Abstract
The objective is to determine if pain and disability outcomes of patients treated with neural mobilisation differ for sub-classifications of low back and leg pain (LB&LP). Radiating leg pain is a poor prognostic factor for recovery in patients with LBP. To improve outcome, a new pathomechanism-based classification system was proposed: neuropathic sensitization (NS), denervation (D), peripheral nerve sensitization (PNS) and musculoskeletal (M). Seventy-seven patients with unilateral LB&LP were recruited. Following classification, all subjects were treated seven times with neural mobilisation techniques. A successful outcome was defined as achieving a minimal clinically important change in pain intensity (11-point numerical rating scale), physical function (Roland Morris disability questionnaire) and global perceived change (7-point Likert scale: from 1 = “completely recovered” to 7 = “worse than ever”). The proportion of responders was significantly greater in PNS (55.6%) than the other three groups (NS 10%; D 14.3% and M10%). After adjusting for baseline differences, mean magnitude of improvement of the outcome measures were significantly greater in PNS compared to the other groups. Patients classified as PNS have a more favourable prognosis following neural mobilisation compared to the other groups.
Keywords: Low back pain, Sciatica, Classification, Musculoskeletal manipulations, Diagnosis
Introduction
Leg pain is a common complaint in relation to low back pain (LBP), present in up to 65% of all patients with LBP [1]. Radiating leg pain is an important predictor of chronicity of LBP and an indicator of the severity of the disorder [2], consequently patients with back and leg pain account for a disproportionate amount of the costs of medical care and disability compensation caused by LBP [3].
Despite the array of randomised controlled studies investigating the effectiveness of various conservative treatments for LBP with or without leg pain, consensus regarding the effectiveness of many interventions has not been attained. Heterogeneity among study samples is one reason why randomised controlled trials often report conflicting results [4]. It follows that identifying more homogenous subgroups of patients would enable targeting each subgroup with the intervention most likely to be effective. Evidence of the validity of this concept is emerging, as studies are demonstrating that classification-based treatment is more effective than non-classification management [5].
There are numerous published classification systems for LBP. The majority are founded on biomedical classification paradigms, most frequently based on pathoanatomical rational for symptoms or simply on clusters of signs and symptoms without presumption of their relationship to pathoanatomical causes [6]. Although patients with back and leg pain are included in a number of these classification systems, none focus on patients who have pain that extends beyond the gluteal fold. There are two notable reliable classification systems that include patients with leg pain [7, 8] with established predictive validity [5]. However, these validation studies did not address validity in subjects with leg pain. Brennan et al. [9] demonstrated improved outcomes when treatment was matched to subjects’ subgroups, defined as manipulation, stabilization and specific exercise according to criteria based on those proposed by Delitto et al. [7]. As patients with signs of nerve root compression were excluded from this study, no conclusions can be drawn about this group of patients. The effectiveness of McKenzie exercises based on directional preference has been examined in a well designed RCT that included subjects with leg pain. However, the presence or absence of leg pain was not a selection criterion and subjects with more than one neurological sign were excluded [10]. Consequently the results of these studies cannot be generalised to patients with leg pain with or without neurological signs.
Our classification system expands on the existing classification systems by focussing on neural-related leg pain while recognising that leg pain can also radiate from somatic structures. For patients with complex pain conditions related to nerve injury, it has been suggested that a mechanism based classification system may be most appropriate, as it provides a better understanding of the presenting complaint, along with direction for treatments which interact with specific neurophysiologic pain mechanisms [11]. Ours is the first classification system to apply this approach to low back and leg pain (LB&LP), taking into account central and peripheral pain mechanisms. We employed a judgemental method based on the clinical experience of the authors as well as a review of the relevant observational and experimental research literature to develop the classification criteria. The rationale for the classification system and the corresponding signs and symptoms for each of the groups has been presented in detail previously [12].
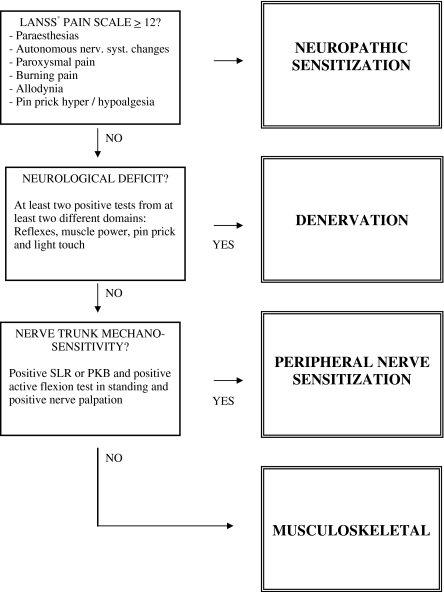
Patients were classified according to the findings of simple clinical tests into four distinct categories: (1) neuropathic sensitization (NS) comprising major features of neuropathic pain with sensory sensitization such as allodynia, hyperalgesia and paroxysmal pain; (2) denervation (D) arising from significant axonal compromise with marked sensory and motor deficits; (3) peripheral nerve sensitization (PNS) arising from nerve trunk inflammation with marked nerve mechanosensitivity; and (4) musculoskeletal (M) with pain referred from non-neural structures such as the disc or facet joints (Fig. 1). The classification system is hierarchical and mutual exclusive, that is, patients who fit the criteria for the first category are assigned to that category regardless of whether they meet the criteria for one of the categories further down the hierarchy (Fig. 1). Inter-rater reliability of the newly developed classification system is good (K = 0.71) [13].
Fig. 1.
Classification algorithm. *LANSS Leeds Assessment of Neuropathic Symptoms and Signs [14]. SLR straight leg raise test, PKBprone knee bend test
The objective of the present study was to commence evaluation of the predictive ability of the classification system, initially focusing on the PNS group. We hypothesize that the proportion of patients with a favourable response to treatment will be greater in the PNS group than the other diagnostic groups.
Materials and methods
Participants
Participants were recruited at a multidisciplinary pain clinic from consecutive patients referred for physiotherapy by medical doctors, who specialized in treatment of musculoskeletal pain disorders in Hamburg, Germany. Patients with LB&LP were informed about the study by administrative staff in the Physiotherapy Department. Those interested in participating were screened for eligibility by one of the research physiotherapists. To be considered for inclusion, volunteers were required to have unilateral LBP and leg pain radiating below the gluteal fold for more than 6 weeks duration and a minimum pain intensity of three on the 11-point numerical rating scale. Patients were excluded if they were <18 or >75 years of age or fulfilled any of the following criteria: (1) history of lower quadrant surgery or trauma within the past 6 months, (2) nerve root block within the past 4 weeks, (3) history of neuropathic pathology such as diabetes or polyneuropathies, (3) history of vascular disease in the lower extremities, (4) systemic disease, (5) inflammatory arthropathies, and (6) contraindications to manual therapy techniques such as trauma, infection or severe osteoporosis.
This study was approved by the Human Research Ethics Committee, Curtin University of Technology, Perth, Western Australia, in accordance with the Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects. All participants provided written informed consent.
Procedure
Classification, treatment and measurement of outcomes were conducted by eight experienced physiotherapists with postgraduate education in manual therapy. Three physiotherapists were involved in the assessment and treatment of each patient. One physiotherapist examined the patients at entry to the study and determined their classification. A second physiotherapist provided treatment to the patient and a third was responsible for post-treatment examination. To maintain blinding, patient classification was concealed from the treating physiotherapist, the patient and the physiotherapist examining the patient post-treatment.
Classification
Subjects were classified into one of four groups following a standardized examination protocol [13]. In brief, the protocol incorporated the interview version of the LANSS questionnaire [14] to screen for positive symptoms and signs indicative of neuropathic pain with sensory sensitization. The physical examination consisted of a standard neurological examination plus nerve provocation tests including the active flexion test in standing, the straight leg raise test and nerve trunk palpation along the course of the femoral and sciatic nerves. Subjects with a score of 12 or higher on the LANSS scale [14] were classified as NS. When there was no evidence of NS, but at least two negative sensory and/or motor signs such as hypoesthesia, muscle weakness or hyporeflexia were present, categorization of D was made. Of the remaining subjects those with positive nerve provocation tests were classified as PNS. Subjects with no suggestion of neural involvement were classified as M (Fig. 1) [13].
Intervention
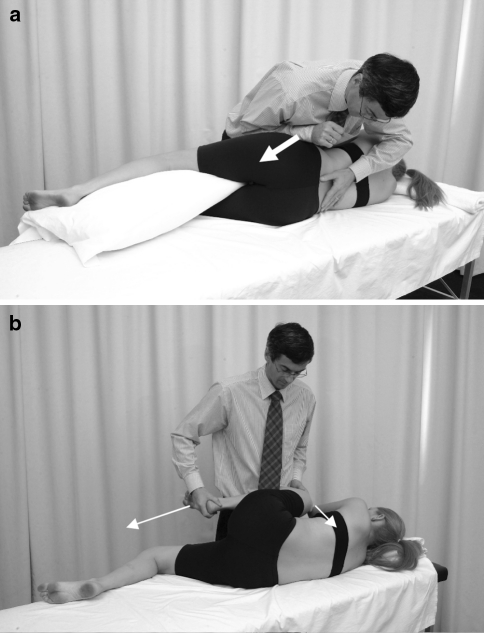
All patients received seven neural mobilisation interventions two times per week that incorporated two passive mobilisation techniques [15] (Fig. 2):
Lumbar lateral flexion in side lying contralateral to the painful side at the affected level for 60 s at a frequency of 1 Hz. Lateral flexion has been shown to increase the size of the intervertebral foramen [16] and is believed to positively influence neural structures [15].
Hip and knee flexion followed by hip and knee extension for 30 s at a frequency of 0.5 Hz (Fig. 2a, b). Both procedures were repeated five times and patients were instructed to conduct progressive neural mobilisation home exercises. Nerve excursion of 6–7 mm during hip and knee extension has been demonstrated in early cadaveric studies [17]. Exercises or treatment techniques using these movements may therefore decrease venous congestion and endoneurial pressure [18].
Fig. 2.
Treatment technique aimed at mobilising neural structures in the intervertebral foramen. a Foraminal opening technique induced by ipsilateral lateral flexion, white arrow indicates direction of force, left thumb fixes the upper spinous process of the affected segment. b Sliding technique for the sciatic nerve incorporating hip and knee flexion followed by hip and knee extension, white arrows indicate directions of movement
This approach to treatment is hypothesized to gradually desensitise the overly sensitive peripheral nervous system [15].
At no point were symptoms provoked. In the event of an exacerbation of symptoms, the treatment programme was discontinued, but patients were encouraged to attend follow-up assessment and to refrain from seeking other forms of treatment until after the final follow-up assessment.
Measurements
The primary outcome for this study was a positive response to treatment, defined as achieving a minimal clinically important change (MCIC) in pain, disability and patient global perceived change. These three domains are considered core criteria for combination to present the results of changes after treatment as a single outcome variable for clinical trials [19].
Pain intensity was measured using an 11-point numerical rating scale (NRS). A minimal clinically important change (MCIC) on the NRS was defined as a reduction of 2.5 points [20]. Functional disability was measured with the Roland Morris Disability Questionnaire (RMDQ). An improvement of 30% was defined as MCIC for the RMDQ [20]. Overall improvement was measured using the Global Perceived Change Scale (GPC) where patients score their perceived change after the treatment on a 7-point scale ranging from: 1 (completely recovered) to 7 (worse than ever) [20]. A self-reported change of 2 (much improved) or 1 (completely recovered) on the GPC was deemed to be a successful response to treatment [20].
The patient’s gender, age, and duration and location of symptoms were recorded. Potential psychological covariates were measured using the Hospital Anxiety and Depression Scale (HADS) and the Fear Avoidance and Beliefs Questionnaire (FABQ). These questionnaires were self-completed prior to clinical examination.
Data analysis
Data were analysed comparing proportions of positive responders to nerve mobilisation across all classifications using Fishers exact test. Where significant differences in proportions of responders were identified, post hoc pair-wise comparisons were conducted with Fishers exact test to specify where differences lay.
In addition, to control for significant baseline differences (p < 0.1) between groups (Table 1), main outcome measures were analysed by implementing univariate analysis of covariance (ANCOVA). Dependent variables were GPC and difference scores from pre to post-treatment for NRS and RMDQ. The independent variable was group (four levels). Baseline scores for NRS, RMDQ and HADS-A were entered into the model as covariates and were eliminated if not significant (p > 0.1). In this way, only statistically significant covariates were selected and controlled for. For significant between group differences, post hoc pair-wise comparisons were performed with the least significant difference (LSD) procedure. All statistical analyses were performed using SPSS software, version 13.0 for MacOS 10 (SPSS Inc., Chicago, Illinois).
Table 1.
Characteristics of the study subjects at baseline
| Total | NS | D | PNS | M | p | |
|---|---|---|---|---|---|---|
| N | 74 | 19 | 27 | 9 | 19 | |
| Age | 47.8 (13.1) | 47.5 (13.4) | 48.2 (12.2) | 44.3 (14.0) | 49.2 (14.2) | 0.83* |
| Gender (% male) | 40 | 35 | 39 | 41 | 45 | 0.92a |
| Pain below knee (%) | 76.3 | 80.0 | 71.4 | 88.9 | 73.7 | 0.71a |
| Pain duration current episode (months) | 7.5 (15.5) | 7 (18.4) | 7.3 (11.3) | 6.0 (12.5) | 10.6 (12.2) | 0.76b |
| Pain (NRS) | 5.0 (1.6) | 5.8 (1.7)c | 4.6 (1.5)c | 5.3 (1.7) | 4.6 (1.4)c | 0.031* |
| Disability (RMDQ) | 8.0 (4.4) | 10.5 (4.0)c | 7.1 (4.8)c | 8.7 (4.5) | 6.5 (3.3)c | 0.014* |
| Fear avoidance (FABQ) | 34.7 (19.1) | 39.1 (19.1) | 34.3 (19.0) | 36.4 (18.8) | 29.8 (21.2) | 0.51* |
| Anxiety (HADS-A) | 6.8 (4.1) | 9.1 (4.6)d | 5.6 (3.6)d | 4.9 (2.5)d | 7.2 (4.0) | 0.013* |
| Depression (HADS-D) | 5.3 (3.6) | 6.5 (3.5) | 4.5 (3.8) | 5.0 (3.2) | 5.3 (3.6) | 0.37* |
| Number of treatments | 6.7 (1.6) | 6.6 (1.1) | 7 (0) | 6.7 (0.9) | 0.93* | |
| Time to follow-up (days) | 25 (14) | 33 (20) | 28 (19) | 28 (18) | 0.21b |
NS neuropathic sensitization, D denervation, PNS peripheral nerve sensitization, M musculoskeletal, NRS numerical rating scale, RMDQ Roland Morris Disability Questionnaire, FABQ Fear Avoidance and Beliefs Questionnaire, HADS Hospital Anxiety and Depression Scale–Anxiety/Depression subscales
* One way ANOVA; values presented are means (standard deviations)
aχ2 test; data are percentage
bKruskall–Wallis test (distribution was not normal); data are median (interquartile range)
cSignificant pair-wise differences (LSD post hoc comparisons) between groups C/D and C/M
dSignificant pair-wise differences (LSD post hoc comparisons) between groups C/D and C/PNS
Sample size
The sample size estimates were based on a power of 0.8 and a p value of 0.05 for χ2 analysis. Based on anecdotal experience, we assumed that 30% of subjects would be classified as PNS and that 60% of the PNS group would positively respond to treatment, whereas only 25% in the other groups would respond. Accordingly, 77 patients were needed, including 5% patients lost to follow-up, to test the hypothesis that a greater proportion of patients from the peripheral sensitisation group will improve in response to neural mobilisation.
Results
Subjects
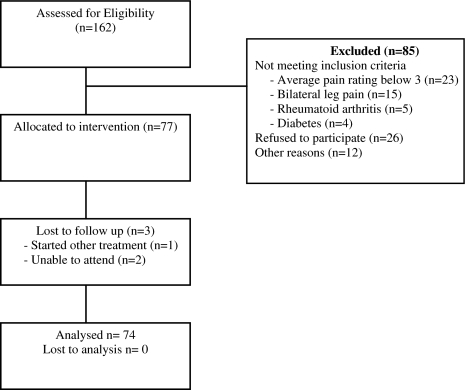
In order to recruit the calculated sample size of 77 subjects, 162 consecutive subjects were screened for eligibility (Fig. 3). The proportion of patients in each classification was 26% NS, 36% D, 12% PNS and 26% M (Table 1). Three patients were lost to follow-up, one sought other forms of treatment and two were unable to attend post-treatment measurements.
Fig. 3.
Flow diagram
Groups were comparable in most baseline parameters (Table 1). The PNS group did not differ from any of the other groups in pain, disability, fear avoidance or depression. However, group NS had a significantly higher pain intensity than group D (mean difference MD = 1.2; 95% CI 0.3, 2.1) and M (MD = 1.2; 95% CI 0.2, 2.2), significantly higher disability than group D (RMDQ MD = 3.5; 95% CI 1.0, 5.9) and M (RMDQ MD = 4.1; 95% CI 1.4, 6.7) as well as significantly higher anxiety than group D (HADS-A MD = 3.4; 95% CI 1.1, 5.7) and PNS (HADS-A MD = 4.1; 95% CI 1.1, 7.3) at baseline (Table 1).
Response following intervention
The proportion of responders was significantly greater in group PNS (56%) than in any of the other three groups (NS 11%, p = 0.02; D 15%, p = 0.026 and M 11%, p = 0.016). There were no differences in response rates between the NS, D and M groups (p > 0.48) (Table 2). A similar pattern was seen when proportions of patients achieving an MCIC for each of the outcomes were considered individually. Fishers exact test revealed significant differences between groups for GPC (p = 0.005) and a strong trend for significance for pain intensity (p = 0.057). However, for functional disability the difference was not statistically significant (p = 0.104) (Table 2).
Table 2.
Proportion of subjects from each group that attain the minimal clinically important change (MCIC) in each and all three of the outcome measures
| MCIC | NS (n = 19) | D (n = 27) | PNS (n = 9) | M (n = 19) | χ2 b | p valueb |
|---|---|---|---|---|---|---|
| Pain NRS | 11% (n = 2) | 15% (n = 4) | 56% (n = 5) | 16% (n = 3) | 7.29 | 0.057 |
| GPC | 16% (n = 3) | 33% (n = 9) | 78% (n = 7) | 16% (n = 3) | 12.20 | 0.005 |
| RMDQ | 67% (n = 12)a | 67% (n = 18) | 89% (n = 8) | 42% (n = 8) | 6.10 | 0.104 |
| Responder | 11% (n = 2) | 15% (n = 4) | 56% (n = 5) | 11% (n = 2) | 7.98 | 0.031c |
NS central sensitization, D denervation, PNS peripheral nerve sensitization, M musculoskeletal, NRS numerical rating scale, GPC global perceived change, RMDQ Roland Morris Disability Questionnaire, Responder achieved MCIC in all three of the outcome measures
aMissing data n = 1
bFishers exact test values and p values
cp values for pair-wise comparisons (Fisher’s exact test): NS-PNS: 0.02; D-PNS: 0.026; M-PNS: 0.016. All other pair-wise comparisons were not significant with p > 0.48
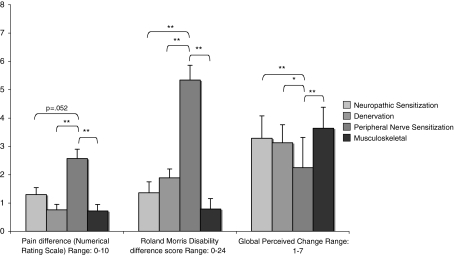
Significant baseline variables initially added to the ANCOVA model were intensity of pain, functional disability and anxiety. As the main effect for anxiety was not significant (p > 0.1), this covariate was eliminated from the model. After correction for functional disability and intensity of pain at baseline, there were significant differences between groups in pain intensity, disability and GPC (Table 3). Post hoc pair-wise comparisons with LSD tests revealed that GPC and reduction in functional disability were greater in the PNS group than all of the other groups (p ≤ 0.015). Reduction in the intensity of pain was also greatest in the PNS group (p ≤ 0.02); however, the difference did not achieve significance for the comparison with NS (p = 0.052) (Fig. 4). For mean reductions, 95% CI, F and p values, please see Table 3.
Table 3.
Final scores (mean and SD) and change scores (mean and 95% confidence interval) for the main outcome variables
| NS | D | PNS | M | F | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Final score (SD) | Change score (95% CI) | Final score (SD) | Change score (95% CI) | Final score (SD) | Change score (95% CI) | Final score (SD) | Change score (95% CI) | |||
| Pain 0–10 | 4.6 (2.4) | 1.30 (0.53, 2.07) | 3.8 (2.4) | 0.76 (0.13, 1.38) | 2.8 (2.1) | 2.57 (1.52, 3.63)a | 3.8 (2.1) | 0.72 (−0.11, 1.45) | 3.3 | 0.025 |
| RMDQ 0–24 | 8.7 (4.5) | 1.36 (−0.21, 2.92) | 5.4 (5.1) | 1.89 (0.62, 3.16) | 3.4 (3.0) | 5.34 (3.21, 7.48)b | 6.1 (4.5) | 0.79 (−0.71, 2.26) | 4.4 | 0.007 |
| GPC 1–7 | 3.29 (2.80, 3.77) | 3.13 (2.74, 3.52) | 2.25 (1.59, 2.91)c | 3.64 (3.18, 4.10) | 4.1 | 0.01 | ||||
Change scores of pain (numerical rating scale 1–10), disability (Roland Morris Disability Questionnaire 0–24) and patient perceived global change GPC (1 = completely recovered to 7 = worse than ever) are estimated marginal means adjusted for baseline pain intensity and disability. F and p values are from univariate analysis of covariance (ANCOVA)
NS neuropathic sensitization, D denervation, PNS peripheral nerve sensitization, M musculoskeletal
ap values for pair-wise comparisons (LSD): NS-PNS: 0.053; D-PNS: 0.002; PNS-M: 0.001; b p values for pair-wise comparisons (LSD): NS-PNS: 0.003; D-PNS: 0.007; PNS-M: 0.001; cp values for pair-wise comparisons (LSD): NS-PNS: 0.004; D-PNS: 0.015; PNS-M: < 0.001; all other pair-wise comparisons were not significant with p > 0.21
Fig. 4.
Patient perceived global change (Global Perceived Change scale) and improvement from baseline in pain and disability. Data are estimated marginal means (error bars represent standard error of measurement) adjusted for baseline pain intensity (Numerical Rating Scale) and disability (Roland Morris Disability Questionnaire). *p < 0.05; **p < 0.01 of pair-wise comparisons with post hoc LSD tests. Lower numbers for GPC indicate a more favourable outcome (1 = completely recovered to 7 = worse than ever)
Discussion
A greater proportion of subjects with LB&LP classified as PNS responded favourably following a specific neural mobilisation programme. These findings indicate that among patients receiving a specific neural mobilisation programme the new classification system identified a subgroup of patients, PNS, with a more favourable prognosis. Although a single-arm study cannot provide evidence for the prediction of treatment effects, the results appear plausible as response to treatment is consistent with presumed mechanisms of neural mobilisation treatment and presumed pathomechanisms in each of the groups.
Only 15% of patients classified as D had a favourable outcome following neural mobilisation interventions. This is consistent with expectations and the findings of previous studies [21, 22]. Denervation may result from compromise of nerve microcirculation. A minimal stretch of perturbed nerve tissue may add further hypoxia and concomitant nerve damage [23]. It seems therefore reasonable to propose that if a nerve root were compressed, hypoxic and oedematous, using a mobilisation technique implying further tensile stress would have a detrimental effect. Also in chronic cases with long-term damage to the nervous system such as scar formation, endoneurial fibrosis and Wallerian degeneration [24], it is unlikely that any form of nerve mobilisation will be of benefit. Instead, if a nerve root is acutely compressed and oedematous, interventions (such as traction), aimed at unloading nerve structures to promote resolution of the effects of compression could be helpful [25].
In group NS only 11% of patients had a favourable outcome following the intervention period. It is not surprising that neural mobilisation treatment alone presumably targeting peripheral mechanisms at the nerve root and surrounding structures seemed ineffective for the majority of patients classified as NS. In this group responsible mechanisms are likely to be multiple and located at a number of sites, possibly requiring a combination of different drugs acting through different targets for adequate management [26].
In Group M the pain experienced by patients was likely to have been referred from a variety of musculoskeletal structures. Therefore, as a group, they would not be expected to respond to specific neural mobilisation. In the present study, only 11% of patients in group M were responders. These patients would potentially benefit from a treatment-based classification system [9] to select the most appropriate management targeting distinct symptomatic structures.
Moderate to low level evidence from clinical trials and case studies indicates that neural mobilisation has beneficial effects in patients with evidence of PNS in the lumbar spine [27–29] and in the cervical spine [30–33]. Patients included in these studies were comparable to the subjects in the present study: all had radiating pain in the extremities and positive nerve provocation tests in the absence of neurological deficits. However, treatment effects found in two controlled trials were low [27, 30]. Also, a recent systematic review [34] of the therapeutic efficacy of neural mobilisation for a variety of conditions concludes that there is limited evidence to support its use. One reason for low treatment effects and inconsistent results could be heterogeneity in patient samples. For example, in none of the above-mentioned studies screening for signs and symptoms indicative of neuropathic pain mechanisms was carried out. As physical treatment modalities are unlikely to have a beneficial effect in patients with neuropathic pain (which present a substantial proportion in patients with LBP [1]), a diluted estimate of treatment effects is likely. By classifying patients in defined, pathomechanism-based categories, homogenous samples of patients with a higher likelihood of positive response to neural mobilisation could be achieved. The use of the classification system [12] could therefore lead to more consistent results in future trials and could help to improve effectiveness of neural mobilisation interventions in clinical practice.
However, several limitations of the present study should be pointed out. First of all, the lack of a control group precludes conclusions being drawn regarding the effect of treatment. Other factors, such as a possible greater rate of natural recovery in group PNS, could have contributed to the more favourable response. Also, the sample size in each of the groups was relatively small; consequently, the power to detect statistically significant baseline differences (Table 1) was low. Furthermore, a high percentage (>50%) of patients were not eligible for inclusion (Fig. 3) limiting the external validity of the results to patients fulfilling the inclusion and exclusion criteria. As subjects were recruited at a multidisciplinary pain clinic, generalizability of the results to a primary care setting may be compromised. Additionally, the lack of long-term follow-up limits the prognostic ability of the classification system to a confined period of time immediately after the treatment period.
This study demonstrated that a clinical classification system can differentiate a group of patients with a more favourable outcome following an intervention targeting PNS, that is evidence as a prognostic factor for that subgroup alone. Further research is needed to provide prognostic evidence also for the other subgroups. Ultimately, an RCT should be conducted to establish predictive validity of the classification system. For this it is critical to have appropriate selection criteria so that a reproducibly homogenous group of subjects likely to respond can be recruited. Our study provides evidence for such selection criteria as well as information on response rates and proportions of subjects in each of the groups. Such information is necessary to conduct power calculations for future trials. The present study is a starting point for the evolvement and refinement of the classification system for LB&LP, as other classification systems have evolved as well [35]. After providing evidence for feasibility, reliability and plausibility [12, 13], content and construct validity of the classification system still need to be demonstrated.
References
- 1.Freynhagen R, Baron R, Gockel U, Tölle TR. Screening of neuropathic pain components in patients with chronic back pain associated with nerve compression: a prospective observational study (MIPORT) Curr Med Res Opin. 2006;22:529–537. doi: 10.1185/030079906X89874. [DOI] [PubMed] [Google Scholar]
- 2.Grotle M, Brox JI, Glomsrod B, Lonn JH, Vollestad NK. Prognostic factors in first-time care seekers due to acute low back pain. Eur J Pain. 2007;11:290–298. doi: 10.1016/j.ejpain.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Ren XS, Selim AJ, Fincke G, Deyo RA, Linzer M, Lee A, et al. Assessment of functional status, low back disability, and use of diagnostic imaging in patients with low back pain and radiating leg pain. J Clin Epidemiol. 1999;52:1063–1071. doi: 10.1016/S0895-4356(99)00094-3. [DOI] [PubMed] [Google Scholar]
- 4.Luijsterburg PA, Verhagen AP, Ostelo RW, Os TA, Peul WC, Koes BW. Effectiveness of conservative treatments for the lumbosacral radicular syndrome: a systematic review. Eur Spine J. 2007;16:881–899. doi: 10.1007/s00586-007-0367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kent P, Mjosund HL, Petersen DH. Does targeting manual therapy and/or exercise improve patient outcomes in nonspecific low back pain? A systematic review. BMC Med. 2010;8:22. doi: 10.1186/1741-7015-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billis EV, McCarthy CJ, Oldham JA. Subclassification of low back pain: a cross-country comparison. Eur Spine J. 2007;16:865–879. doi: 10.1007/s00586-007-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delitto A, Erhard RE, Bowling RW. A treatment-based classification approach to low back syndrome: identifying and staging patients for conservative treatment. Phys Ther. 1995;75:470–485. doi: 10.1093/ptj/75.6.470. [DOI] [PubMed] [Google Scholar]
- 8.McKenzie RA (1981) The lumbar spine: mechanical diagnosis and therapy. Spinal Publications, Waikanae, N.Z
- 9.Brennan GP, Fritz JM, Hunter SJ, Thackeray A, Delitto A, Erhard RE. Identifying subgroups of patients with acute/subacute “nonspecific” low back pain: results of a randomized clinical trial. Spine. 2006;31:623–631. doi: 10.1097/01.brs.0000202807.72292.a8. [DOI] [PubMed] [Google Scholar]
- 10.Long A, Donelson R, Fung T. Does it matter which exercise? A randomized control trial of exercise for low back pain. Spine. 2004;29:2593–2602. doi: 10.1097/01.brs.0000146464.23007.2a. [DOI] [PubMed] [Google Scholar]
- 11.Woolf CJ, Bennett GJ, Doherty M, Dubner R, Kidd B, Koltzenburg M, et al. Towards a mechanism-based classification of pain? Pain. 1998;77:227–229. doi: 10.1016/S0304-3959(98)00099-2. [DOI] [PubMed] [Google Scholar]
- 12.Schäfer A, Hall T, Briffa K. Classification of low back-related leg pain—a proposed patho-mechanism-based approach. Man Ther. 2009;14:222–230. doi: 10.1016/j.math.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Schäfer A, Hall T, Lüdtke K, Mallwitz J, Briffa K. Interrater reliability of a new classification system for patients with low back related leg pain. J Man Manip Ther. 2009;17:109–117. doi: 10.1179/106698109790824730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92:147–157. doi: 10.1016/S0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 15.Hall TM, Elvey RL. Management of mechanosensitivity of the nervous system in spinal pain syndromes. In: Boyling JD, Jull G, editors. Grieves modern manual therapy. 3. Edinburgh: Churchill Livingstone; 2004. pp. 413–433. [Google Scholar]
- 16.Panjabi MM, Takata K, Goel VK. Kinematics of lumbar intervertebral foramen. Spine. 1983;8:348–357. doi: 10.1097/00007632-198305000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Breig A, Troup JDG. Biomechanical considerations in the straight-leg-raising test cadaveric and clinical studies of the effects of medial hip rotation. Spine. 1979;4:242–250. doi: 10.1097/00007632-197905000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Coppieters MW, Butler DS. Do ‘sliders’ slide and ‘tensioners’ tension? An analysis of neurodynamic techniques and considerations regarding their application. Man Ther. 2008;13:213–221. doi: 10.1016/j.math.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Pham T, Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthr Cartil. 2004;12:389–399. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Ostelo RW, Vet HC. Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol. 2005;19:593–607. doi: 10.1016/j.berh.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Cibulka MT, Aslin K. How to use evidence-based practice to distinguish between three different patients with low back pain. J Orth Sports Phys Ther. 2001;31:678–688. doi: 10.2519/jospt.2001.31.12.678. [DOI] [PubMed] [Google Scholar]
- 22.Kitteringham C. The effect of straight leg raise exercises after decompression surgery—a pilot study. Physiotherapy. 1996;82:115–123. doi: 10.1016/S0031-9406(05)66966-0. [DOI] [Google Scholar]
- 23.Kwan MK, Wall EJ, Massie J, Garfin SR. Strain, stress and stretch of peripheral nerve. Rabbit experiments in vitro and in vivo. Acta Orthop Scand. 1992;63:267–272. doi: 10.3109/17453679209154780. [DOI] [PubMed] [Google Scholar]
- 24.Yoshizawa H, Kobayashi S, Morita T. Chronic nerve root compression. Pathophysiologic mechanism of nerve root dysfunction. Spine. 1995;20:397–407. doi: 10.1097/00007632-199502001-00001. [DOI] [PubMed] [Google Scholar]
- 25.Fritz JM, Lindsay W, Matheson JW, Brennan GP, Hunter SJ, Moffit SD, et al. Is there a subgroup of patients with low back pain likely to benefit from mechanical traction? Results of a randomized clinical trial and subgrouping analysis. Spine. 2007;32:E793–E800. doi: 10.1097/BRS.0b013e31815d001a. [DOI] [PubMed] [Google Scholar]
- 26.Dickenson AH, Bee LA (2008) Neurobiological mechanisms of neuropathic pain and its treatment. In: Castro-Lopes J, Raja S, Schmelz M (eds) Pain 2008: an updated review. IASP press, Seattle, pp 277–286
- 27.Cleland JA, Childs JD, Palmer JA, Eberhart S. Slump stretching in the management of non-radicular low back pain: a pilot clinical trial. Man Ther. 2006;11:279–286. doi: 10.1016/j.math.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Cleland JA, Hunt GC, Palmer JA. Effectiveness of neural mobilization in the treatment of a patient with lower extremity neurogenic pain: a single case design. J Manual Manipul Ther. 2004;12:143–152. doi: 10.1179/106698104790825211. [DOI] [Google Scholar]
- 29.George SZ. Characteristics of patients with lower extremity symptoms treated with slump stretching: a case series. J Orthop Sports Phys Ther. 2002;32:391–398. doi: 10.2519/jospt.2002.32.8.391. [DOI] [PubMed] [Google Scholar]
- 30.Allison GT, Nagy BM, Hall T. A randomized clinical trial of manual therapy for cervico-brachial pain syndrome—a pilot study. Man Ther. 2002;7:95–102. doi: 10.1054/math.2002.0453. [DOI] [PubMed] [Google Scholar]
- 31.Coppieters MW, Stappaerts KH, Wouters LL, Janssens K. The immediate effects of a cervical lateral glide treatment technique in patients with neurogenic cervicobrachial pain. J Orthop Sports Phys Ther. 2003;33:369–378. doi: 10.2519/jospt.2003.33.7.369. [DOI] [PubMed] [Google Scholar]
- 32.Cowell IM, Phillips DR. Effectiveness of manipulative physiotherapy for the treatment of a neurogenic cervicobrachial pain syndrome: a single case study—experimental design. Man Ther. 2002;7:31–38. doi: 10.1054/math.2001.0429. [DOI] [PubMed] [Google Scholar]
- 33.Hall T, Elvey R, Davies N, Dutton L, Moog M (1997) Efficacy of manipulative physiotherapy for the treatment of cervicobrachial pain. In: 10th Manipulative physiotherapists association of Australia conference, Melbourne, pp 73–74
- 34.Ellis RF. Neural mobilization: a systematic review of randomized controlled trials with an analysis of therapeutic efficacy. J Man Manip Ther. 2008;16:8. doi: 10.1179/106698108790818594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fritz JM, Cleland JA, Childs JD. Subgrouping patients with low back pain: evolution of a classification approach to physical therapy. J Orthop Sports Phys Ther. 2007;37:290–302. doi: 10.2519/jospt.2007.2498. [DOI] [PubMed] [Google Scholar]