar]
- 36.Merlin T, Weston A, Tooher R (2009) Extending an evidence hierarchy to include topics other than treatment: revising the Australian ‘levels of evidence’. BMC Med Res Methodol 9:34 [DOI] [PMC free article] [PubMed]
- 37.Mortimer M, Pernold G, Wiktorin C. Low back pain in a general population. Natural course and influence of physical exercise—a 5-year follow-up of the Musculoskeletal Intervention Center-Norrtalje Study. Spine. 2006;31:3045–3051. doi: 10.1097/01.brs.0000250324.03210.3f. [DOI] [PubMed] [Google Scholar]
- 38.Motl RW, McAuley E, Snook EM, Gliottoni RC. Physical activity and quality of life in multiple sclerosis: Intermediary roles of disability, fatigue, mood, pain, self-efficacy and social support. Psychol Health Med. 2009;14:111–124. doi: 10.1080/13548500802241902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oleske DM, Lavender SA, Andersson GBJ, Morrissey MJ, Zold-Kilbourn P, Allen C, Taylor E. Risk factors for recurrent episodes of work-related low back disorders in an industrial population. Spine. 2006;31:789–798. doi: 10.1097/01.brs.0000207017.30490.28. [DOI] [PubMed] [Google Scholar]
- 40.Perruccio AV, Power JD, Badley EM. Arthritis onset and worsening self-rated health: a longitudinal evaluation of the role of pain and activity limitations. Arthritis Care Res. 2005;53:571–577. doi: 10.1002/art.21317. [DOI] [PubMed] [Google Scholar]
- 41.Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity. 2007;15:2371–2379. doi: 10.1038/oby.2007.281. [DOI] [PubMed] [Google Scholar]
- 42.Pols MA, Peeters PHM, Bueno De-Mesquita HB, Ocke MC, Wentink CA, Kemper HCG, Collette HJA. Validity and repeatability of a modified Baecke questionnaire on physical activity. Int J Epidemiol. 1995;24:381–388. doi: 10.1093/ije/24.2.381. [DOI] [PubMed] [Google Scholar]
- 43.Rasmussen-Barr E, Lundqvist L, Nilsson-Wikmar L, Ljungquist T. Aerobic fitness in patients at work despite recurrent low back pain: a cross-sectional study with healthy age- and gender-matched controls. J Rehabil Med. 2008;40:359–365. doi: 10.2340/16501977-0176. [DOI] [PubMed] [Google Scholar]
- 44.Refshauge KM, Maher CG. Low back pain investigations and prognosis: a review. Br J Sports Med. 2006;40:494–498. doi: 10.1136/bjsm.2004.016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smeets RJEM, Wittink H. The deconditioning paradigm for chronic low back pain unmasked? Pain. 2007;130:201–202. doi: 10.1016/j.pain.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 46.Smeets RJE, Wittink H, Hidding A, Knottnerus JA. Do patients with chronic low back pain have a lower level of aerobic fitness than healthy controls? Are pain, disability, fear of injury, working status, or level of leisure time activity associated with the difference in aerobic fitness level? Spine. 2006;31:90–98. doi: 10.1097/01.brs.0000192641.22003.83. [DOI] [PubMed] [Google Scholar]
- 47.Smeets RJ, Geel KD, Verbunt JA. Is the fear avoidance model associated with the reduced level of aerobic fitness in patients with chronic low back pain? Arch Phys Med Rehabil. 2009;90:109–117. doi: 10.1016/j.apmr.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Smith MD, Russell A, Hodges PW. Disorders of breathing and continence have a stronger association with back pain than obesity and physical activity. Aust J Physiother. 2006;52:11–16. doi: 10.1016/s0004-9514(06)70057-5. [DOI] [PubMed] [Google Scholar]
- 49.Steenstra IA, Verbeek JH, Heymans MW, Bongers PM. Prognostic factors for duration of sick leave in patients sick listed with acute low back pain: a systematic review of the literature. Occup Environ Med. 2005;62:851–860. doi: 10.1136/oem.2004.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steenstra IA, Anema JR, Bongers PM, Vet HC, Knol DL, Mechelen W. The effectiveness of graded activity for low back pain in occupational healthcare. Occup Environ Med. 2006;63:718–725. doi: 10.1136/oem.2005.021675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Storheim K, Ivar Brox J, Holm I, Bø K. Predictors of return to work in patients sick listed for sub-acute low back pain: a 12-month follow-up study. J Rehabil Med. 2005;37:365–371. doi: 10.1080/16501970510040344. [DOI] [PubMed] [Google Scholar]
- 52.Berg-Emons RJ, Schasfoort FC, Vos LA, Bussmann JB, Stam HJ. Impact of chronic pain on everyday physical activity. Eur J Pain. 2007;11:587–593. doi: 10.1016/j.ejpain.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Tulder M, Malmivaara A, Esmail R, Koes B. Exercise therapy for low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine. 2000;25:2784–2796. doi: 10.1097/00007632-200011010-00011. [DOI] [PubMed] [Google Scholar]
- 54.Tulder M, Becker A, Bekkering T, Breen A, Del Real MTG, Hutchinson A, Koes B, Laerum E, Malmivaara A. Chapter 3: European guidelines for the management of acute nonspecific low back pain in primary care. Eur Spine J. 2006;15:S169–S191. doi: 10.1007/s00586-006-1071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weering MGH, Vollenbroek-Hutten MMR, Tonis TM, Hermens HJ. Daily physical activities in chronic lower back pain patients assessed with accelerometry. Eur J Pain. 2008;13:649–654. doi: 10.1016/j.ejpain.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Verbunt JA, Westerterp KR, Heijden GJ, Seelen HA, Vlaeyen JW, Knottnerus JA. Physical activity in daily life in patients with chronic low back pain. Arch Phys Med Rehabil. 2001;82:726–730. doi: 10.1053/apmr.2001.23182. [DOI] [PubMed] [Google Scholar]
- 57.Verbunt JA, Sieben JM, Seelen HA, Vlaeyen JW, Bousema EJ, Heijden GJ, Knottnerus JA. Decline in physical activity, disability and pain-related fear in sub-acute low back pain. Eur J Pain. 2005;9:417–425. doi: 10.1016/j.ejpain.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 58.Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. Can Med Assoc J. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wessels T, Tulder M, Sigl T, Ewert T, Limm H, Stucki G. What predicts outcome in non-operative treatments of chronic low back pain? A systematic review. Eur Spine J. 2006;15:1633–1644. doi: 10.1007/s00586-006-0073-4. [DOI] [PubMed] [Google Scholar]
- 60.Wittink H, Hoskins Michel T, Wagner A, Sukiennik A, Rogers W. Deconditioning in patients with chronic low back pain: fact or fiction? Spine. 2000;25:2221–2228. doi: 10.1097/00007632-200009010-00013. [DOI] [PubMed] [Google Scholar]