Abstract
Over the past 20 years, the center of pressure (COP) has been commonly used as an index of postural stability in standing. While many studies investigated COP excursions in low back pain patients and healthy individuals, no comprehensive analysis of the reported differences in postural sway pattern exists. Six online databases were systematically searched followed by a manual search of the retrieved papers. The selection criteria comprised papers comparing COP measures derived from bipedal static task conditions on a force-plate of non-specific low back pain (NSLBP) sufferers to those of healthy controls. Sixteen papers met the inclusion criteria. Heterogeneity in study designs prevented pooling of the data so only a qualitative data analysis was conducted. The majority of the papers (14/16, 88%) concluded that NSLBP patients have increased COP mean velocity and overall excursion as compared to healthy individuals. This was statistically significant in the majority of studies (11/14, 79%). An increased sway in anteroposterior direction was also observed in NSLBP patients. Patients with NSLBP exhibit greater postural instability than healthy controls, signified by greater COP excursions and a higher mean velocity. While the decreased postural stability in NSLBP sufferers further appears to be associated with the presence of pain, it seems unrelated to the exact location and pain duration. No correlation between the pain intensity and the magnitude of COP excursions could be identified.
Keywords: Balance, Center of pressure, Force-plate, Low back pain, Healthy controls, Systematic review
Background
Body sway can be assessed by measuring the deviations in the location of the center of pressure (COP) on the supporting surface by means of a force platform. COP refers to the point at which the pressure of the body over the soles of the feet would be if it were concentrated in one spot. It is, however, not a true record of body sway but rather a measure of the activity of the motor system in moving the COP.
The cause of sway is attributed to many factors such as inherent noise within the human neuromotor system, as reflective of an active anticipatory search process, or as an output of a control process to maintain postural control [1–3]. Many uncontrollable factors may contribute to the degradation of the balance system such as decreased performance of the sensory-motor system with aging, neurological or musculoskeletal disorders such as low back pain (LBP) [4].
Low back pain is a common condition with a reported 1-year prevalence ranging from 22 to 65% [5]. While the majority of these cases resolve within 6 weeks without medical intervention [6], a minority of around 20% may progress to become chronic and constitute the western World’s most prevalent and costly health problem [7]. Recent evidence showed that while age is a major determinant for balance, low back pain might account for up to 9% of the variance in balance [8].
A variety of theories exists about the potential effect of NSLBP on postural stability. Ideally, the body should be able to generate quick COP transitions that just exceed the current position of the center of mass (COM) [3] and accelerate it into the opposite direction in order to maintain balance. On a basic level (chronic) damage of sensory tissues in the lumbar spine, trunk [9] or lower extremities [10] may affect postural stability. Deterioration of this proprioceptive information from these areas may be the determining factor in reducing the accuracy in the sensory integration process. The resulting imprecise estimation of the COM position especially in chronic LBP sufferers may then lead to an increase in the safety margin of the adaptive COP shifts with regard to the predicted COM oscillations [11].
Another possible mechanism behind balance alterations is acute “pain inhibition” [12]. In this case, discharge from high-threshold nociceptive afferents interferes with spinal motor-pathways [13] as well as the motor cortex [14]. In addition, it has been shown that pain may cause an increased presynaptic inhibition of muscle afferents [15] as well as affecting the central modulation of proprioceptive spindles of muscles [16], causing prolonged latencies by the decrease in muscle spindle feedback. These alterations may lead to decreased muscle control and result in increased postural sway.
This literature review will attempt to identify possible differences in COP pattern between NSLBP sufferers and healthy controls that may relate to the mechanisms described above. This step is fundamental before investigating whether a connection between the magnitude of these differences and the LBP intensity or location exists.
To our knowledge, no systematic review has been conducted to investigate the possible impact of low back pain on COP pattern and the possible association of this effect with pain intensity or disability.
Aims
The aims of this systematic literature review were: (1) to determine if there are significant differences in COP between LBP patients and healthy controls, (2) to investigate whether the magnitude of these COP excursions is related to the level of pain perception or (3) to the perceived level of disability.
Methods
Search strategy
A comprehensive search strategy was developed by identifying all potentially relevant search terms, categorizing these terms into specific search phases and subsequently combining them by using Boolean terms. This search strategy was applied to six different electronic databases: PubMed, MEDLINE, EMBASE, Web of Science, ScienceDirect, Digital Dissertations and the Cochrane library. The detailed search strategy will be made available upon contacting the corresponding author.
Electronic searches
All databases were searched using the search strategy described above. Appropriate minor modifications to the basic search template were made to optimize the strategy in each of the six databases. Papers were limited to human studies published between January 1980 and July 2009.
Searching other resources
The hand search included analyzing references cited in studies selected from the original online search. Citation searches of relevant studies were conducted using the PubMed, MEDLINE, and ScienceDirect databases.
Selection criteria
Papers were limited to peer-reviewed journals and dissertations without restrictions regarding language. Wide inclusion and exclusion criteria for study designs were in order to avoid limitation of potentially relevant papers.
The inclusion criteria were: papers in any language that were fully or partially concerned with COP measures of subjects with NSLBP derived from bipedal static tasks on a force-plate, compared to measures of healthy controls. For the purpose of this review, NSLBP was broadly defined as pain of musculoskeletal etiology in the absence of any neurological symptomatology or structural damage due to trauma or serious pathology such as cancer or infection.
All COP measures, experimental setups, and statistical models fitting these criteria were considered. No limitations of the type of patient demographics applied. We excluded studies with insufficient documentation of patient demographics or experimental setup where this rendered data extraction impossible. In addition, papers that were anecdotal, speculative, or editorial in nature or studies that employed dynamic task conditions such as one-leg hopping, walking, or some form of translation of the force platform were excluded.
Data extraction and management
For the purpose of this review, AR acted as the principal reviewer. A colleague was involved independently in the process of identifying relevant studies and did not participate in further analysis of the finally included papers.
About the research question, the data extraction consisted of five main areas regarding low back pain and disability: (1) location and origin of the pain, (2) LBP duration prior to the measurements, (3) number of previous painful episodes, (4) perceived pain intensity, and (5) any reported disability level.
For the purpose of this review, a p value at or below 0.05 (p ≤ 0.05) was considered statistically significant.
Assessment of methodology
Recently it was suggested that combined quality scores should not be incorporated into systematic reviews and instead the accuracy should be assessed by an investigation into individual quality scores [17].
The reviewers specifically assessed the application, documentation, and association of six individual items concerning differences in COP measures between LBP patients and healthy controls. The reviewed criteria for experimental setups consisted of (1) subject demographics and morphology, (2) sample duration, (3) number of trial repetitions, (4) visual condition (eyes open or closed), (5) stance, and (6) type of platform surface.
Results
Literature search results
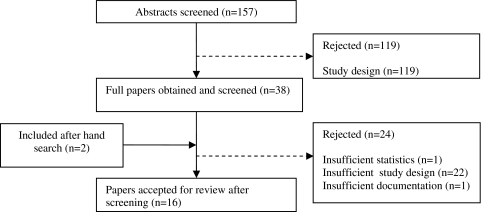
Initially, the online search strategy identified 157 studies of which the reviewers screened abstracts individually. The application of inclusion/exclusion criteria and consensus by the reviewers on the titles and abstracts eliminated a further 119 papers. The most common reason for rejection was not meeting the selection criteria such as static or bipedal tasks. From the titles and abstracts of papers selected (n = 38), full papers were reviewed and the same two reviewers (AR and TB) applied the inclusion criteria to the full text. Of these, 16 studies met the inclusion criteria and were included in this review; 2 of these 16 were added after the hand search of reference lists of included papers (Fig. 1).
Fig. 1.
Flowchart of papers
Study results
Characteristics of participants and methods
There was no blinding of the examiners to the participant’s health status described. Most authors described the baseline demographics in appropriate detail by including weight, height, age and gender (12/16, 75%), eight studies (50%) included a physical examination in order to validate their health status prior to study enrollment. Only one of the included studies reported calibration procedures of the force-plate [18], another one described procedures to ensure that the participants resumed an identical foot position throughout the trials [19].
Both subject demographics and health status for all studies are shown in Table 1. With regard to patient demographics, less than half of the included studies (41%) enrolled mixed gender groups of healthy and NSLBP participants. The studies employed rather broad age ranges of participants, with the most commonly enrolled age range being 21–40 years (76%).
Table 1.
Participant demographics and health status
| Study | Healthy status and number of participants | Gender | Age in years (SD) | Weight in kg (SD) | Height in cm (SD) | |
|---|---|---|---|---|---|---|
| Female | Male | |||||
| Luoto et al. [20] | Moderate LBP: 68 | 35 | 33 | 20–60 | – | – |
| Severe LBP: 31 | 18 | 13 | 20–60 | – | – | |
| Healthy: 61 | 29 | 32 | 20–60 | – | – | |
| Mientjes and Frank [21] | LBP: 8 | 3 | 5 | 38.4 | – | 179 |
| Healthy: 8 | 3 | 5 | 37.1 | – | 171 | |
| Kuukkanen and Malkia [22] | LBP: 90 | – | – | 39.9 (7.9) | – | – |
| Hamaoui et al. [23] | LBP: 10 | 0 | 10 | 33 | 77 | 181 |
| Healthy: 10 | 0 | 10 | 31 | 69 | 178 | |
| Grimstone and Hodges [24] | LBP: 10 | – | – | 32 (8.3) | 69 (14.7) | 173 (10.0) |
| Healthy: 10 | – | – | 26 (5.4) | 66 (15.1) | 171 (10.0) | |
| Brumagne et al. [25] | LBP: 10 | – | – | 25 | – | – |
| Healthy: 10 | – | – | 25 | – | – | |
| LBP: 10 | – | – | 63 | – | – | |
| Healthy: 10 | – | – | 63 | – | – | |
| Hamaoui et al. [26] | LBP: 10 | 0 | 10 | 33 | 77 | 181 |
| Healthy: 10 | 0 | 10 | 31 | 69 | 178 | |
| Mok et al. [27] | LBP: 24 | – | – | 36.6 (10.0) | 71.2 (11.5) | 171 (9.0) |
| Healthy: 24 | – | – | 36.9 (10.5) | 65.3 (11.6) | 169 (8.0) | |
| Smith et al. [28] | Healthy/induced LBP: 12 | 4 | 8 | 26 (4.0) | 71 (12.0) | 176 (12.0) |
| della Volpe et al. [2] | LBP: 12 | 5 | 7 | 35.4 | – | 174.9 |
| Healthy: 12 | – | – | – | – | – | |
| Popa et al. [29] | LBP: 13 | 6 | 7 | 35.1 (11.9) | 76.5 (17.9) | 174.3 (9.1) |
| Healthy: 13 | – | – | 32.2 (7.2) | 69.5 (12.7) | 174.4 (7.5) | |
| Brumagne et al. [30] | LBP: 21 | 14 | 7 | 23.5 (1.0) | 64.5 (12.9) | 171.2 (10.2) |
| Healthy: 24 | 13 | 11 | 23.0 (1.6) | 63.4 (10.1) | 172.9 (9.5) | |
| Lafond et al. [31] | LBP: 12 | – | – | 41.5 | 74.6 | 172.0 |
| Healthy: 12 | – | – | 40.0 | 68.5 | 167.3 | |
| Harringe et al. [32] | LBP: 11 | 11 | 0 | 15.0 | 49.9 | 161 |
| Healthy: 18 | 11 | 0 | 13.8 | 48.1 | 160 | |
| Mann et al. [19] | LBP: 10 | 10 | 0 | 57.6 (0.6) | 57.6 (0.6) | 165 (4.0) |
| Healthy: 10 | 10 | 0 | 20.27 (1.7) | 56.7 (0.2) | 166 (3.0) | |
| Salavati et al. [33] | LBP: 22 | 9 | 13 | 26.1 (6.2) | 67.1 (11.2) | 172 (10.0) |
| Healthy: 22 | 9 | 13 | 25.0 (5.5) | 66.5 (12.1) | 173 (10.0) | |
LBP low back pain
While the majority of studies defined neurological pathologies such as nerve root irritations in their exclusion criteria, few studies specifically addressed excluding vestibular conditions [21, 22, 26]. Other neurological conditions affecting balance were not addressed. Only one study investigated whether NSLBP sufferers were under the influence of pain medication [14] and consequently excluded those patients.
Table 2 shows the study characteristics and the results of the most commonly used COP parameters. There is a marked heterogeneity present in the in the included studies in terms of sample duration, number of trials or choice of COP parameters used.
Table 2.
Study characteristics and selected COP parameters measured on a firm surface
| Study | Condition | Duration (s) | Number of trials | Parameter | Low back pain result (SD) |
Healthy controls result (SD) |
p Value |
|---|---|---|---|---|---|---|---|
| Luoto et al. [20] | Normal stance, EO/F | 25 | 1 | mVel | Male: A: 14 mm/s B: 13 mm/s Female: A: 10 mm/s B: 20 mm/s |
Male: C: 12 mm/s Female: C: 11 mm/s |
>0.05 <0.05 |
| Mientjes and Frank [21] | Normal stance, EO/EC, F/C | Unclear | 3 | mPos RMS (ML) RMS (AP) |
– – – |
– – – |
0.099 0.016 0.031 |
| Kuukkanen and Malkia [22] | Unclear stance, EC/F | 20 (40) | 1 | mVel (AP) mVel (ML) |
17.1 mm/s (3.7) 12.3 mm/s (2.7) |
– – |
– – |
| Hamaoui et al. [23] | Normal stance, EO/F | 20 | 5 | mPos (AP) mPos (ML) |
2.9 mm (0.5) 1.6 mm (0.7) |
1.9 mm (0.8) 1.1 mm (0.6) |
0.002 0.032 |
| Grimstone and Hodges [24] | Normal stance, EO/F | 120 | 1 | Mean displacement | 3.2 mm | 2.4 mm | – |
| Brumagne et al. [25]a | Normal stance, Unclear visual condition/F |
60 | 1 | RMS (AP) | Young: ~8 mm Elderly: ~7.5 mm |
Young: ~5 mm Elderly: ~5 mm |
<0.05 <0.05 |
| Hamaoui et al. [26] | Normal stance, EC/F Narrow stance, EC/F |
20 | 5 | Mean displacement | AP 4.3 mm (1.6) ML 2.0 mm (1.2) AP 5.5 mm (1.5) ML 4.7 mm (1.6) |
AP 2.7 mm (0.9) ML 1.3 mm (0.6) AP 3.0 mm (0.6) ML 3.7 mm (0.9) |
<0.05 >0.05 <0.001 >0.05 |
| Mok et al. [27]b | Normal stance, EC/F | 70 | 1 | mVel | 4.3 mm/s (2.17) | 5.03 mm/s (2.8) | >0.05 |
| Smith et al. [28]a | Normal stance, EC/EO/F | 120 | 1 | Mean displacement | EC: ~2.9 mm | EC: ~2.75 mm | – |
| della Volpe et al. [2] | Normal stance, EO/F | 20 | 3 | mVel (AP) RMS length |
12.18 mm/s (1.2) 0.19 mm (0.01) |
10.32 mm/s (0.6) 0.16 mm (0.01) |
– – |
| Popa et al. [29] | Normal stance, EC/F | 20 | 3 | Mean displacement | 2.85 mm (0.024) | 2.09 mm (0.01) | <0.05 |
| Brumagne et al. [30] | Normal stance, EO/EC, F/C | 60 | 1 | RMS (AP) | EC/F: 8.8 mm EO/F: 8.2 mm EC/C: 7.5 mm EO/C:.7.8 mm |
EC/F: 5.4 mm EO/F: 6.2 mm EC/C: 8.7 mm EO/C: 10.5 mm |
>0.05 >0.05 >0.05 <0.05 |
| Lafond et al. [31]a | Normal stance, EC/F Normal stance, EC/F |
60 1,800 |
1 | mVel (AP) RMS length Area mVel (AP) RMS length Area |
~5 mm/s ~1.3 mm ~8.0 cm² ~13.5 mm/s ~11 mm ~18.5 cm² |
~3 mm/s ~4.3 mm ~4.7 cm² ~17.5 mm/s ~17.5 mm ~25.0 cm² |
<0.05 <0.05 <0.05 >0.05 <0.05 >0.05 |
| Harringe et al. [32] | Normal stance, EC/F | 120 | 2 | RMS Vel Area |
2.2 mm/s (0.59) 7.11 cm² (3.04) |
2.06 mm/s (0.6) 6.92 cm² (3.91) |
>0.05 >0.05 |
| Mann et al. [19]a | Normal stance, EC/F | 30 | 1–3 | SD vel m displ AP m displ ML |
~6.7 mm/s ~7.6 mm ~4.5 mm |
~5 mm/s ~3.3 mm ~1.7 mm |
0.015 <0.001 0.007 |
| Salavati et al. [33] | Normal stance, EC/F | 30 | 3 | SD vel mVel |
AP: 13.0 mm/s ML: 15.2 mm/s 13.7 mm/s (0.35) |
AP: 14.8 mm/s ML: 17.2 mm/s 15.9 mm/s (0.33) |
– – – |
AP anteroposterior, BP bipedal, C compliant surface, displ displacement, EC eyes closed, EO eyes open, F firm surface, m displ mean displacement; ML medial–lateral, mPos mean position, mVel mean velocity, RMS root mean square, SD vel standard deviation of velocity
aThe results presented have been extracted from bar-charts
bThe results from unilateral and bilateral static task conditions were not differentiated
About 53% of the trials were performed under both eyes closed (EC) and eyes open (EO) conditions. Most of the authors conducted less than three repetitions of postural sway recordings (9/16, 56%). Mean velocity (mVel), mean distance/displacement, root mean square (RMS) as well as sway area accounted for most of the COP parameters selected (Table 2).
Although both height and weight have been shown to affect the reliability of COP measures [34, 35], none of the presented results was subject to a normalizing process for these factors. Normalizing refers to statistically removing the dependence of stabilometric parameters on biomechanical factors as originally proposed by Mok et al. [27].
Reliability of COP data
Table 3 gives an overview of how the studies included meet the ideal experimental setup for reliable data [36]. Generally, the most important factors for reliable data appear to be sampling duration, number of trials and visual condition. Irrespective of sampling frequency and cut-off frequency, a sufficient sampling duration (<90 s) in combination with the appropriate number of recordings (3–5) showed to yield reliable data for most COP parameters such as mean velocity (mVel) or area [32, 36, 37].
Table 3.
Reliability criteria
| Study | Sampling frequency | Cut-off frequency | Duration | Number of repetitions | Visual condition | Surface | Total |
|---|---|---|---|---|---|---|---|
| Recommended | ~100 Hz | 10 Hz | ≥90 s | 3–5 | Eyes closed | Firm | |
| Luoto et al. [20] | 0 | 0 | 0 | 0 | 0 | + | + |
| Mientjes and Frank [21] | 0 | 0 | Unclear | + | + | + | +++ |
| Kuukkanen and Malkia [22] | Unclear | Unclear | 0 | 0 | + | + | ++ |
| Hamaoui et al. [23] | 0 | Unclear | 0 | 0 | 0 | + | ++ |
| Grimstone and Hodges [24] | 0 | Unclear | + | 0 | 0 | + | ++ |
| Brumagne et al. [25] | 0 | 0 | 0 | 0 | Unclear | + | + |
| Hamaoui et al. [26] | Unclear | Unclear | 0 | + | + | + | +++ |
| Mok et al. [27] | + | + | 0 | 0 | + | + | ++++ |
| Smith et al. [28] | + | Unclear | + | 0 | + | + | ++++ |
| della Volpe et al. [2] | Unclear | Unclear | 0 | + | 0 | + | ++ |
| Popa et al. [29] | + | 0 | 0 | + | + | + | ++++ |
| Brumagne et al. [30] | + | 0 | 0 | 0 | + | + | +++ |
| Lafond et al. [31] | + | + | + | 0 | 0 | + | ++++ |
| Harringe et al. [32] | 0 | + | + | 0 | + | + | ++++ |
| Mann et al. [19] | + | Unclear | 0 | + | + | + | ++++ |
| Salavati et al. [33] | + | + | 0 | + | + | + | +++++ |
With few exceptions [2, 20, 23, 31], most of the studies conducted the trials under visual deprivation while only four [24, 28, 32, 38] applied a sampling duration that has shown sufficient reliability [36]. A minority used three or more trial repetitions [2, 19, 21, 26, 29, 33].
Pain characteristics
Only half the studies (8/16, 50%) stated the total low back pain duration prior to the test (ranging from 1 to 10.5 years); the long-term implications of this factor on COP excursions cannot be assessed. Of all the studies only, a minority (6/16, 38%) correlated this duration to pain intensity (Table 4).
Table 4.
Pain definition, intensity and characteristics of included studies
| Study | Physical examination | Low back paina | Pain presence in years (SD) | Pain present at time of trial (n) | Pain intensity evaluation (pre-trial) | Score (SD) |
|---|---|---|---|---|---|---|
| Luoto et al. [20] | Yes | Chronic | – | Yes (99/99) | VAS | Unclear |
| Mientjes and Frank [21] | – | Chronic | 10.9 | Yes (8/8) | VAS | 2.6 |
| Kuukkanen and Malkia [22] | Yes | Subacute | 10 (8.4) | Yes (58/58) | – | – |
| Hamaoui et al. [23] | – | Chronic | – | Yes (10/10) | – | – |
| Grimstone and Hodges [24] | – | Chronic | 3.54 | Yes (10/10) | VAS | <2 |
| Brumagne et al. [25] | – | – | – | Unclear | – | – |
| Hamaoui et al. [26] | Yes | Chronic | – | Yes (10/10) | – | – |
| Mok et al. [27] | – | Chronic | 10.5 (8) | Yes (24/24) | VAS | 2.0 (1.6) |
| Smith et al. [28] | – | Acute | – | Yes (12/12) | VAS | 4.4 (1.9) |
| della Volpe et al. [2] | Yes | Chronic | 5.2 | Yes (12/12) | NRS-11 | 2–5/10 |
| Popa et al. [29] | Yes | Chronic | 5.2 (3.3) | Yes (13/13) | – | – |
| Brumagne et al. [30] | – | Chronic | 3.4 (2.5) | Yes (21/21) | VAS | 2.2 (1.5) |
| Lafond et al. [31] | Yes | Chronic | – | Yes (10/10) | VAS | 2.5 |
| Harringe et al. [32] | – | – | – | Mostly (7/11) | – | – |
| Mann et al. [19] | Yes | Chronic | – | Yes (10/10) | VAS | 6 (2) |
| Salavati et al. [33] | – | Episodic | 1.0 | No (22/22) | VAS | <2.0 |
Visual analogue scale (VAS) ranging 0–10, 0–2 light pain; 3–5 light-to-moderate pain; 6–7 moderate-to-intense pain; 8–10 unbearable pain
aChronic pain is defined as pain presence for at least 3 months
Pain assessment
Due to the described heterogeneity in the experimental setups, a direct comparison of data sets is problematic. Only about half of the studies described some form of physical examination prior to the recordings (9/16, 57%). While all investigated the effect of NSLBP on COP measures, not all studies (9/16, 57%) assessed the pain level in some form e.g., by means of a visual analogue scale (VAS). Luoto et al. [20] mentioned collecting VAS data of their participants but this data is missing in the published paper.
The participants of two studies did not experience any pain at the time of recording [24, 33, 39], neither did four individuals of another [32]. While Brumagne et al. [30] stated that their participants were not in an acute recurrence of NSLBP; they nevertheless reported VAS scores of 2.2 ± 1.5 and were consequently counted as in pain. The perceived pain levels were similar throughout the studies at around 2.5 (VAS), indicating mild-to-moderate pain (Table 4).
Low back pain and postural sway
Generally, there is a great variability in the reported COP measures irrespective of the parameter chosen. The results of the included studies indicate that patients suffering from NSLBP exhibited a greater postural instability than healthy controls. This difference was statistically significant in the majority of studies (14/16, 88%). Only two studies found significantly lesser COP excursions in patients suffering from low back pain [27, 33].
Compared to healthy controls, participants with NSLBP exhibited a greater sway area [31, 32], which varied greatly between 7.11 [32] and 18.5 cm² [31]. The NSLBP patients also showed an increased COP mean displacement [23, 24, 26, 28, 29]. This difference was significant in the AP direction [23, 26, 29]. The general trend towards an increased AP sway in pain sufferers was also present when considering the root mean square (RMS) for anteroposterior sway [30, 40], an effect that was found to increase with longer sampling durations [31].
Additionally, a higher COP sway velocity was found in non-specific LBP cases [2, 19, 20, 31, 32]. The mean velocities ranged from about 2.23 [32] to 17.1 mm/s [22] throughout the studies. For comparison, Table 5 shows the results for the parameter mean velocity.
Table 5.
The effect of NSLBP on postural sway for the COP parameter mean velocity (mVel)
| Study | Duration (s) | Number of trials | Healthy controls Result (SD) |
LBP patients (SD) | Pain severity (SD) |
|---|---|---|---|---|---|
| Luoto et al. [20] | 15 | 1 | Male: | Male: | |
| 12 mm/s | 14 mm/s | Moderate | |||
| 13 mm/s | Severe | ||||
| 15 | 1 | Female: | Female: | ||
| 11 mm/s | 10 mm/s | Moderate | |||
| 20 mm/s | Severe | ||||
| della Volpe et al. [2] | 20 | 3 | AP: 12.2 mm/s (1.2) | AP: 10.3 mm/s (0.6) | 2–5 NRS-11 |
| Lafond et al. [31]a | 60 | 1 | ~3 mm/s | ~5 mm/s | 2.5 VAS |
| Mann et al. [19]a | 30 | 1–3 | ~5 mm/s | ~6.7 mm/s | 6 (2) VAS |
| Salavati et al. [33] | 30 | 3 | 15.9 mm/s (0.33) | 13.7 mm/s (0.35) | <2.0 VAS |
Visual analogue scale (VAS) ranging 0–10, 0–2 light pain; 3–5 light-to-moderate pain; 6–7 moderate-to-intense pain; 8–10 unbearable pain
NRS-11 ranging from 0 “no pain” to 10 “worst possible pain”
aThe results presented have been extracted from bar-charts
The contribution of visual information
The results show that the differences in COP pattern between LBP sufferers and healthy controls gain significance under visual deprivation. An increase in postural sway in the absence of visual input has been observed by numerous studies of healthy participants [19, 22, 29, 41]. In a study enrolling patients suffering from lumbar disc pathologies, the level of significance between those and healthy controls increased from p < 0.05 (~12 mm/s compared to ~8 mm/s) under eyes open to p < 0.01 (~23 and ~13 mm/s, respectively) under eyes closed condition for COP mean velocity [41]. Mann et al. [19] reported that the presence of visual input did not influence COP mean velocity in healthy subjects and no difference between healthy controls and LBP patients was observed under eyes open condition. With closed eyes, however, a significant difference became apparent (5 mm/s compared to 6.7 mm/s, p = 0.015).
Sampling duration
Most studies focused on investigating COP excursions of NSLBP sufferers during relatively short sampling durations of up to 120 s, observing the described increased postural instability. Only one study assessed body sway during prolonged standing of 30 min [31].
Disability assessment
The study designs and variable health characteristics of the participants render any direct comparison of results problematic (Table 6). The majority of the included studies (12/16, 75%) investigated the perceived level of disability of the participants. Two of the papers [20, 22] failed to document the results; another one only assessed post-trial disability levels [21]. In addition to the Roland and Morris [42] questionnaire, the Oswestry [43] questionnaire was the most commonly used (8/12, 67%). The scores generally show great variability ranging 1–32/50 (Oswestry) and 3.2–17/24 (Roland–Morris).
Table 6.
Disability definition and characteristics of included studies
| Study | Disability assessed | Questionnaire | Score (SD) |
|---|---|---|---|
| Luoto et al. [20] | Yes | Oswestry | Unclear |
| Mientjes and Frank [21] | Yes | Oswestry (post-trial) Roland–Morris (post-trial) |
9–32/50 (mean 15.6) 3–17/24 (mean 7.5) |
| Kuukkanen and Malkia [22] | No | Oswestry | Unclear |
| Hamaoui et al. [23] | No | – | – |
| Grimstone and Hodges [24] | No | – | – |
| Brumagne et al. [25] | Yes | Oswestry | 20/50 |
| Hamaoui et al. [26] | No | – | – |
| Mok et al. [27] | Yes | Roland–Morris | 3.2 (3.5)/24 |
| Smith et al. [28] | No | – | – |
| della Volpe et al. [2] | Yes | Oswestry | 1–24/50 (mean 7.8) |
| Popa et al. [29] | Yes | Oswestry | 0–24/50 (mean 7.08) |
| Brumagne et al. [30] | Yes | Oswestry | 7.3 (7.6)/100 |
| Lafond et al. [31] | Yes | Oswestry FABQ |
12.6/50 (7.3) 20.4 (16.2) |
| Harringe et al. [32] | No | – | – |
| Mann et al. [19] | No | – | – |
| Salavati et al. [33] | Yes | Roland–Morris | 3.4/24 (3.2) |
Roland–Morris Disability Questionnaire: 24 items, 0 (no disability)–24 (severe disability)
Oswestry Disability Questionnaire: 50 points. 0–10 minimal disability, 11–20 mild disability, 21–30 severe disability, 31–40 crippling disability, 41–50 bed bound
FABQ Fear Avoidance Belief Questionnaire. 0–96 the higher the scale scores the greater the degree of fear and avoidance beliefs shown by the patient
Discussion
Due to the heterogeneous study designs and experimental setups, pooling of data was not possible. However, despite the great variability across the included studies our systematic review showed that patients suffering from NSLBP exhibit a significantly increased COP sway. Unfortunately, the magnitude of these differences in postural sway cannot be summarily expressed in terms of specific percentages or values. As a result, only a general trend is noted.
The reliability of COP measurements is determined by factors such as sampling duration, sampling frequency and number of trials [36]. In our critical review, only about half the included studies fulfilled three or more of these recommended reliability criteria. However, there was a trend towards better methodological reporting in the more recent studies. Despite this fact, it is worth bearing in mind that studies with less than all six criteria may still present fairly reliable results.
About vision, an increase in COP excursions has been observed under visual deprivation as compared to EO patients suffering from NSLBP. This supports the previously mentioned proprioceptive deficits in NSLBP patients. An existing impaired sensory input from muscles and joints is more severely challenged with closed eyes. Vision is primarily used in controlling low frequency disturbances [44], as occurring during quiet stance. In conjunction with vestibular information, it is essential for stabilizing upright posture. In patients with a reduction in proprioceptive input, as seen in chronic NSLBP, it is therefore common to find a greater reliance on visual and vestibular cues to maintain postural stability. Visual obstruction will therefore exhibit a profound effect on balance as the system is deprived of two major contributors for postural control.
The pronounced anteroposterior sway with the resulting raised ankle stiffness [11] observed in NSLBP sufferers [23, 26, 29] may be seen as a compensatory mechanism to enhance sensory discrimination and thereby compensate for the deterioration of the feedback loop [29].
Interestingly, the magnitude of COP excursions varies depending on the location of the pain. Experimentally induced pain into the biceps muscle, for example, did not exhibit any significant effect on postural sway [2], while a similar injection of levo-ascorbic acid (l-AS) into the feet elicited the same basic COP pattern found in chronic LBP sufferers. As the pain level was increased, so did the COP mean velocity and range in anterior–posterior direction [45].
Clinical application of COP measures
While this literature review shows that statistically significant differences in postural sway are present, the clinical application of COP measurements still remains limited for five major reasons described below.
Firstly, the causative factor for the altered postural sway is still unknown. The question remains whether the increased COP excursions are related to the previously described physiological changes due to chronic pain perception or rather acute “pain inhibition” [12]. If the latter mechanism is mainly responsible, monitoring NSLBP sufferers during their treatment and rehabilitation process may aid as an objective tool in assessing the patient’s progress. If long-term neurophysiological changes are primarily involved, individually varying recovery time frames may render such measurements less useful.
To address this question, future research is recommended to compare groups of participants suffering from (a) acute LBP without previous pain history to (b) those asymptomatic but with a long pain history to (c) healthy controls. This way, the direct effect of acute pain on postural stability can be assessed in the absence of physiological and neurological changes postulated with chronicity.
Secondly, the data available is insufficient to determine whether some form of linear or non-linear correlation between the perceived pain intensity and the magnitude of postural sway exists. At similar VAS scores, the reported results for COP mean velocity vary considerably. While one study reported a 100% increase in sway velocity with increasing pain perception [20], other studies showed no significant difference [19, 31].
Thirdly, the effect of pain duration, episodes of LBP and disability on COP excursions remain unclear. Due to the heterogeneous patient groups with a wide variety of pain durations and no information on the number of previous painful episodes being available, no conclusions can be drawn. Another contributing factor may be that self-reporting of LBP is prone to recall bias [46] and the definitions of NSLBP contained some variation throughout the studies. Both Oswestry and Roland–Morris results showed equally great variability which, in addition to the heterogeneous experimental setups, prevents interpretation. Further research is necessary to answer this question.
Fourthly, it has been shown that there is a steady natural increase in COP excursions with aging [47]. The rather broad age range of participants throughout the studies prevents an analysis of whether this also applies to pain-induced postural instability and how this magnitude correlates to specific age groups.
Finally, “normal” values are largely unknown and only one large-scale study offers reference values of healthy individuals for various COP parameters [47]. Similarly, reference data needs to be established for different LBP subgroups as a foundation for any intervention study. Until then, the identification of different COP patterns may be considered of academic rather than of clinical value at this time.
Conclusion
Patients with non-specific LBP exhibit greater postural instability than healthy controls. This difference is more pronounced under visual obstruction and can be attributed to either acute pain inhibition or diminished proprioceptive input from the lumbar spine and trunk muscles due to long-term neurological adaptations.
The decreased postural stability in NSLBP sufferers further appears to be associated with the acute presence of pain. There is insufficient data to suggest a relationship between pain intensity, previous pain duration or the level of perceived disability and the magnitude of COP excursions.
The clinical application of COP measures is limited by the unknown origin of the altered sway pattern, as well as a lack of COP reference values for different gender and age groups under both healthy and NSLBP. Further research is necessary to address these issues.
Limitations
A limitation of this literature review is the search strategy and its limitation to six databases, which might not have identified all relevant papers. To overcome this, a dynamic search strategy was employed with selected hand searches of reference lists. Another limitation is the fact that only very few papers allowed for any direct inter-study comparison of results and many conclusions had to be drawn from those studies.
Acknowledgments
The author(s) like to thank Tino Bos (TB) for his valuable contribution during the first phase of the literature search.
Conflict of interest The author(s) declare that they have no competing interests. No funding was received for this review.
References
- 1.Gatev P, Thomas S, Kepple T, Hallett M. Feedforward ankle strategy of balance during quiet stance in adults. J Physiol. 1999;514(Pt 3):915–928. doi: 10.1111/j.1469-7793.1999.915ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.della Volpe R, Popa T, Ginanneschi F, Spidalieri R, Mazzocchio R, Rossi A (2006) Changes in coordination of postural control during dynamic stance in chronic low back pain patients. Gait Posture 24:349–355. doi:10.1016/j.gaitpost.2005.10.009 [DOI] [PubMed]
- 3.Baratto L, Morasso PG, Re C, Spada G. A new look at posturographic analysis in the clinical context: sway-density versus other parameterization techniques. Mot Control. 2002;6:246–270. doi: 10.1123/mcj.6.3.246. [DOI] [PubMed] [Google Scholar]
- 4.Madeleine P, Prietzel H, Svarrer H, Arendt-Nielsen L. Quantitative posturography in altered sensory conditions: a way to assess balance instability in patients with chronic whiplash injury. Arch Phys Med Rehabil. 2004;85:432–438. doi: 10.1016/j.apmr.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Walker BF. The prevalence of low back pain: a systematic review of the literature from 1966 to 1998. J Spinal Disord. 2000;13:205–217. doi: 10.1097/00002517-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Waddell G. 1987 Volvo award in clinical sciences. A new clinical model for the treatment of low-back pain. Spine (Phila Pa 1976) 1987;12:632–644. doi: 10.1097/00007632-198709000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 8.Liu-Ambrose T, Eng JJ, Khan KM, Mallinson A, Carter ND, McKay HA. The influence of back pain on balance and functional mobility in 65–75-year-old women with osteoporosis. Osteoporos Int. 2002;13:868–873. doi: 10.1007/s001980200119. [DOI] [PubMed] [Google Scholar]
- 9.Brumagne S, Cordo P, Lysens R, Verschueren S, Swinnen S. The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain. Spine (Phila Pa 1976) 2000;25:989–994. doi: 10.1097/00007632-200004150-00015. [DOI] [PubMed] [Google Scholar]
- 10.Mazzocchio R, Scarfo GB, Mariottini A, Muzii VF, Palma L. Recruitment curve of the soleus H-reflex in chronic back pain and lumbosacral radiculopathy. BMC Musculoskelet Disord. 2001;2:4. doi: 10.1186/1471-2474-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casadio M, Morasso PG, Sanguineti V. Direct measurement of ankle stiffness during quiet standing: implications for control modelling and clinical application. Gait Posture. 2005;21:410–424. doi: 10.1016/j.gaitpost.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Moseley GL, Hodges PW. Are the changes in postural control associated with low back pain caused by pain interference? Clin J Pain. 2005;21:323–329. doi: 10.1097/01.ajp.0000131414.84596.99. [DOI] [PubMed] [Google Scholar]
- 13.Rossi A, Decchi B, Ginanneschi F. Presynaptic excitability changes of group Ia fibres to muscle nociceptive stimulation in humans. Brain Res. 1999;818:12–22. doi: 10.1016/S0006-8993(98)01253-0. [DOI] [PubMed] [Google Scholar]
- 14.Rossi S, della Volpe R, Ginanneschi F, Ulivelli M, Bartalini S, Spidalieri R, Rossi A. Early somatosensory processing during tonic muscle pain in humans: relation to loss of proprioception and motor ‘defensive’ strategies. Clin Neurophysiol. 2003;114:1351–1358. doi: 10.1016/S1388-2457(03)00073-7. [DOI] [PubMed] [Google Scholar]
- 15.Sibley KM, Carpenter MG, Perry JC, Frank JS. Effects of postural anxiety on the soleus H-reflex. Hum Mov Sci. 2007;26:103–112. doi: 10.1016/j.humov.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Capra NF, Ro JY. Experimental muscle pain produces central modulation of proprioceptive signals arising from jaw muscle spindles. Pain. 2000;86:151–162. doi: 10.1016/S0304-3959(00)00231-1. [DOI] [PubMed] [Google Scholar]
- 17.Whiting P, Harbord R, Kleijnen J (2005) No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Med Res Methodol 5 [DOI] [PMC free article] [PubMed]
- 18.Byl NN, Sinnott PL. Variations in balance and body sway in middle-aged adults. Subjects with healthy backs compared with subjects with low-back dysfunction. Spine (Phila Pa 1976) 1991;16:325–330. doi: 10.1097/00007632-199103000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Mann L, Kleinpaul JF, Moro ARP, Mota CB, Carpes FP (2009) Effect of low back pain on postural stability in younger women: Influence of visual deprivation. J Bodyw Mov Ther. doi:10.1016/j.jbmt.2009.06.007 [DOI] [PubMed]
- 20.Luoto S, Taimela S, Hurri H, Aalto H, Pyykko I, Alaranta H. Psychomotor speed and postural control in chronic low back pain patients—a controlled follow-up study. Spine (Phila Pa 1976) 1996;21:2621–2627. doi: 10.1097/00007632-199611150-00012. [DOI] [PubMed] [Google Scholar]
- 21.Mientjes MI, Frank JS. Balance in chronic low back pain patients compared to healthy people under various conditions in upright standing. Clin Biomech (Bristol, Avon) 1999;14:710–716. doi: 10.1016/S0268-0033(99)00025-X. [DOI] [PubMed] [Google Scholar]
- 22.Kuukkanen TM, Malkia EA. An experimental controlled study on postural sway and therapeutic exercise in subjects with low back pain. Clin Rehabil. 2000;14:192–202. doi: 10.1191/026921500667300454. [DOI] [PubMed] [Google Scholar]
- 23.Hamaoui A, Mc Do, Poupard L, Bouisset S. Does respiration perturb body balance more in chronic low back pain subjects than in healthy subjects? Clin Biomech (Bristol, Avon) 2002;17:548–550. doi: 10.1016/S0268-0033(02)00042-6. [DOI] [PubMed] [Google Scholar]
- 24.Grimstone SK, Hodges PW. Impaired postural compensation for respiration in people with recurrent low back pain. Exp Brain Res. 2003;151:218–224. doi: 10.1007/s00221-003-1433-5. [DOI] [PubMed] [Google Scholar]
- 25.Brumagne S, Cordo P, Verschueren S. Proprioceptive weighting changes in persons with low back pain and elderly persons during upright standing. Neurosci Lett. 2004;366:63–66. doi: 10.1016/j.neulet.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Hamaoui A, Do MC, Bouisset S. Postural sway increase in low back pain subjects is not related to reduced spine range of motion. Neurosci Lett. 2004;357:135–138. doi: 10.1016/j.neulet.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 27.Mok NW, Brauer SG, Hodges PW. Hip strategy for balance control in quiet standing is reduced in people with low back pain. Spine (Phila Pa 1976) 2004;29:E107–E112. doi: 10.1097/01.brs.0000115134.97854.c9. [DOI] [PubMed] [Google Scholar]
- 28.Smith M, Coppieters MW, Hodges PW. Effect of experimentally induced low back pain on postural sway with breathing. Exp Brain Res. 2005;166:109–117. doi: 10.1007/s00221-005-2352-4. [DOI] [PubMed] [Google Scholar]
- 29.Popa T, Bonifazi M, Della Volpe R, Rossi A, Mazzocchio R. Adaptive changes in postural strategy selection in chronic low back pain. Exp Brain Res. 2007;177:411–418. doi: 10.1007/s00221-006-0683-4. [DOI] [PubMed] [Google Scholar]
- 30.Brumagne S, Janssens L, Knapen S, Claeys K, Suuden-Johanson E. Persons with recurrent low back pain exhibit a rigid postural control strategy. Eur Spine J. 2008;17:1177–1184. doi: 10.1007/s00586-008-0709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lafond D, Champagne A, Descarreaux M, Dubois JD, Prado JM, Duarte M (2009) Postural control during prolonged standing in persons with chronic low back pain. Gait Posture 29:421–427. doi:10.1016/j.gaitpost.2008.10.064 [DOI] [PubMed]
- 32.Harringe ML, Halvorsen K, Renstrom P, Werner S. Postural control measured as the center of pressure excursion in young female gymnasts with low back pain or lower extremity injury. Gait Posture. 2008;28:38–45. doi: 10.1016/j.gaitpost.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Salavati M, Mazaheri M, Negahban H, Ebrahimi I, Jafari AH, Kazemnejad A, Parnianpour M. Effect of dual-tasking on postural control in subjects with nonspecific low back pain. Spine (Phila Pa 1976) 2009;34:1415–1421. doi: 10.1097/BRS.0b013e3181a3a917. [DOI] [PubMed] [Google Scholar]
- 34.Hue O, Simoneau M, Marcotte J, Berrigan F, Dore J, Marceau P, Marceau S, Tremblay A, Teasdale N. Body weight is a strong predictor of postural stability. Gait Posture. 2007;26:32–38. doi: 10.1016/j.gaitpost.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Demura S-I, Kitabayashi T, Noda M, Aoki H. Age-stage differences in body sway during a static upright posture based on sway factors and relative accumulation of power frequency. Percept Mot Skills. 2008;107:89–98. doi: 10.2466/pms.107.1.89-98. [DOI] [PubMed] [Google Scholar]
- 36.Ruhe A, Fejer R, Walker BF (2010) The test-retest reliability of centre of pressure measures in bipedal static task conditions: a systematic review of the literature. Gait Posture (under review) [DOI] [PubMed]
- 37.Santos BR, Delisle A, Lariviere C, Plamondon A, Imbeau D. Reliability of centre of pressure summary measures of postural steadiness in healthy young adults. Gait Posture. 2008;27:408–415. doi: 10.1016/j.gaitpost.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Lafond D, Champagne A, Descarreaux M, Dubois J-D, Prado JM, Duarte M. Postural control during prolonged standing in persons with chronic low back pain. Gait Posture. 2009;29:421–427. doi: 10.1016/j.gaitpost.2008.10.064. [DOI] [PubMed] [Google Scholar]
- 39.Salavati M, Hadian MR, Mazaheri M, Negahban H, Ebrahimi I, Talebian S, Jafari AH, Sanjari MA, Sohani SM, Parnianpour M (2009) Test–retest reliability of center of pressure measures of postural stability during quiet standing in a group with musculoskeletal disorders consisting of low back pain, anterior cruciate ligament injury and functional ankle instability. Gait Posture 29:460–464. doi:10.1016/j.gaitpost.2008.11.016 [DOI] [PubMed]
- 40.Brumagne S, Janssens L, Janssens E, Goddyn L. Altered postural control in anticipation of postural instability in persons with recurrent low back pain. Gait Posture. 2008;28:657–662. doi: 10.1016/j.gaitpost.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Leinonen V, Kankaanpaa M, Luukkonen M, Kansanen M, Hanninen O, Airaksinen O, Taimela S. Lumbar paraspinal muscle function, perception of lumbar position, and postural control in disc herniation-related back pain. Spine (Phila Pa 1976) 2003;28:842–848. [PubMed] [Google Scholar]
- 42.Roland M, Morris R. A study of the natural history of back pain. Part I. Development of a reliable and sensitive measure of disability in low-back pain. Spine (Phila Pa 1976) 1983;8:141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Fairbanks J, Couper J, Davies J, O’Brian J. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 44.Gill J, Allum JH, Carpenter MG, Held-Ziolkowska M, Adkin AL, Honegger F, Pierchala K. Trunk sway measures of postural stability during clinical balance tests: effects of age. J Gerontol A Biol Sci Med Sci. 2001;56:M438–M447. doi: 10.1093/gerona/56.7.m438. [DOI] [PubMed] [Google Scholar]
- 45.Corbeil P, Blouin JS, Teasdale N (2004) Effects of intensity and locus of painful stimulation on postural stability. Pain 108:43–50. doi:10.1016/j.pain.2003.12.001 [DOI] [PubMed]
- 46.Vet HC, Heymans MW, Dunn KM, Pope DP, Beek AJ, Macfarlane GJ, Bouter LM, Croft PR. Episodes of low back pain: a proposal for uniform definitions to be used in research. Spine (Phila Pa 1976) 2002;27:2409–2416. doi: 10.1097/01.BRS.0000030307.34002.BE. [DOI] [PubMed] [Google Scholar]
- 47.Era P, Sainio P, Koskinen S, Haavisto P, Vaara M, Aromaa A (2006) Postural balance in a random sample of 7,979 subjects aged 30 years and over. Gerontology 52:204–213. doi:10.1159/000093652 [DOI] [PubMed]