Abstract
Background
A classification system with four types of infected TKAs has been commonly used to determine treatment, especially with regard to whether the prosthesis should be removed or retained.
Questions/purposes
We asked whether (1) the classification-dictated treatment of the four types of infection after TKA would control infection and maintain functional TKA; (2) repeated débridement and two-stage TKA would further improve the infection control rate after initial treatment; and (3) fixation of TKA prosthesis to the host bone was achieved.
Methods
We retrospectively reviewed 114 patients with 116 infected TKAs. We determined the infection control rate after initial treatment, repeated débridement and two-stage TKA. We evaluated the functional and radiographic results using the Knee Society and Hospital for Special Surgery knee scoring systems. The minimum followup was 2 years (mean, 5.6 years; range, 2–8 years).
Results
The overall infection control rate was 100% in all patients. All patients with early superficial postoperative infection, 94% of patients with early deep postoperative infection, 96% of patients with late chronic infection, and 86% of patients with acute hematogenous infection maintained functioning knee prosthesis at the final followup. One hundred nine of the 114 patients could walk with no or only slight pain and maintained functioning knee prostheses. These 109 patients had stable fixation of the TKA prosthesis to host bone.
Conclusions
The techniques proposed by the classification effectively controlled infection and maintained functional TKA with firm fixation of the TKA prosthesis in most patients. Repeated débridement and two-stage TKA further improved the control of infection and functional TKA after initial treatment.
Level of Evidence
Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Several schemes have been proposed for classifying and staging of infection after TKA [2, 7, 14, 20, 31] and these systems have been used to determine treatment, especially with regard to whether the prosthesis should be removed or retained. These classification systems [2, 7, 14, 20, 31] are based on the clinical presentation of four types of infections after TKA or THA: (1) positive intraoperative culture; (2) early postoperative infection (superficial and deep); (3) late chronic infection; and (4) acute hematogenous infection. These systems have been used to determine treatment, especially with regard to whether the prosthesis should be removed or retained. Using the most appropriate treatment for a given type of infection after TKA is paramount for the control of infection, as reported by Segawa et al. [26]. In their study, the overall control rate of infection was 97% treated according to the four types of infection [26].
The reported control rates of treating deep infection of TKA have ranged from 20–68% for surgical débridement with retention of the prosthesis, 50–87% for one-stage exchange, and 56–100% for two-stage exchange [2–5, 11, 15–17, 19, 22, 24–28, 31–35]. Some authors suggest infection control rates are worse (around 83%) for chronic infections [6, 8, 14, 23, 26, 35]. However, all of these reports combined infection control rates for primary and revision TKAs and their infection control rates were variable. Relatively few studies [19] to date have focused on repeated débridement and two-stage TKA for infected TKAs.
To confirm previous reports we asked whether (1) the treatment according to the types of infection after TKA would control infection and maintain functional TKA; (2) repeated débridement and repeated two-stage revision would further improve the control rate of infection of the initial treatment; (3) durable fixation of a TKA prosthesis to the host bone would be achieved.
Patients and Methods
We retrospectively reviewed data for 121 prospectively followed patients with 123 infected TKAs who underwent revision TKAs between January 2001 and January 2007. Of the 121 patients, seven were lost to followup before 1 year, leaving 114 patients (116 knees) for review. The records of the 114 patients had been entered into an ongoing computerized database that was updated continuously. There were 30 men and 84 women with a mean age of 65 ± 9.4 years (range, 37–90 years). Their mean body mass index was 26.18 ± 3.56 kg/m2 (range, 18.9–40.4 kg/m2). The minimum duration of followup monitoring was 2 years (mean, 5.6 ± 0.92 years; range, 2–8 years). The study was approved by the Institutional Review Board, and all patients provided written informed consent.
Infection after TKA was diagnosed if the patients fulfilled any one of the following criteria: (1) an abscess or sinus tract communicating with the joint space; (2) positive preoperative aspiration culture findings on solid media; (3) positive cultures on two or more intraoperative cultures; or (4) one positive culture on solid media in conjunction with the presence of gross purulence. In patients with negative cultures, infection was diagnosed when all or any of these following findings were present: elevated white blood cell (WBC) count and leukocyte differential of the aspirated fluid [30], or an abnormal serology (erythrocyte sedimentation rate ([ESR] > 20 mm/hr, C-reactive protein [CRP] level > 0.5 mg/dL). Seventy-four of the 116 knees had positive preoperative and/or intraoperative cultures. The remaining 42 knees had negative cultures. Of these 42 knees with negative cultures, 29 knees (with early superficial infection) had no gross purulence, six knees (with early deep and chronic infection) had gross purulence intraoperatively, and seven other knees (with early deep and chronic infection) had sinus tract communication into the joint. All 13 infected knees had an elevated WBC count and differential and abnormal serology. Staphylococci accounted for 30 of the 74 (41%) bacterial isolates. Overall, aerobic Gram-positive cocci accounted for 48 of the 74 (65%) isolates and Gram-negative bacilli accounted for 19 (26%). The fungal and mycobacterium isolates came from late chronic infections (Table 1).
Table 1.
Bacterial isolates according to types of infection
| Pathogen | Group | |||
|---|---|---|---|---|
| Early superficial (29) | Early deep (32) | Late chronic (48) | Acute hematogenous (7) | |
| Gram-positive cocci (n = 48) | ||||
| Coagulase-positive staphylococci (n = 19) | 7 | 10 | 2 | |
| Methicillin-susceptible | 5 | 6 | 1 | |
| Methicillin-resistant | 2 | 4 | 1 | |
| Coagulase-negative staphylococci (n = 15) | 5 | 8 | 2 | |
| Methicillin-susceptible | 4 | 6 | 0 | |
| Methicillin-resistant | 1 | 2 | 2 | |
| Streptococcus (n = 7) | 3 | 4 | ||
| Enterococcus (n = 7) | 4 | 3 | ||
| Gram-negative bacilli (n = 19) | ||||
| Enterobacter (n = 11) | 5 | 5 | 1 | |
| Acinobacter baumanii complex (n = 8) | 4 | 4 | ||
| Fungus (n = 2) | – | 2 | ||
| Mycobacterium (n = 5) | – | 5 | ||
| No growth (n = 42) | 29 | 4 | 7 | 2 |
We defined four types of infection in our study according to the classification system of Tsukayama et al. [31]: (I) Positive intraoperative culture; (II) Early postoperative infection; (III) Acute hematogenous; (IV) Late chronic. For their first class based only on positive cultures, a patient was considered to have an infection only when the same organism grew on culture of at least two intraoperative specimens obtained at the time of a revision; for this first class all of the revisions had to be for presumed aseptic loosening. In our series, however, no patient had aseptic loosening for revision and therefore no patient was classified as Type I. Tsukayama et al. [31] defined an early postoperative infection as a wound infection (superficial or deep) that developed less than 4 weeks after the index operation. An early superficial postoperative infection was diagnosed when, on direct visualization of an infected wound at the time of the operation, there was no extension of inflammation into the joint. We defined an early superficial postoperative infection as evidence of cellulitis and redness over the wound site with an elevated ESR and CRP level, but the findings on analysis of the aspirated joint fluid for absolute WBC count and percentage of neutrophils were normal and the culture results of aspirated joint fluid were negative. Twenty-nine knees (25%) had an early superficial infection. An early deep postoperative infection was defined as extension of inflammation into the joint [31]. Thirty-two knees (28%) had an early deep postoperative infection. A late chronic infection was one that developed ≥ 4 weeks after the index operation and that had an insidious clinical presentation. Forty-eight knees (41%) had a late chronic infection. An acute hematogenous infection was defined as an association with documented or suspected antecedent onset of symptoms (severe pain and swelling within 48 hours) in the affected joint with the prosthesis [9]. Seven knees (6%) had an acute hematogenous infection.
We treated early superficial postoperative infections with an administration of a third-generation cephalosporin (1 g every 12 hours parenterally) for 2 to 6 weeks while monitoring patients’ blood parameters and clinical response. Early deep postoperative infections were treated with débridement, replacement of the polyethylene insert of the tibial component, retention of the prosthesis, and intravenous administration of antibiotics for 6 weeks (Table 2). Late chronic infections were treated with two-stage revision. First, we performed débridement, removal of all of the prosthetic components and bone cement, and placement of a tobramycin-impregnated (1.2 g per 40-g batch of bone cement) mobile cement spacer. Antibiotics were administered intravenously for 6 weeks (Table 2). After completion of antibiotic therapy, ESR, CRP levels, and total WBC count and differential in the joint aspirates were obtained and the patient was observed for 2 more weeks. If results of these tests showed no evidence of inflammation and there was no clinical evidence of recurrent infection, we performed delayed-exchange arthroplasty. Multiple cultures of specimens obtained during revision operations were performed to confirm infection eradication. The antibiotic-impregnated spacer was removed and a Legacy Constrained Condylar Knee prosthesis (LCCK; Zimmer, Warsaw, IN) was inserted and fixed with antibiotic-impregnated bone cement (1.2 g tobramycin mixed with 40 g of cement). Acute hematogenous infections were treated with débridement, replacement of the polyethylene insert, retention of the prosthesis if it was not loose, and intravenous administration of antibiotics for six weeks.
Table 2.
Treatment of infection with antibiotics
| Microorganism | Antimicrobial agent | Dosage | Route |
|---|---|---|---|
| Staphylococcus aureus coagulate-positive or -negative | |||
| Methicillin-susceptible | Nafcillin or floxacillin plus | 2 g every 6 hours | IV |
| Rifampin for 2 weeks followed by | 450 mg every 12 hours | PO | |
| Rifampin plus | 450 mg every 12 hours | PO | |
| Ciprofloxacin or | 750 mg every 12 hours | PO | |
| Levofloxacin | 750 mg every 24 hours | PO | |
| Methicillin-resistant | Vancomycin plus | 1 g every 12 hours | IV |
| Rifampin for 2 weeks | 450 mg every 12 hours | PO | |
| Rifampin plus | 450 mg every 12 hours | PO | |
| Ciprofloxacin or | 750 mg every 12 hours | PO | |
| Levofloxacin or | 750 mg every 24 hours | PO | |
| Teicoplanin or | 400 mg every 24 hours | IV | |
| Fusidic acid | 500 mg every 8 hours | PO | |
| Streptococcus and Enterococcus | Penicillin G or | 5 million units every 6 hours | IV |
| Ampicillin or amoxicillin | 2 g every 4-6 hours | IV | |
| Enterobacter and Actinobacter | Ceftazidime or cefepime plus | 2 g every 8 hours | IV |
| Aminoglycoside for 2 weeks followed by ciprofloxacin | 750 mg every 12 hours | PO | |
IV = intravenously; PO = by mouth.
Specimens of periprosthetic tissue (including the joint capsule, synovial lining, intramedullary material from curettage, granulation tissue, and bone fragments) were obtained during the revision procedure, or when the prosthesis was removed because of an infection, and were evaluated histologically. We used the criterion of acute inflammation (more than five polymorphonuclear leukocytes per high-power field) described by Mirra et al. [18]. Cultures of material obtained from the knee and by swabbing of the prosthesis as well as cultures of periprosthetic tissue were obtained. A minimum of five cultures was obtained for each patient, and all cultures were evaluated for aerobic, anaerobic, fungus, and tuberculous bacilli growth. Cultures that showed growth only in broth were not considered to be a positive finding.
On the second day after surgery, a continuous passive motion machine was used for passive ROM exercise twice daily for 30 minutes each time. The machine settings were advanced incrementally under the supervision of a physical therapist. Also, patients performed active ROM exercises under the supervision of a physical therapist. On the second postoperative day they began standing at the bedside or walking with crutches or a walker twice daily for 30 minutes, each time under the supervision of a physical therapist. Patients used crutches or a walker with full weightbearing for 6 weeks and then with a cane as needed thereafter. No patient received outpatient physiotherapy after discharge from the hospital.
Routine followup evaluation was scheduled at postoperative intervals of 6 weeks, 3 months, 6 months, 1 year, and yearly thereafter. At these intervals, we evaluated the patients and obtained radiographs. Preoperative and postoperative review data were recorded according to the systems of the Knee Society (KS) [12] and the Hospital for Special Surgery (HSS) [13]. All of the knees were evaluated by one observer (YWC) who was not connected with the original surgery, and the data were entered into a computerized record.
The criteria for infection control were: no pain or swelling; no wound drainage; normal serology (ESR < 20 mm/hr, CRP level < 0.5 mg/dL); a synovial fluid leukocyte differential of less than 65% neutrophils (or a leukocyte count < 1.7 × 103/uL); and a normal radiographic finding for at least 2 years after the end of antibiotic therapy along with a functional knee with a knee prosthesis in place at the time of the latest followup [30]. For a knee to be considered functional, there had to be no or only slight pain upon walking (with or without the use of a cane) and no radiographic findings, such as progressive osteolysis or loosening of the prosthetic components that indicated a need for immediate or impending surgical intervention. If the initial treatment failed, we recorded the number and type of subsequent courses of treatment that were attempted. In some instances, infection eradication was achieved only after multiple courses of treatment.
One (YHK) of us evaluated the final radiographs. We defined radiographic loosening as a complete radiolucent line of ≥ 2 mm in width at the bone-cement or prosthesis-cement interface or shift in position of a component or components on serial radiographic examination [14].
The differences among the four groups for discrete variables (including age, period of followup, CRP, ESR, leukocyte count, and leukocyte differential) were compared using Fisher’s exact probability two-tailed test. Logistic regression was used to assess the rate of failure associated with the different types of infection. One-way analysis of variance was used to confirm differences among the four groups with respect to the final clinical score. The level of significance was set at p < 0.05. All analyses were performed with SPSS, Version 14.0 (SPSS Inc, Chicago, IL).
Results
The initial course of treatment was successful for 28 of 29 knees (97%) with early superficial infection (Table 3), 27 of 32 knees (84%) with early deep postoperative infection (Table 4), 38 of 48 knees (79%) with late chronic postoperative infection (Table 5), and two of seven knees with acute hematogenous infection (Table 6).
Table 3.
Early superficial postoperative infections
| Treatment | Number of infections | Result | |
|---|---|---|---|
| Success | Failure | ||
| First treatment | |||
| Intravenous antibiotics only for 2–6 weeks | 29 knees | 28 knees (97%) | 1 knee |
| Second treatment | |||
| Débridement | – | 1 knee | – |
| Final result | 29 knees | 29 knees (100%) | – |
Table 4.
Early deep postoperative infections
| Treatment | Number of infections | Result | |
|---|---|---|---|
| Success | Failure | ||
| First treatment | |||
| Débridement | 32 knees | 27 knees (84%) | 5 knees (16%) |
| Second treatment | |||
| Débridement | 5 knees (16%) | 3 knees (9%) | 2 knees (6%) |
| Third treatment | |||
| Delayed-exchange arthroplasty | 2 knees | – | 2 knees |
| Fourth treatment | |||
| Arthrodesis | 2 knees | – | 2 knees |
| Final result | 32 knees | 30 knees (94%) | 2 knees (6%) |
Table 5.
Late chronic postoperative infections
| Treatment | Number of infections | Result | |
|---|---|---|---|
| Success | Failure | ||
| First treatment | |||
| Delayed-exchange arthroplasty | 48 knees | 38 knees (79%) | 10 knees (21%) |
| Second treatment | |||
| Delayed-exchange arthroplasty | 10 knees (21%) | 7 knees | 3 knees |
| Third treatment | |||
| Arthrodesis | 3 knees | – | 3 knees |
| Fourth treatment | |||
| Total knee arthroplasty after fusion takedown | 1 knee | 1 knee | – |
| Final result | 48 knees | 46 knees (96%) | 2 knees |
Table 6.
Acute hematogenous infection
| Treatment | Number of infections | Result | |
|---|---|---|---|
| Success | Failure | ||
| First treatment | |||
| Débridement | 7 knees | 2 knees | 5 knees |
| Second treatment | |||
| Delayed-exchange arthroplasty | 5 knees | 4 knees | 1 knee |
| Third treatment | |||
| Delayed-exchange arthroplasty | 1 knee | – | 1 knee |
| Fourth treatment | |||
| Arthrodesis | 1 knee | – | 1 knee |
| Final result | 7 knees | 6 knees | 1 knee |
Infection control and maintenance of functional TKA after first treatment were further improved by repeated débridement and repeated two-stage TKA (Table 7). At the last evaluation, all 29 knees (100%) with early superficial infection, 30 of 32 knees (94%) with early deep infections, 46 of 48 knees (96%) with late chronic infections, and six of seven knees with acute hematogenous infections maintained functioning total knee prostheses (Table 7). The mean KS knee and functional scores and HSS knee scores in the entire group were 87, 77, and 84 points, respectively, at the most recent evaluation. One hundred nine patients were able to walk with no or only slight pain (some with the assistance of a cane).
Table 7.
Results for types of infection
| Result | Group | ||||
|---|---|---|---|---|---|
| Early superficial postoperative infection (n = 29) | Early deep postoperative infection (n = 32) | Late chronic postoperative infection (n = 48) | Acute hematogenous infection (n = 7) | Overall (n = 116) | |
| Successful first treatment | 28 knees (97%) | 27 knees (84%) | 38 knees (79%) | 2 knees (29%) | 95 (82%) |
| Infection eradicated | 29 knees (100%) | 32 knees (100%) | 48 knees (100%) | 7 knees (100%) | 116 (100%) |
| Functional knee | 29 knees (100%) | 30 knees (94%) | 46 knees (96%) | 6 knees (86%) | 111 (96%) |
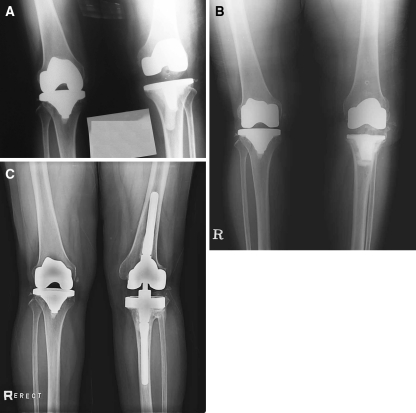
All of the 111 knees that obtained functioning TKA prostheses had stable fixation of the prosthesis to host bone. None of these 111 knees had evidence of a shift in the position of a component or of progressive osteolysis that would have indicated a need for immediate or impending surgical intervention. A 63-year-old woman with advanced osteoarthritis of both knees underwent a bilateral simultaneous TKA with an Omnifit prosthesis (Stryker, Mahwah, NJ). Early postoperative deep infection (1 week after surgery) developed in the left knee. The knee was treated with débridement, tibial insert exchange, and intravenous antibiotics for 6 weeks. The infection recurred, and she underwent delayed-exchange revision TKA with an LCS rotating platform prosthesis (DePuy, Warsaw, IN) (Fig. 1A). However, the infection recurred yet again, and she underwent a second delayed-exchange revision TKA with a NexGen PS prosthesis (Zimmer, Warsaw, IN) (Fig. 1B). Infection recurred again, and arthrodesis of the knee was performed. One year after arthrodesis of the left knee, TKA was carried out using a Legacy Condylar Constrained Knee (LCCK) prosthesis (Zimmer) after a fusion takedown (Fig. 1C).
Fig. 1A–C.
A 63-year-old woman with advanced osteoarthritis of both knees is shown. (A) An AP view of both knees reveals a revised left TKA with an LCS rotating platform prosthesis. (B) An AP view of both knees demonstrates a rerevised left TKA with a NexGen (PS) prosthesis. (C) An AP view of both knees shows a left TKA with an LCCK prosthesis after a fusion takedown.
Discussion
Using the most appropriate treatment for a given type of infection after TKA is paramount for controlling the infection. Classification using four types of infected TKA has been used to guide treatment, especially with regard to whether the prosthesis should be removed or retained. We asked whether (1) treatment according to the four types of infection after TKA would control infection and maintain functional TKA; (2) repeated débridement and repeated two-stage TKA would further improve the infection control rate and functional TKA after the initial treatment; and (3) fixation of the TKA prosthesis to host bone would be achieved.
We note several limitations. First, although all patients in this study were prospectively followed, the design of the study was to retrospectively test our classification-based treatment algorithm for infected TKA with a hypothesis that it would be successful. Second, owing to limited patient numbers, we were unable to analyze data for patients stratified according to infecting organism. It is possible that the infection control after infected TKA was influenced by the virulence of the infecting organism. Third, the pain and function scores of a revision of TKA are difficult to assess because they are influenced by many factors: the etiology of failure, the extent of bone loss, the quality of the soft tissues, the reconstruction technique, the adequacy of rehabilitation, patient compliance, the duration of followup, and the mode of assessment. None of these factors were analyzed in this study because the sample size was too small. Thus, more cases must be studied to analyze the pain and function scores of revisions of infected TKA. Finally, considering that the number of reinfections increased with time [8], the followup of some patients may be too short to ensure all infections were indeed controlled. However, we included all patients regardless of the cause or presentation of infection and had complete followup on all patients. We presume the percentage of any subsequent infections would be small and not alter our conclusions.
Because of the risk of subsequent deep infection [16, 26], superficial infection should be treated intensively [17, 22, 33] (Table 8). For the 28 of 29 patients with early superficial postoperative infection in our study, 2 to 6 weeks of antibiotics was sufficient to eradicate infection. In our series, the overall infection control rate of the initial treatment was 72%. This infection control rate is substantially worse than 81% reported in other series [26]. One reason for the worse result is the high incidence (28%) of methicillin-resistant Staphylococcus aureus and Gram-negative bacilli among our patients. Another possible reason is the problems inherent in soft tissue healing. A knee that has undergone surgery lies superficially beneath the skin and the fascia envelope and is covered by a limited amount of well-vascularized muscle. The so-called watershed area of the vascular supply to the skin that lies anteriorly in the path of the typical skin incisions is a potential area of impaired wound healing [1].
Table 8.
Infection Control Rate in the Four Types of Infected TKA
| Authors | Number of Cases | Age* (years) | Followup* (years) | Infection control rate |
|---|---|---|---|---|
| 1. Early Superficial Infection | ||||
| Johnson and Bannister [16] | 23 | NA | 2.8 (1–5) | 23/23 (100%) |
| Rasul et al. [22] | 13 | 65.2 (59–82) | 2.7 (2–4) | 6/6 (100%) |
| Segawa et al. [26] | 6 | NA | 4.0 (0.3–14) | 13/13 (100%) |
| Weiss and Krackow [33] | 8 | 68.1 (59–75) | 4.3 (1.5–9.5) | 8/8 (100%) |
| Kim et al. [current study] | 29 | 65 (37–90) | 5.6 (2–8) | 29/29 (100%) |
| 2. Early Deep Infection | ||||
| Borden and Gearen [2] | 6 | 61 (28–78) | 3.8 (2.3–9.2) | 6/6 (100%) |
| Hartman et al. [10] | 33 | 71.2 (44–85) | 4.5 (2–9) | 21/33 (64%) |
| Mont et al. [19] | 24 | 66 (46–80) | 4 (2–11.7) | 20/24 (83%) |
| Rand and Bryan [21] | 14 | 61 (24–86) | 6.9 (3.8–12.2) | 8/14 (57%) |
| Schoifet and Morrey [25] | 31 | 59 (36–83) | 5.4 (0.6–12.8) | 7/31 (23%) |
| Segawa et al. [26] | 10 | 67 (32–87) | 3.7 (0.3–8.8) | 9/10 (90%) |
| Teeny et al. [29] | 24 | 5.8 (30–74) | 4 (2–12) | 20/24 (83%) |
| Kim et al. [current study] | 32 | 65 (37–90) | 5.6 (2–8) | 32/32 (100%) |
| 3. Late Chronic Infection | ||||
| Borden and Gearen [2] | 15 | 61 (28–78) | 3.8 (2.3–9.2) | 12/15 (80%) |
| Goldman et al. [6] | 64 | 67 (37–89) | 7.5 (2–17) | 62/64 (97%) |
| Hart and Jones [9] | 48 | 68.2 (37.2–81.3) | 4 (2.2–7.1) | 42/48 (88%) |
| Insall et al. [14] | 11 | 67.3 (60–86) | 2.8 (1–6) | 11/11 (100%) |
| Rosenberg et al. [23] | 26 | 67 (54–80) | 2.4 (1–4.8) | 26/26 (100%) |
| Segawa et al. [26] | 24 | 67 (32–87) | 3.3 (0.4–8.5) | 26/29 (90%) |
| Windsor et al. [35] | 38 | 67.3 (60–86) | 4 (2.5–10) | 37/38 (97.4%) |
| Kim et al. [current study] | 48 | 65 (37–90) | 5.6 (2–8) | 48/48 (100%) |
| 4. Acute Hematogenous Infection | ||||
| Mont et al. [19] | 14 | 66 (46–80) | 4 (2–11.7) | 10/14 (71%) |
| Segawa et al. [26] | 7 | 67 (32–87) | 3.3 (0.3–5.5) | 5/7 (71%) |
| Kim et al. [current study] | 7 | 65 (37–90) | 5.6 (2–8) | 7/7 (100%) |
* Values are expressed as mean, with range in parentheses; NA = not applicable.
In various series ranging in size from five to 35 infections, the overall infection control rate associated with the use of irrigation and débridement for the treatment of early deep postoperative infection ranged from 0% to 100% [2, 10, 19, 21, 25, 29] (Table 8). Mont et al. [19] reported that a protocol of multiple débridements was successful for the treatment of 10 of 10 early deep postoperative infections (defined as those that occurred < 4 weeks after the index arthroplasty). In our study, the success rate of initial treatment in the early deep postoperative infection was 84%, which is higher than the 50% reported for other series [26]. The infection control rate was improved to 100% after repeated débridement and repeated two-stage revision. The infection control rate of the initial treatment in the late chronic infection in our series (79%) was slightly worse than the 83% reported for other series [26]. After repeated débridement and repeated two-stage revision, the infection control rate improved to 100%. The infection control rate in our study (100%; 48 of 48 infections) is better than the rates reported by others [2, 6, 8, 9, 14, 23, 35] (Table 8). In our study, seven knees with late chronic infection had good control of infection after the second two-stage revision. We believe at least two attempts at two-stage revision are worthwhile for the treatment of late chronic infection. The infection control rate (29%) of the first course of treatment (retention of the prosthesis, débridement, and antibiotics for 6 weeks) for acute hematogenous infection in our series was lower than the rates reported by others [1, 19, 26] (Table 8). We are not certain why this was the case.
Segawa et al. [26] reported five of 10 patients with early deep postoperative infection, all 13 patients with early superficial postoperative infections, and 24 of 29 with late chronic postoperative infections were able to walk with no or only slight pain (some with the assistance of a cane). None of their patients had radiographic findings (such as evidence of implant migration or progressive osteolysis) that would have indicated a need for immediate or impending surgical intervention. In our study, 109 patients (111 knees) of 114 patients (116 knees) were able to walk with no or only slight pain (some with the assistance of a cane). None of our patients had component migration or progressive osteolysis that would have indicated a need for immediate or impending surgical intervention.
The data suggest that treatment according to four types of infection after TKA controlled infection and maintained functional TKA with a firm level of fixation for most patients. Repeated débridement and two-stage TKA further improved the infection control rate after the initial treatment and increased the likelihood of maintaining a functional TKA.
Acknowledgments
We thank Sang-Mi Lee, BA for compiling the medical records.
Footnotes
Each author certifies that he or she has no commercial association (eg, consultancies, stock ownership, equity interest, patent, licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that written informed consent for participation in the study was obtained.
References
- 1.Bengtson S, Knutson K. The infected knee arthroplasty. A 6-year follow-up of 357 cases. Acta Orthop Scand. 1991;62:301–311. doi: 10.3109/17453679108994458. [DOI] [PubMed] [Google Scholar]
- 2.Borden LS, Gearen PF. Infected total knee arthroplasty. A protocol for management. J Arthroplasty. 1987;2:27–36. doi: 10.1016/S0883-5403(87)80028-1. [DOI] [PubMed] [Google Scholar]
- 3.Brandt CM, Duffy MC, Berbari EF, Hanssen AD, Steckelberg JM, Osmon DR. Staphylococcus aureus prosthetic joint infection treated with prosthesis removal and delayed reimplantation arthroplasty. Mayo Clin Proc. 1999;74:553–558. doi: 10.4065/74.6.553. [DOI] [PubMed] [Google Scholar]
- 4.Burger RR, Basch T, Hopson CN. Implant salvage in infected total knee arthroplasty. Clin Orthop Relat Res. 1991;273:105–112. [PubMed] [Google Scholar]
- 5.Deirmengian C, Greenbaum J, Stern J, Braffman M, Lotke PA, Booth RE, Jr, Lonner JH. Open débridement of acute gram-positive infections after total knee arthroplasty. Clin Orthop Relat Res. 2003;416:129–134. doi: 10.1097/01.blo.0000092996.90435.35. [DOI] [PubMed] [Google Scholar]
- 6.Goldman RT, Scuderi GR, Insall JN. 2-stage reimplantation for infected total knee replacement. Clin Orthop Relat Res. 1996;331:118–124. doi: 10.1097/00003086-199610000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Gristina AG, Kolkin J. Current concepts review. Total joint replacement and sepsis. J Bone Joint Surg Am. 1983;65:128–134. [PubMed] [Google Scholar]
- 8.Hanssen AD, Trousdale RT, Osmon DR. Patient outcome with reinfection following reimplantation for the infected total knee arthroplasty. Clin Orthop Relat Res. 1995;321:55–67. [PubMed] [Google Scholar]
- 9.Hart WJ, Jones RS. Two-stage revision of infected total knee replacements using articulating cement spacers and short-term antibiotic therapy. J Bone Joint Surg Br. 2006;88:1011–1015. doi: 10.2106/JBJS.E.01077. [DOI] [PubMed] [Google Scholar]
- 10.Hartman MB, Fehring TK, Jordan L, Norton HJ. Periprosthetic knee sepsis. The role of irrigation and débridement. Clin Orthop Relat Res. 1991;273:113–118. [PubMed] [Google Scholar]
- 11.Hirakawa K, Stulberg BN, Wilde AH, Bauer TW, Secic M. Results of 2-stage reimplantation for infected total knee arthroplasty. J Arthroplasty. 1998;13:22–28. doi: 10.1016/S0883-5403(98)90071-7. [DOI] [PubMed] [Google Scholar]
- 12.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 13.Insall JN, Ranawat CS, Aglietti P, Shine J. A comparison of four models of total knee-replacement prostheses. J Bone Joint Surg Am. 1976;58:754–765. [PubMed] [Google Scholar]
- 14.Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65:1087–1098. [PubMed] [Google Scholar]
- 15.Jacobs MA, Hungerford DS, Krackow KA, Lennox DW. Revision of septic total knee arthroplasty. Clin Orthop Relat Res. 1989;238:159–166. [PubMed] [Google Scholar]
- 16.Johnson DP, Bannister GC. The outcome of infected arthroplasty of the knee. J Bone Joint Surg Br. 1986;68:289–291. doi: 10.1302/0301-620X.68B2.3958017. [DOI] [PubMed] [Google Scholar]
- 17.McLaren AC, Spooner CE. Salvage of infected total knee components. Clin Orthop Relat Res. 1996;331:146–150. doi: 10.1097/00003086-199610000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Mirra JM, Amstutz HC, Matos M, Gold R. The pathology of the joint tissues and its clinical relevance in prosthesis failure. Clin Orthop Relat Res. 1976;117:221–240. [PubMed] [Google Scholar]
- 19.Mont MA, Waldman B, Banerjee C, Pacheco IH, Hungerford DS. Multiple irrigation, débridement, and retention of components in infected total knee arthroplasty. J Arthroplasty. 1997;12:426–433. doi: 10.1016/S0883-5403(97)90199-6. [DOI] [PubMed] [Google Scholar]
- 20.Morrey BF, Westholm F, Schoifet S, Rand JA, Bryan RS. Long-term results of various treatment options for infected total knee arthroplasty. Clin Orthop Relat Res. 1989;248:120–128. [PubMed] [Google Scholar]
- 21.Rand JA, Bryan RS. Reimplantation for the salvage of an infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65:1081–1086. [PubMed] [Google Scholar]
- 22.Rasul AT, Jr, Tsukayama D, Gustilo RB. Effect of time of onset and depth of infection on the outcome of total knee arthroplasty infections. Clin Orthop Relat Res. 1991;273:98–104. [PubMed] [Google Scholar]
- 23.Rosenberg AG, Haas B, Barden R, Marquez D, Landon GC, Galante JO. Salvage of infected total knee arthroplasty. Clin Orthop Relat Res. 1988;226:29–33. [PubMed] [Google Scholar]
- 24.Saleh K, Olson M, Resig S, Bershadsky B, Kuskowski M, Gioe T, Robinson H, Schmidt R, McElfresh E. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20:506–515. doi: 10.1016/S0736-0266(01)00153-X. [DOI] [PubMed] [Google Scholar]
- 25.Schoifet SD, Morrey BF. Treatment of infection after total knee arthroplasty by débridement with retention of the components. J Bone Joint Surg Am. 1990;72:1383–1390. [PubMed] [Google Scholar]
- 26.Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81:1434–1445. doi: 10.2106/00004623-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Sierra RJ, Trousdale RT, Pagnano MW. Above-the-knee amputation after a total knee replacement: prevalence, etiology, and functional outcome. J Bone Joint Surg Am. 2003;85:1000–1004. doi: 10.2106/00004623-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Simmons TD, Stern SH. Diagnosis and management of the infected total knee arthroplasty. Am J Knee Surg. 1996;9:99–106. [PubMed] [Google Scholar]
- 29.Teeny SM, Dorr L, Murata G, Conaty P. Treatment of infected total knee arthroplasty. Irrigation and débridement versus two-stage reimplantation. J Arthroplasty. 1990;5:35–39. doi: 10.1016/S0883-5403(06)80007-0. [DOI] [PubMed] [Google Scholar]
- 30.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004;117:556–562. doi: 10.1016/j.amjmed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 31.Tsukayama DT, Goldberg VM, Kyle R. Diagnosis and management of infection after total knee arthroplasty. J Bone Joint Surg Am. 2003;85(Suppl 1):75–80. doi: 10.2106/00004623-200300001-00014. [DOI] [PubMed] [Google Scholar]
- 32.Wang CJ, Huang TW, Wang JW, Chen HS. The often poor clinical outcome of infected total knee arthroplasty. J Arthroplasty. 2002;17:608–614. doi: 10.1054/arth.2002.32700. [DOI] [PubMed] [Google Scholar]
- 33.Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8:285–289. doi: 10.1016/S0883-5403(06)80091-4. [DOI] [PubMed] [Google Scholar]
- 34.Wilde AH, Ruth JT. Two-stage reimplantation in infected total knee arthroplasty. Clin Orthop Relat Res. 1988;236:23–35. [PubMed] [Google Scholar]
- 35.Windsor RE, Insall JN, Urs WK, Miller DV, Brause BD. Two-stage reimplantation for the salvage of total knee arthroplasty complicated by infection. Further follow-up and refinement of indications. J Bone Joint Surg Am. 1990;72:272–278. [PubMed] [Google Scholar]