Abstract
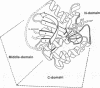
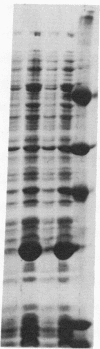
The G domain of elongation factor Tu (EF-Tu), representing the N-terminal half of the factor according to its three-dimensional model traced at high resolution, has been isolated by genetic manipulation of tufA and purified to homogeneity. The G domain, whose primary structure shares homology with the eukaryotic protein p21, is capable of supporting the basic activities of the intact molecule (guanine nucleotide binding in 1:1 molar ratio and GTPase activity). However, it is no longer exposed to the allosteric mechanisms regulating EF-Tu. The G-domain complexes with GTP and GDP display similar K'd values in the microM range, in contrast to EF-Tu that binds GDP much more tightly than GTP. Its GTPase shows the characteristics of a slow turnover reaction (0.1 mmol X sec-1 X mol-1 of G domain), whose rate closely corresponds to the initial hydrolysis rate of EF-Tu X GTP in the absence of effectors and lies in the typical range of GTPase of the p21 protein. Of the EF-Tu ligands only the ribosome displays a clear effect enhancing the G-domain GTPase. Our results suggest that the middle and C-terminal domain play an essential role in regulating the activity of the N-terminal domain of the intact molecule as well as in the interactions of EF-Tu with aminoacylated tRNA, elongation factor Ts, and kirromycin. With the isolation of the G domain of EF-Tu, a model protein has been constructed for studying and comparing common characteristics of the guanine nucleotide-binding proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Kawakita M., Kaziro Y. Studies on the polypeptide elongation factors from E. coli. V. Properties of various complexes containing EF-Tu and EF-Ts. J Biochem. 1974 Aug;76(2):293–306. doi: 10.1093/oxfordjournals.jbchem.a130571. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A., Weber K. Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci U S A. 1972 May;69(5):1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchini V., Parlato G., De Vendittis E., Sander G., Parmeggiani A. Energetic aspects of the EF-Tu-dependent GTPase activity. A study using the antibiotic kirromycin. Eur J Biochem. 1980 Dec;113(1):53–60. doi: 10.1111/j.1432-1033.1980.tb06138.x. [DOI] [PubMed] [Google Scholar]
- Bosch L., Kraal B., Van der Meide P. H., Duisterwinkel F. J., Van Noort J. M. The elongation factor EF-Tu and its two encoding genes. Prog Nucleic Acid Res Mol Biol. 1983;30:91–126. doi: 10.1016/s0079-6603(08)60684-4. [DOI] [PubMed] [Google Scholar]
- Bourne H. R. GTP-binding proteins. One molecular machine can transduce diverse signals. 1986 Jun 26-Jul 2Nature. 321(6073):814–816. doi: 10.1038/321814a0. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano O., Bruns W., Crechet J. B., Sander G., Parmeggiani A. Modification of elongation-factor-Tu . guanine-nucleotide interaction by kirromycin. A comparison with the effect of aminoacyl-tRNA and elongation factor Ts. Eur J Biochem. 1978 Sep 1;89(2):557–565. doi: 10.1111/j.1432-1033.1978.tb12560.x. [DOI] [PubMed] [Google Scholar]
- Fasano O., Crechet J. B., Parmeggiani A. Preparation of nucleotide-free elongation factor Tu and its stabilization by the antibiotic kirromycin. Anal Biochem. 1982 Jul 15;124(1):53–58. doi: 10.1016/0003-2697(82)90218-4. [DOI] [PubMed] [Google Scholar]
- Fasano O., De Vendittis E., Parmeggiani A. Hydrolysis of GTP by elongation factor Tu can be induced by monovalent cations in the absence of other effectors. J Biol Chem. 1982 Mar 25;257(6):3145–3150. [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Halliday K. R. Regional homology in GTP-binding proto-oncogene products and elongation factors. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(6):435–448. [PubMed] [Google Scholar]
- Jurnak F. Structure of the GDP domain of EF-Tu and location of the amino acids homologous to ras oncogene proteins. Science. 1985 Oct 4;230(4721):32–36. doi: 10.1126/science.3898365. [DOI] [PubMed] [Google Scholar]
- Kramer W., Schughart K., Fritz H. J. Directed mutagenesis of DNA cloned in filamentous phage: influence of hemimethylated GATC sites on marker recovery from restriction fragments. Nucleic Acids Res. 1982 Oct 25;10(20):6475–6485. doi: 10.1093/nar/10.20.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberman R., Egner U. Homologies in the primary structure of GTP-binding proteins: the nucleotide-binding site of EF-Tu and p21. EMBO J. 1984 Feb;3(2):339–341. doi: 10.1002/j.1460-2075.1984.tb01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F., Clark B. F., la Cour T. F., Kjeldgaard M., Norskov-Lauritsen L., Nyborg J. A model for the tertiary structure of p21, the product of the ras oncogene. Science. 1985 Oct 4;230(4721):78–82. doi: 10.1126/science.3898366. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Möller W., Amons R. Phosphate-binding sequences in nucleotide-binding proteins. FEBS Lett. 1985 Jul 1;186(1):1–7. doi: 10.1016/0014-5793(85)81326-0. [DOI] [PubMed] [Google Scholar]
- Parmeggiani A., Sander G. Properties and regulation of the GTPase activities of elongation factors Tu and G, and of initiation factor 2. Mol Cell Biochem. 1981 Mar 27;35(3):129–158. doi: 10.1007/BF02357085. [DOI] [PubMed] [Google Scholar]
- Parmeggiani A., Swart G. W. Mechanism of action of kirromycin-like antibiotics. Annu Rev Microbiol. 1985;39:557–577. doi: 10.1146/annurev.mi.39.100185.003013. [DOI] [PubMed] [Google Scholar]
- Reddy P., Miller D., Peterkofsky A. Stimulation of Escherichia coli adenylate cyclase activity by elongation factor Tu, a GTP-binding protein essential for protein synthesis. J Biol Chem. 1986 Sep 5;261(25):11448–11451. [PubMed] [Google Scholar]
- Remaut E., Tsao H., Fiers W. Improved plasmid vectors with a thermoinducible expression and temperature-regulated runaway replication. Gene. 1983 Apr;22(1):103–113. doi: 10.1016/0378-1119(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Papageorge A. G., Shih T. Y. Guanine nucleotide-binding activity as an assay for src protein of rat-derived murine sarcoma viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5355–5359. doi: 10.1073/pnas.76.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Papageorge A. G., Stokes P. E., Weeks M. O., Scolnick E. M. Guanine nucleotide-binding and autophosphorylating activities associated with the p21src protein of Harvey murine sarcoma virus. Nature. 1980 Oct 23;287(5784):686–691. doi: 10.1038/287686a0. [DOI] [PubMed] [Google Scholar]
- Sigal I. S., Gibbs J. B., D'Alonzo J. S., Temeles G. L., Wolanski B. S., Socher S. H., Scolnick E. M. Mutant ras-encoded proteins with altered nucleotide binding exert dominant biological effects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):952–956. doi: 10.1073/pnas.83.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart G. W., Kraal B., Bosch L., Parmeggiani A. Allosteric changes of the guanine nucleotide site of elongation factor EF-Tu: a comparative study of two kirromycin-resistant EF-Tu species. FEBS Lett. 1982 Jun 1;142(1):101–106. doi: 10.1016/0014-5793(82)80228-7. [DOI] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Tamanoi F., Walsh M., Kataoka T., Wigler M. A product of yeast RAS2 gene is a guanine nucleotide binding protein. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6924–6928. doi: 10.1073/pnas.81.22.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temeles G. L., Gibbs J. B., D'Alonzo J. S., Sigal I. S., Scolnick E. M. Yeast and mammalian ras proteins have conserved biochemical properties. Nature. 1985 Feb 21;313(6004):700–703. doi: 10.1038/313700a0. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Hol W. G. Predicted nucleotide-binding properties of p21 protein and its cancer-associated variant. Nature. 1983 Apr 28;302(5911):842–844. doi: 10.1038/302842a0. [DOI] [PubMed] [Google Scholar]
- Wolf H., Chinali G., Parmeggiani A. Kirromycin, an inhibitor of protein biosynthesis that acts on elongation factor Tu. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4910–4914. doi: 10.1073/pnas.71.12.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. M., Lindahl L. A secondary promoter for elongation factor Tu synthesis in the str ribosomal protein operon of Escherichia coli. Mol Gen Genet. 1982;185(3):487–492. doi: 10.1007/BF00334145. [DOI] [PubMed] [Google Scholar]
- la Cour T. F., Nyborg J., Thirup S., Clark B. F. Structural details of the binding of guanosine diphosphate to elongation factor Tu from E. coli as studied by X-ray crystallography. EMBO J. 1985 Sep;4(9):2385–2388. doi: 10.1002/j.1460-2075.1985.tb03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]