Abstract
Background
Revision of the infected hip arthroplasty with major bone loss is difficult. Attempts to restore bone stock with structural allograft are controversial.
Questions/purposes
We assessed the (1) reinfection rate; (2) rerevision rate; (3) radiographic graft union, resorption, and implant migration; (4) Harris hip scores at 1 year and at last followup compared with before surgery; and (5) other major complications associated with the use of bulk structural allograft to treat massive bone loss in infected hip arthroplasty.
Methods
We retrospectively reviewed 27 patients who underwent two-stage revision arthroplasty using structural allograft to treat massive bone defects in infected hip arthroplasty. There were 17 proximal femoral grafts, three acetabular major column grafts, two acetabular minor column grafts, and 10 cortical strut grafts used. Five patients had combinations of two allografts. The minimum followup was 1.1 years (mean, 8.2 years; range, 1.1–16.8 years).
Results
One of 27 patients had reinfection. The Kaplan-Meier survivorship was 93% at 10 years with rerevision for aseptic loosening as the end point. Radiographically, three patients had nonunion at the graft–host junction. All patients except two had graft resorption, of which all were mild except two, which were severe. Three patients had implant migration. The mean modified Harris hip scores were 39.2 points (range, 25–60) preoperatively, 67.3 points (range, 40–91) at 1-year followup, and 70.3 points (range, 46–81) at last followup. Other major complications included one patient with dislocation and one patient with transient sciatic nerve injury.
Conclusions
Based on our data, we believe the use of structural allografts is a reasonable option for treating massive bone loss in infected hip arthroplasties.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The failed hip arthroplasty with concurrent deep infection and massive bone loss is a complex situation and may present as one of the most challenging aspects of hip surgery. Treatment options are few. Excision arthroplasty eradicates infection in 86% to 100% of patients [26, 29] and relieves pain in 30% to 59% of patients [26, 29], but it functionally impairs the patient with major leg length discrepancy and poor walking ability [5, 6, 15]. It is now reserved as a salvage technique. The use of a tumor megaprosthesis after eradication of infection may restore function, but it does not restore bone stock and has been associated with a higher rate of dislocation (28%) and poorer implant survivorship (58% at 10 years) [42] when compared with proximal femoral allograft prosthetic composite (76% at 10 years, 0% dislocation) [42]. The use of total femur replacement has been associated with higher hip instability and dislocation rates [12, 42]. For large uncontained segmental acetabular defects that consisted of more than 30% of the host acetabulum, options include impaction bone grafting, reinforcement ring, oblong cup and trabecular metal cup, and augment and structural allograft. With such defects, there are concerns over cup migration and early failure with impaction grafting [7, 39], survivorships of the reinforcement ring (80%–90% with 6 years mean followup) [13, 36], and uncemented oblong cup (86%–93% at 6–9 years followup) [18, 21]. These studies, however, also involved patients with contained defects. The survivorship at 3 years on trabecular metal cups and augments were above 95%, but these were short-term studies [22, 37]. The cup survivorship associated with acetabular structural allograft has been comparable with 78% to 80% and graft survivorship 94% to 100% at 10 years mean followup [38, 41]. In these studies, the majority of rerevisions with cup-only exchanges indicated the added advantage of restoring host bone stock to aid future rerevisions.
The concept of eradicating infection while restoring function with arthroplasty is appealing. However, with massive bone loss, it is not feasible to use autograft and with uncontained segmental bone loss, impaction bone grafting may be limited because of concerns over implant migration and periprosthetic fractures [28, 30]. The use of bulk structural allografts to restore bone stock in a previously infected environment is controversial [1, 3, 33, 40] with reinfection rates ranging from 0% to 14% [1, 3].
We therefore assessed the (1) reinfection rate; (2) implant survivorship; (3) radiographic graft union, resorption, and cup migration; (4) Harris hip scores at 1 year followup and at last followup compared with preoperative scores; and (5) other major complications associated with the use of bulk structural allograft for treating infected hip arthroplasty with massive bone loss.
Patients and Methods
From our prospectively collected database, we identified all patients who had undergone two-stage revision using bulk structural allograft for a failed infected hip arthroplasty with massive bone loss at our institution between 1986 and 2005. Some of the patients have been involved in an earlier report (n = 11) [1]. Twenty-seven patients were identified with a minimum followup of 1.1 years (mean, 8.2 years; range, 1.1–16.8 years). The mean age was 62 years (range, 28–83 years). The underlying cause for primary hip arthroplasty was osteoarthritis (14 patients) followed by infection (five), developmental hip dysplasia (three), fracture (two), avascular necrosis (one), Perthes disease (one), and slipped upper femoral epiphysis (one). The mean total number of previous operations before the index revision arthroplasty was 2.4 (range, 1–7). Two patients died of unrelated causes after a followup of 2 and 9 years, respectively. Six of the 27 patients were lost to followup after a minimum followup of 1.1 years (mean, 6.2 years; range, 1.1–14.2 years). For survivorship analysis, we have excluded patients with less than 2 years followup. This left 26 patients for analysis at a mean followup of 8.8 years (range, 2.1–16.8 years).
All wound swab or hip aspirate growth cultures were routinely taken after 2 weeks of stopping antibiotics before the first stage of revision. All patients had a positive wound or hip aspirate growth culture except five. These five patients had negative growth cultures but had been on long-term antibiotic treatment for deep infection manifested by persistently discharging wounds or sinuses with elevated inflammatory markers. The most common organism isolated was Staphylococcus aureus. Others included Staphylococcus epidermidis, Streptococcus milleri, Enterococcus faecalis, Escherichia coli, Actinobacteria, and Candida. Four patients had positive cultures for multiple organisms.
Our indications for revision of an infected hip arthroplasty include: (1) a positive culture from persistent wound discharge; (2) a positive culture from a hip aspirate; or (3) a persistently discharging wound with elevated inflammatory markers despite long-term antibiotic treatment and a negative wound culture. With reference to our previously published classifications on acetabular and femoral bone defects [35], our indications for using bulk structural allograft were as follows: (1) a minor column acetabular allograft was indicated in an uncontained segmental defect size that consisted more than 30% but less than 50% of the host acetabulum (Type 3); (2) a major column acetabular allograft was indicated in an uncontained segmental defect that consisted more than 50% of the host acetabulum (Type 4); (3) a proximal femoral allograft for an uncontained proximal femoral segmental defect extending more than 5 cm distal to the lesser trochanter (Type 4); and (4) a cortical strut allograft for a femoral defect that causes difficulty and instability in seating the femoral stem in the femur (Types 3 and 4). The contraindications for surgery were: (1) unresolved infection after first-stage implant removal, débridement, and washout; (2) patients who were medically unfit to undergo prolonged surgery; and (3) patients who were unable to comply with the postoperative rehabilitation program.
We used a two-stage revision procedure to treat patients with infected THAs. In the first stage of the revision, we removed all hardware and cement, thoroughly débrided any visibly devitalized tissue, and obtained multiple tissue samples for microbiologic culture and sensitivity tests. We washed out the wound repeatedly and sequentially with 5% povidone–iodine solution (Rougier Pharma, Montreal, Canada), 1% hydrogen peroxide solution (Jedmon Products Ltd, Toronto, Canada), and Bacitracin solution (50,000 U/L of 0.9% normal saline solution). The host bone defect was assessed and the decision to use bulk structural allograft in the second stage was confirmed. We routinely insert an articulating antibiotic-impregnated cement spacer to maintain range of movement, leg length, and soft tissue tension. The antibiotics used were specific to culture results if positive and broad spectrum if culture results were negative. We used up to 4 g of powdered antibiotics per bag of cement. The patients routinely continued intravenous antibiotics for at least 6 weeks.
The second stage was usually scheduled at more than 3 months after the first stage. Before the second stage, antibiotics were discontinued for 2 weeks and then hip aspiration was performed and tests for inflammatory markers repeated (C-reactive protein, erythrocyte sedimentation rate). If the aspirate was positive on growth culture and the inflammatory markers abnormal, then another wound débridement and washout with reinsertion of a second antibiotic-impregnated spacer was scheduled. We had previously published our surgical techniques for revision arthroplasty [2, 16]. We used allograft from our hospital bone bank, which was accredited by the Musculoskeletal Council of the American Association of Tissue Banks [11, 31]. Intraoperatively, multiple tissue samples were sent for Gram stain, frozen section, and microscopy under high-power field to assess for polymorphonuclear cell count. If there was any suggestion of a persisting infection during surgery such as more than five polymorphonuclear cell count per high-power field on frozen section [24], we would abandon insertion of the definitive prosthesis, conduct another thorough wound débridement and washout, reinsert another antibiotic-impregnated cement spacer, repeat antibiotic treatment for another 6 weeks, and reschedule the second stage of the revision arthroplasty. We routinely give broad-spectrum antibiotics based on general references during the second stage of the revision after tissue sampling. The mean duration between the first and second stages was 5.5 months (range, 1.9–14 months). One patient had necrotizing fasciitis treated with excision arthroplasty and required skin grafting to the whole flank. Wound healing was protracted and the duration between excision arthroplasty and the definitive arthroplasty was 4 years 3 months. We excluded this patient from the mean duration calculation to avoid skewing the data.
The allografts used included 17 proximal femoral grafts, three major column acetabular grafts, two minor column acetabular grafts, and 10 cortical strut grafts. One patient with major column grafts had a concurrent proximal femoral graft, another with major column graft had a concurrent strut graft, and three patients with proximal femoral grafts had concurrent cortical strut grafts. Twelve patients had additional morselized allograft for contained defects but were not considered for the purpose of this study. The femoral stem used that was cemented into a proximal femoral allograft was a Long Stem Hip (Johnson & Johnson, Warsaw, IN). Without the use of proximal femoral graft, femoral stems used were uncemented modular distally fit porous coat ZMR (Zimmer, Warsaw, IN). The acetabular components used were mainly uncemented Trabecular Metal (Zimmer), Harris-Galante (Zimmer), and PCA (Howmedica, Rutherford, NJ) and occasionally cemented Mueller (Biomet, Budapest, Hungary) cups.
We routinely started prophylactic intravenous antibiotics at induction and continued for 5 days after surgery. Deep vein thrombosis prophylaxis with subcutaneous enoxaparin was continued for 21 days after surgery; coumarin was used early in the series before enoxaparin became available. Patients were instructed to avoid active abduction for 6 weeks, but passive assisted abduction with a physiotherapist was permitted. Nonweightbearing mobilization with two crutches was allowed, but weightbearing was avoided for 3 months until radiographic evidence of graft–host union was confirmed. This was evidenced radiographically by bridging trabecular bone between allograft and host bone. Physiotherapy to improve the gait pattern was occasionally prescribed thereafter, for example, for hip extensor or abductor strengthening for 2 to 6 months.
All patients were routinely followed up at 6 weeks, 3 months, 6 months, 1 year, and then annually. Routine clinical assessment included gait, leg length, wound, ROM, and neurologic status. Radiographic assessment included an AP pelvic view and a lateral view of the hip and entire prosthesis. For modified Harris hip scores, we were able to obtain preoperative and at 1-year followup scores on 15 patients and preoperative and at last followup scores on 10 patients. We recorded the: (1) reinfection rate; (2) radiographic graft union, resorption, and cup migration; (3) modified Harris hip scores [32] at 1 year and last followup compared with preoperative scores; and (4) other major complications. We defined major complication as one that required further surgery, one that involved significant disability, for example deep infection or nerve injury, or death.
Two of us (PTHL, OAS) evaluated 14 of 26 available radiographs at last followup. Followup radiographs for the 12 patients who had revision surgery in the 1990s and 1980s were not available since the institution converted to digital imaging in 2003 and this resulted in a loss of the hard-copy radiographic archives. We assessed graft union, implant migration, and graft resorption. Graft union was considered to occur when there was radiographic evidence of trabecular bridging at the graft–host interface. We judged clinically important implant migration to occur when more than 5 mm of migration when comparing radiographs obtained at the last followup with those obtained soon after the index operation [27]. Acetabular graft resorption was assessed and classified using the techniques described by Jasty and Harris [20]. Graft resorption was considered to occur when there was an area of lucency within the graft on an AP pelvic radiograph. Resorption was considered “mild” when the area of resorption involved, seen as lucency, was less than one-third of the length of the graft; “moderate” when it involved more than a third but less than half of the length of the graft; and “severe” when it involved more than half of the length of the graft. Assessments were made by comparing radiographs obtained soon after the index operation and radiographs obtained at the last followup visit. Femoral graft resorption was assessed and classified using the techniques described by Gross and Hutchinson [17]. “Mild” resorption involved partial-thickness resorption of less than 1 cm in length, “moderate” resorption involved partial-thickness resorption of greater than 1 cm in length, and “severe” resorption involved full-thickness resorption at any length. We expressed interobserver variability as a percentage of the difference in observations between the two observers for each radiographic observation. Our interobserver error for graft union was 14% (two of 14), implant migration was 14% (two of 14), and graft resorption was 50% (seven of 14), of which five of seven were the result of discrepancies between “no” and “mild” graft resorption.
The implant survivorship was calculated with Kaplan-Meier analysis with revision for aseptic loosening as the end point. The Harris hip scores were analyzed with the Mann-Whitney U test.
Results
The reinfection rate was 3.7% (one of 27). One patient had a postoperative infection treated with wound débridement, washout, and long-term suppressive antibiotics.
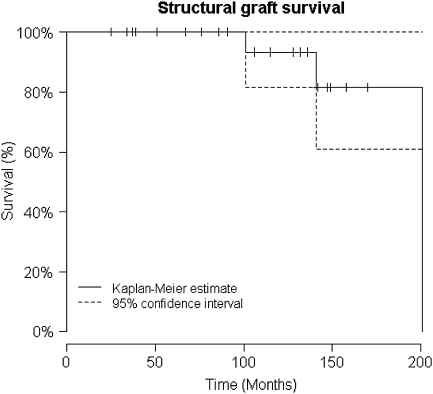
The Kaplan-Meier survivorship was 93% at 10 years with rerevision for aseptic loosening as the end point (Fig. 1). The three patients with clinical and radiographic implant loosening had rerevision surgery; one had a stem exchange and retained the previous proximal femoral allograft at 14.8 years after the index operation, one had a major column graft revised with a contour ring at 1.8 years, and one had a rerevision of the stem and cortical strut in addition to a liner exchange at 4.7 years.
Fig. 1.
The Kaplan-Meier survivorship for implant with rerevision for aseptic loosening as end point is shown; the survivorship was 93% at 10 years.
Radiographically, three patients had nonunions at the graft–host junction (one major column graft and two proximal femoral grafts). All patients except two had some degree of radiographic graft resorption; all were mild except for two with severe resorption. Of the three patients who had rerevision surgery with radiographic loosening (mentioned previously), two had no (cortical strut) or mild (proximal femoral) graft resorption, whereas the third had severe (major column) graft resorption. With regard to implant migration, there were three patients with implant migration. Of the three patients, one had a major column allograft with concurrent graft nonunion (one of the three mentioned previously), severe graft resorption (one of the two mentioned), and required rerevision (one of the three mentioned). The other two patients with implant migration had proximal femoral graft with nonunions at the graft–host junction (two of the three mentioned) and required open reduction and internal fixation with a plate and cortical strut graft. The mean preoperative modified Harris hip score was 39.2 points (range, 25–60), the mean score at 1 year followup was 67.3 points (range, 40–91, p = 0.19), and at the last followup was 70.3 points (range, 46–81, p = 0.15).
Other major complications included one patient with dislocation that required revision to a constrained cup, one patient with sciatic nerve injury that recovered with nonoperative management with observation only, and two unrelated deaths.
Discussion
The use of bulk structural allografts to restore bone stock in a previously infected environment is controversial. We therefore assessed the (1) reinfection rate; (2) implant survivorship; (3) radiographic graft union, resorption, and cup migration; (4) modified Harris hip scores at 1-year followup and at last followup compared with preoperative scores; and (5) other major complications associated with the use of bulk structural allograft for treating infected hip arthroplasty with massive bone loss.
There were several limitations to our study. First, the cohort was a heterogeneous group of patients. The number of previous hip operations the patients had before the index revision surgery ranged from one to seven and there was a range of difficulties in performing revision surgery for different patients (Fig. 2). Although all patients had bulk structural allografts, the types ranged from proximal femoral graft, major and minor acetabular column grafts, and cortical strut grafts with five patients receiving a combination of two structural grafts and 12 patients receiving concurrent morselized bone graft. Nevertheless, we believed the cohort gave a realistic overall impression of the risks of reinfection in the use of bulk structural allograft for the treatment of infected hip arthroplasty with massive bone loss and associated complications. Second, we included patients with a minimum followup of 2 years, although bulk structural graft may take longer to fail. We believed this cohort of patient with deep infection and severe bone loss poses a very difficult challenge with generally poorer overall outcomes even at the early postoperative period to justify the 2-year minimum followup. Third, only 14 of 26 sets of radiographs were available for assessment. This was because all of the 12 patients who were without radiographs for graft assessment had the index revision surgery in the 1990s and 1980s. The institution converted to digital imaging in 2003 and this resulted in a loss of a proportion of the hard-copy radiographic archive. Fourth, our interobserver errors for graft union and implant migration were both 14% (two of 14), but our error on graft resorption was 50% (seven of 14). We found difficulty in differentiating graft with no resorption from graft with mild resorption on the acetabular and femoral sides. These comprised the majority of the error on assessing graft resorption (five of seven). We found no reports on interobserver variability in interpreting radiographs for union and extent of resorption for structural allograft with which to compare our findings. Fifth, for modified Harris hip scores, we obtained preoperative and 1-year followup scores on 15 patients and preoperative and at last followup scores on 10 patients. This was accepted as part of the limitation for a retrospective study.
Fig. 2A–H.
Preoperative anteroposterior radiographs of the (A) proximal and (B) distal femur show an infected THA. The anteroposterior (C) pelvic and (D) femoral radiographs show an initial postoperative first-stage revision with a cement spacer. The radiographs show the (E) anterior and (F) lateral femur after further débridement and washout with temporary excision arthroplasty. Radiographic appearances of the (G) proximal and (H) distal femur 14 months after a second-stage revision are shown.
One of the main concerns with the use of bulk structural allograft to treat massive bone loss in revision hip arthroplasty for infection is the theoretical increased risk for reinfection. Bulk structural allograft is a large-volume nonvascularized organic tissue and may theoretically impair access of antibiotics and the host’s cellular defense mechanisms to organisms attached to it. We have previously published this technique on a smaller series [1] with no reinfections (n = 11) at a minimum followup of 2 years (mean, 4 years; range, 2–6 years). Nusem and Morgan [33] reported a 5.6% (n = 18) reinfection rate at a minimum followup of 5 years (mean, 9 years; range, 5–14 years). Both studies involved the use of bulk structural allograft for large bone defects in revision hip arthroplasty for infection. In this study, our reinfection rate of 3.7% was comparable to the previously mentioned studies and other studies involving the use of morselized allograft or a mixed cohort of morselized and structural allografts. English et al. [9] reported a 7.5% (n = 53) reinfection rate, whereas Ammon and Stockley [3] reported a 14% (n = 57) reinfection rate, both of which used a two-stage revision and impaction allografting technique. Hsieh et al. [19] reported no reinfections, whereas Wang and Chen [40] reported a 9% reinfection rate, both of which used two-stage revision in a mixed cohort of patients receiving morselized or structural allografts at 2 to 7 years followup.
Deep infection after the use of bulk structural allograft is devastating with potentially hazardous consequences. In the majority of cases, complete resection of the allograft, including resection arthroplasty, with long-term antibiotic treatment is necessary to control the infection [8, 25]. In one patient with reinfection, we were able to manage the infection to a clinically acceptable level with fairly conservative measures. The patient had several comorbidities when she had a two-stage revision arthroplasty with proximal femoral and cortical strut grafts. She had a bladder infection 4 years later with subsequent reinfection of her hip. On surgical exploration, the infection extended deep to the fascia lata, but the implants were well-fixed and allograft incorporated. We opted for a trial of wound débridement, washout, and long-term suppressant antibiotics to optimize functional outcomes and reserved excision arthroplasty as a salvage procedure. The patient is currently more than 6 years postoperative and well. Her wound has healed, the pain has resolved, the implant remains stable, she walks with one stick, and she improved in her modified Harris hip scores by 52 at the last followup compared with preoperative scores.
Our rerevision rate for aseptic loosening was 11.5% and the allograft nonunion rate was 15.4% at a mean followup of 8.8 years. These figures were comparable to other studies in which revisions using bulk structural allografts were performed for aseptically loosened THA with aseptic loosening rates ranging from 4% to 14.3% and nonunion rates ranging from 6% to 28.6% at 4.4 to 16 years followup [14, 23, 34].
The mean modified Harris hip scores improved by 28.1 points at 1-year followup and 31.1 points at the last followup. There are only two other reports of using bulk structural allograft for revising infected hip arthroplasty. The improvement on Harris hip scores in this study was less than our previous report on 11 patients in which the mean improvement was 45.1 [1] and slightly less than the other report of 37 points [33]. The previous studies have not reported on any statistical tests for functional scores.
With regard to major complications, one patient had a dislocation and required a change to a constrained liner. Similarly, in the report by Nusem and Morgan [33] (n = 18), one of the two patients with postoperative dislocations required change to a constrained liner. The dislocation by the patient with proximal femoral allograft in the present study gave a dislocation rate of 3.7%, which was lower than other studies using a megaprosthesis for revision surgery without infection [42].
The use of bulk structural allograft allows restoration of host-depleted bone stock to facilitate future revision surgery for aseptic loosening. It also facilitates the preservation and reconstruction of the soft tissue envelope, including the greater trochanter and abductor mechanism. Several comparative studies to revision arthroplasty using tumor megaprostheses demonstrate superior functional outcomes in revision arthroplasties with the use of bulk structural allografts [4, 10, 42]. We believe the data support the use of bulk structural allografts in two-stage revisions for infected hip arthroplasties with massive bone loss in selected patients.
Acknowledgment
We thank Mathew MacDonald for help with data processing.
Footnotes
One or more of the authors (PTHL) received funding from the Royal College of Surgeons of England, the British Orthopaedic Association, and the John Charnley Trust.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Alexeef M, Mahomed N, Morsi E, Garbuz D, Gross A. Structural allograft in two-stage revision for failed septic hip arthroplasty. J Bone Joint Surg Br. 1996;78:213–216. [PubMed] [Google Scholar]
- 2.Allan DG, Lavoie GJ, McDonald S, Oakeshott R, Gross AE. Proximal femoral allografts in revision hip arthroplasty. J Bone Joint Surg Br. 1991;73:235–240. doi: 10.1302/0301-620X.73B2.2005146. [DOI] [PubMed] [Google Scholar]
- 3.Ammon P, Stockley I. Allograft bone in two-stage revision of the hip for infection. Is it safe? J Bone Joint Surg Br. 2004;86:962–965. doi: 10.1302/0301-620X.86B7.14292. [DOI] [PubMed] [Google Scholar]
- 4.Anract P, Coste J, Vastel L, Jeanrot C, Mascard E, Tomeno B. Proximal femoral reconstruction with megaprosthesis versus allograft prosthesis composite. A comparative study of functional results, complications and longevity in 41 cases [in French] Rev Chir Orthop Reparatrice Appar Mot. 2000;86:278–288. [PubMed] [Google Scholar]
- 5.Bittar ES, Petty W. Girdlestone arthroplasty for infected total hip arthroplasty. Clin Orthop Relat Res. 1982;170:83–87. [PubMed] [Google Scholar]
- 6.Bourne RB, Hunter GA, Rorabeck CH, Macnab JJ. A six-year follow-up of infected total hip replacement managed by Girdlestone’s arthroplasty. J Bone Joint Surg Am. 1985;67:1074–1085. doi: 10.1302/0301-620X.66B3.6725342. [DOI] [PubMed] [Google Scholar]
- 7.Buttaro MA, Comba F, Pusso R, Piccaluga F. Acetabular revision with metal mesh, impaction bone grafting, and a cemented cup. Clin Orthop Relat Res. 2008;466:2482–2490. doi: 10.1007/s11999-008-0442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dick HM, Strauch RJ. Infection of massive bone allografts. Clin Orthop Relat Res. 1994;306:46–53. [PubMed] [Google Scholar]
- 9.English H, Timperley D, Sunlop D, Gie G. Impaction grafting of the femur in two-stage revision for infected total hip replacement. J Bone Joint Surg Br. 2002;84:700–705. doi: 10.1302/0301-620X.84B5.12504. [DOI] [PubMed] [Google Scholar]
- 10.Farid Y, Lin PP, Lewis VO, Yasko AW. Endoprosthetic and allograft-prosthetic composite reconstruction of the proximal femur for bone neoplasms. Clin Orthop Relat Res. 2006;442:223–229. doi: 10.1097/01.blo.0000181491.39048.fe. [DOI] [PubMed] [Google Scholar]
- 11.Fawcett K, Barr AR. Tissue Banking. Arlington, VA: American Association of Tissue Banks; 1987. [Google Scholar]
- 12.Fountain JR, Dalby-Ball J, Carroll FA, Stockley I. The use of total femoral arthroplasty as a limb salvage procedure: the Sheffield experience. J Arthroplasty. 2007;22:663–669. doi: 10.1016/j.arth.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Gerber A, Pisan M, Zurakowski D, Isler B. Ganz reinforcement ring for reconstruction of acetabular defects in revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85:2358–2364. doi: 10.2106/00004623-200312000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Graham NM, Stockley I. The use of structural proximal femoral allograft in complex revision hip arthroplasty. J Bone Joint Surg Br. 2004;86:337–343. doi: 10.1302/0301-620X.86B3.14287. [DOI] [PubMed] [Google Scholar]
- 15.Grauer JD, Amstutz HC, O’Carrol PF, Dorey FJ. Resection arthroplasty of the hip. J Bone Joint Surg Am. 1989;71:669–678. [PubMed] [Google Scholar]
- 16.Gross AE. Revision arthroplasty of the hip using allograft bone. In: Czitrom AA, AE Gross, editors. Allografts in Orthopedic Practice. Baltimore, MD: Williams & Williams; 1992. pp. 147–173. [Google Scholar]
- 17.Gross AE, Hutchinson CR. Proximal femoral allografts for reconstruction of bone stock in revision hip arthroplasty. Orthopedics. 1998;21:999–1001. doi: 10.3928/0147-7447-19980901-23. [DOI] [PubMed] [Google Scholar]
- 18.Herrera A, Martínez AA, Cuenca J, Canales V. Management of types III and IV acetabular deficiencies with the longitudinal oblong revision cup. J Arthroplasty. 2006;21:857–864. doi: 10.1016/j.arth.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh PH, Shih CH. YH Chang, Lee MS, Yang WE, Shih HN. Treatment of deep infection of the hip associated with massive bone loss. J Bone Joint Surg Br. 2005;87:770–775. doi: 10.1302/0301-620X.87B6.15411. [DOI] [PubMed] [Google Scholar]
- 20.Jasty M, Harris W. Salvage total hip reconstruction in patients with major acetabular bone deficiency using structural femoral head allografts. J Bone Joint Surg Br. 1990;72:63–67. doi: 10.1302/0301-620X.72B1.2298796. [DOI] [PubMed] [Google Scholar]
- 21.Koster G, Rading S. Revision of failed acetabular components utilizing a cementless oblong cup: an average 9-year follow-up study. Arch Orthop Trauma Surg. 2009;129:603–608. doi: 10.1007/s00402-008-0624-8. [DOI] [PubMed] [Google Scholar]
- 22.Lakstein D, Backstein DJ, Safir O, Kosashvili Y, Gross AE. Trabecular metal for acetabular defects with 50% of less host bone contact. Clin Orthop Relat Res. 2009;467:2318–2324. doi: 10.1007/s11999-009-0772-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langlais F, Lambotte JC, Collin P, Thomazeau H. Long-term results of allograft composite total hip prosthesis for tumor. Clin Orthop Relat Res. 2003;414:197–211. doi: 10.1097/01.blo.0000079270.91782.23. [DOI] [PubMed] [Google Scholar]
- 24.Lonner JH, Desai P, Dicesare PE, Sreiner G, Zuckerman JD. The reliability of analysis of intraoperative frozen sections for identifying active infection during revision hip or knee arthroplasty. J Bone Joint Surg Am. 1996;78:1553–1558. doi: 10.2106/00004623-199610000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Lord CF, Gerhardt MC, Tomford WW, Mankin HJ. Infection in bone allografts: incidence, nature and treatment. J Bone Joint Surg Am. 1988;70:369–376. [PubMed] [Google Scholar]
- 26.Mallory TH. Excision arthroplasty with delayed wound closure for the infected total hip replacement. Clin Orthop Relat Res. 1978;137:106–111. [PubMed] [Google Scholar]
- 27.Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration: an experimental study. J Arthroplasty. 1989;4:245–251. doi: 10.1016/S0883-5403(89)80020-8. [DOI] [PubMed] [Google Scholar]
- 28.Masterson EL, Duncan CP. Subsidence and the cement mantle in femoral impaction allografting. Orthopedics. 1997;20:821–822. doi: 10.3928/0147-7447-19970901-22. [DOI] [PubMed] [Google Scholar]
- 29.McElwaine JP, Colville J. Excision arthroplasty for infected total hip replacements. J Bone Joint Surg Br. 1984;66:168–171. doi: 10.1302/0301-620X.66B2.6707049. [DOI] [PubMed] [Google Scholar]
- 30.Medling JB, Ritter MA, Keating EM, Faris PM. Impaction bone grafting before insertion of a femoral stem with cement in revision total hip arthroplasty: a minimum two-year follow-up study. J Bone Joint Surg Am. 1997;79:1834–1841. doi: 10.2106/00004623-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Mowe JC. Standards for Tissue Banking. Arlington, VA: American Association of Tissue Banks; 1988. [Google Scholar]
- 32.Murray D. The hip. In: Pynsent D, Fairbank J, Carr A, editors. Outcome Measurements in Orthopaedics. Oxford, UK: Butterworth-Heinemann; 1993. pp. 198–227. [Google Scholar]
- 33.Nusem I, Morgan DAF. Structural allograft for bone stock reconstruction in two-stage revision for infected total hip arthroplasty. Good outcome in 16 of 18 patients followed up for 5–14 years. Acta Orthop. 2006;77:92–97. doi: 10.1080/17453670610045740. [DOI] [PubMed] [Google Scholar]
- 34.Safir O, Kellett CF, Flint M, Backstein D, Gross AE. Revision of the deficient proximal femur with a proximal femoral allograft. Clin Orthop Relat Res. 2009;467:206–212. doi: 10.1007/s11999-008-0573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saleh KJ, Holtzman J, Gafni ASaleh L, Jaroszynski G, Wong P, Woodgate I, Davis A, Gross AE. Development, test reliability and validation of a classification for revision hip arthroplasty. J Orthop Res. 2001;19:50–56. doi: 10.1016/S0736-0266(00)00021-8. [DOI] [PubMed] [Google Scholar]
- 36.Schlegel UJ, Bitsch RG, Pritsch M, Clauss M, Mau H, Breusch SJ. Mueller reinforcement rings in acetabular revision: outcome in 164 hips followed for 2–17 years. Acta Orthop. 2006;77:234–241. doi: 10.1080/17453670610045966. [DOI] [PubMed] [Google Scholar]
- 37.Siegmeth A, Duncan CP, Masri BS, Kim WY, Garbuz DS. Modular tantalum augments for acetabular defects in revision hip arthroplasty. Clin Orthop Relat Res. 2009;467:199–205. doi: 10.1007/s11999-008-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sporer SM, O’Rourke M, Chong P, Paprosky WG. The use of structural distal femoral allografts for acetabular reconstruction Average ten-year follow-up. J Bone Joint Surg Am. 2005;87:760–765. doi: 10.2106/JBJS.D.02099. [DOI] [PubMed] [Google Scholar]
- 39.Haaren EH, Heyligers IC, Alexander FG, Wuisman PI. High rate of failure of impaction grafting in large acetabular defects. J Bone Joint Surg Br. 2007;89:296–300. doi: 10.1302/0301-620X.89B3.18080. [DOI] [PubMed] [Google Scholar]
- 40.Wang JW, Chen CE. Reimplantation of infected hip arthroplasties using bone allografts. Clin Orthop Relat Res. 1997;335:202–210. doi: 10.1097/00003086-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Woodgate I, Gross AE. Minor column structural acetabular allografts in revision hip arthroplasty. Clin Orthop Relat Res. 2000;371:75–85. doi: 10.1097/00003086-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Zehr R, Enneking W, Scarborough M. Allograft-prosthesis composite versus megaprosthesis in proximal femoral reconstruction. Clin Orthop Relat Res. 1996;322:207–223. doi: 10.1097/00003086-199601000-00026. [DOI] [PubMed] [Google Scholar]