Abstract
Background
A two-stage revision total knee arthroplasty is recognized as the gold standard in the treatment of infection. However, traditional spacers limit function in the interval between the two stages and may cause instability, scarring, and bone erosion. The PROSTALAC knee spacer is an antibiotic-loaded cement articulating spacer that allows some movement of the knee between stages. Whether motion enhances long-term function is unknown.
Questions/purposes
We therefore identify the rate of control of infection using the PROSTALAC exchange spacer and to assess the clinical outcome after implantation with a definitive implant.
Methods
We retrospectively reviewed 115 knees that underwent two-stage exchange with the PROSTALAC spacer. Forty-eight of these had a minimum followup of 5 years (mean, 9 years; range, 5–12 years).
Results
At last review, 101 of the 115 knees (88%) had no evidence of infection. Of the 14 knees that became reinfected, four were from the same organism and 10 were with a different organism. After further intervention, using the two-stage approach again, the infection was controlled in 12 of the 14 initially reinfected cases, resulting in a failure to cure in only two cases. We observed improvements in mean WOMAC, Oxford, UCLA, and Patient Satisfaction scores at last review.
Conclusions
The PROSTALAC functional spacer was associated with a 98% rate of control of infection and improvements in the quality-of-life outcomes in the treatment of chronically infected total knee arthroplasties.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
TKA is associated with high survivorship; Konig and Kirschner [22] reported a revision rate of 7% at 10 years, Kelly and Clarke [21] 95% survivorship at 10 years, with failure being revision of either the femoral or tibial component, Ranawat et al. [25] 94.6% survivorship at 15 years, and Gill and Joshi [12] 96.3% survivorship at 15 years and 82% at 23 years. However, between 1% and 2% of knee arthroplasties are complicated by infection [1, 2, 12, 26–28, 31, 37] with its associated patient morbidity. Two-stage exchange arthroplasty with a minimum interval of 6 weeks between stages remains the gold standard in revision arthroplasty for infection [3–5, 8, 13, 18–20, 29, 32–36, 39]. The interval between stages is to ensure control of infection but in many situations, either the clinical situation or laboratory tests, or both, suggest the infection has not resolved and a longer interval is required [4, 29, 32, 35, 36, 39]. This further delay may result in considerable morbidity for the patient with poor ROM, instability, and considerable discomfort [16–18]. Traditionally, static spacers have been used; however, problems of instability, scarring, and bone erosion have been reported in the literature [6]. We previously presented short-term results using an articulated spacer, which demonstrated 91% control of infection in patients at followup of 48 months (range, 48–112 months) [15, 23, 24]. To determine whether these early findings would be durable, we report these patients with a minimum followup of 5 years.
We specifically asked: (1) What is the rate of infection control using the PROSTALAC two-stage exchange arthroplasty at followup from 5 to 12 years? (2) Are quality-of-life outcome scores including the WOMAC, Oxford, and SF-12 sustained after reimplantation? (3) Is there any improvement in the flexion deformity or ROM when using the PROSTALAC spacer? (4) Are there any factors that predispose patients to reinfection? (5) What is the rate of reoperation for reasons other than recurrence of infection?
Patients and Methods
We identified from our database 119 patients treated for an infected knee arthroplasty between September 1996 and January 2004. Nine patients had not undergone a second-stage revision TKA and therefore were not included. We therefore followed 110 patients (60 male and 50 female patients). Five of these patients had bilaterally infected knees leaving a total of 115 knees. The reason for primary arthroplasty was osteoarthritis in 91 patients, rheumatoid arthritis in 17, postseptic arthritis degeneration in two, seronegative inflammatory arthropathy in two, posttraumatic arthritis in one, gout in one, and pseudogout in one. Of the 115 knees, 99 had an infected primary TKA, three had an infected primary unicondylar knee arthroplasty, seven had previous revision knee arthroplasty, one patient had two previous revision knee arthroplasties, and five patients had a previous two-stage revision for infection, which had failed. Twenty-one knees had a single discharging sinus, three knees had two sinuses, and one knee had three sinuses. The infecting organisms were identified in 106 cases (Table 1). Despite clinically evident infection in nine patients, no organisms were identified on culture. The mean age of the patients at the time of the first stage was 68 years (range, 35–86 years). An assessment of the patients’ comorbidities showed 57 patients (50%) were in Charnley Class C [7], 42 (36%) in Class B, and 16 (14%) in Class A. All knee aspirations before the second stage were negative. Thirty-two patients had died before the 5-year followup for reasons that were not directly attributable to the infection. This left 78 patients potentially available for quality-of-life outcome assessment by questionnaire analysis either by mail or by telephone. The infection status of the 32 patients who died was obtained by contacting their last known family physician who had continued to monitor patient progress and outcome of the affected prosthetic joint. The minimum followup for the 48 patients who did take part in the quality-of-life outcomes analysis was 5 years (mean, 9 years; range, 5–12 years). Ethics approval was obtained before the study was started.
Table 1.
Causative organism in 106 infected knees
| Organism | Number |
|---|---|
| Single organisms | |
| Staphylococcus epidermidis (six methicillin-resistant) | 37 |
| Staphylococcus aureus (two methicillin-resistant) | 36 |
| Group B Streptococcus | 8 |
| Enterococcus | 8 |
| Streptococcus viridians | 3 |
| Peptostreptococcus | 2 |
| Staphylococcus lugdunensis | 2 |
| Hemophilus influenzae | 1 |
| Bacteroides | 1 |
| Enterobacter | 1 |
| Mycobacterium goodii | 1 |
| Multiple organisms | |
| S. aureus and S. epidermidis | 1 |
| Enterobacter and S. aureus | 1 |
| Methicillin-resistant S. aureus and Branhamella catarrhalis | 1 |
| S. epidermidis and Streptococcus viridians | 1 |
| S. epidermidis and Enterococcus | 1 |
| S. epidermidis and Corynebacterium | 1 |
Of the 78 available for completion of a quality-of-life outcome questionnaire, 30 patients declined to take part in the outcomes portion of the study. However, for the 30 patients who declined or were unable to complete a questionnaire, we determined their infection status by contacting their family physicians as well as their charts at last followup and they were included in the analysis of infection recurrence. As a result, the infection status was obtained for all patients at the minimum followup.
For purposes of this review, a successful outcome was defined as the absence of symptoms associated with infection in the affected joint (including erythema, sinus, warmth, swelling, cellulitis), documented evidence of acceptable functional performance of the affected joint, and documented normalization of infectious blood work parameters (ie, erythrocyte sedimentation rate [ESR], C-reactive protein [CRP], white blood cell count). A failure was defined as the recurrence of infection indicated by the presence of symptoms of infection as well as raised inflammatory markers.
Patients had a full clinical evaluation before the first stage, specifically noting the presence of sinuses, ROM, and swelling as well as completing the quality-of-life outcome scores. Those patients already on oral antibiotics before the first stage were discussed with the microbiologist after the culture results were obtained intraoperatively to ensure they were on the most appropriate antibiotic postoperatively.
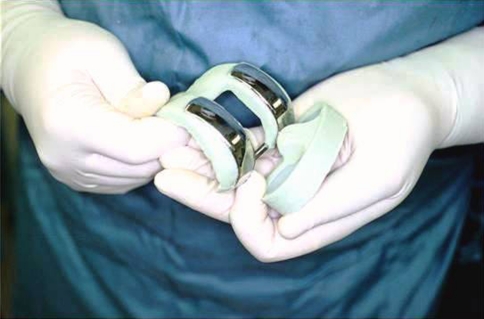
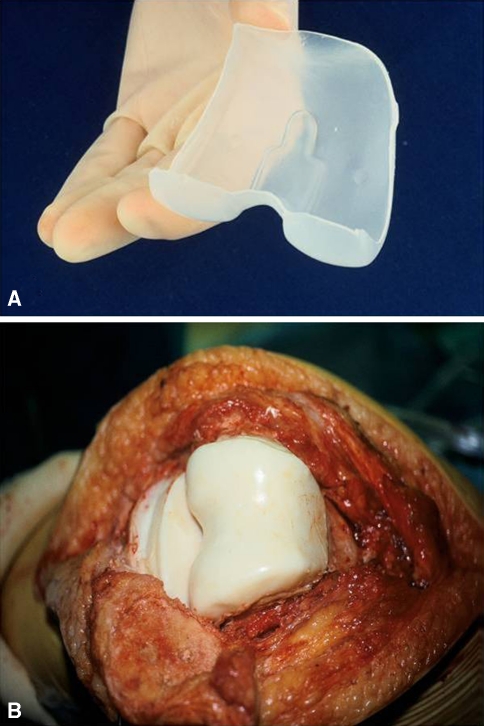
The PROSTALAC knee spacer has two components, femoral and tibial. Each is made of antibiotic-loaded Palacos (Zimmer, Warsaw, IN) acrylic cement within which a small inset prosthesis is placed consisting of low-friction articulation of metal on polyethylene (Fig. 1).
Fig. 1.
The final 1994 PROSTALAC design is shown just before implantation incorporating small polycentric metal runners, small inlaid polyethylene plateaus, a cam mechanism, and a posterior cross bar.
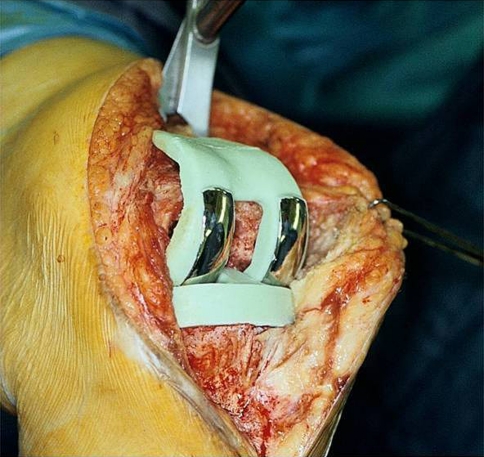
The first stage of the knee revision involved removal of all foreign material as well as thorough débridement of all infected soft tissue. Any bone deficiencies were augmented with additional cement within the variable height and width of the prosthetic molds with the aim of restoring the normal joint line and alignment. Once the components, with the incorporated low-friction runners, were cured in the molds, they were secured to the host femur and tibia with a thin layer of antibiotic-loaded acrylic cement (Fig. 2). Appropriate placement of the spacer was confirmed on an AP and lateral radiograph postoperatively (Fig. 3). Of the 115 knees, 25 had a rectus snip at the first stage, two had a V-Y quadriceps turndown, and one a tibial tubercle osteotomy and rectus snip.
Fig. 2.
The PROSTALAC components are shown implanted.
Fig. 3.
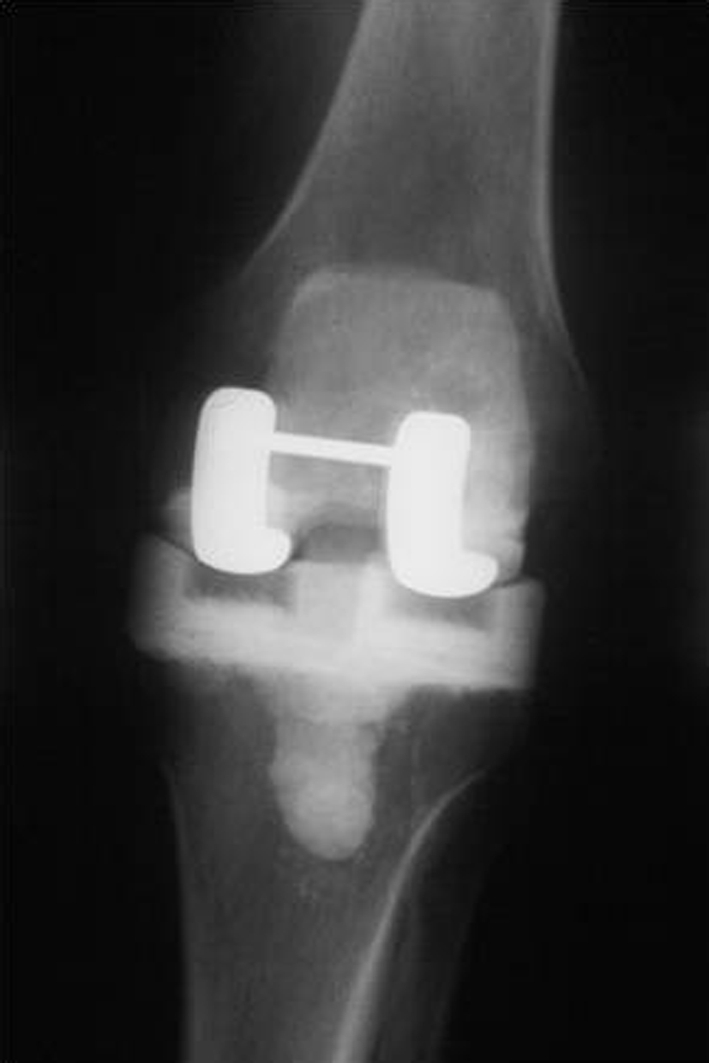
An AP radiograph shows a PROSTALAC articulating knee spacer.
The patients were allowed to weightbear as tolerated with the aid of crutches or a walker postoperatively. All patients were treated with a course of intravenous antibiotics for a minimum of 5 weeks using an antibiotic regimen tailored to the antimicrobial sensitivities of the organism(s). After completion of the antibiotic regimen, all patients were monitored over an additional 4 to 6 weeks to assess for infection control using clinical and laboratory parameters. For reimplantation, we required resolution of symptoms of infection and inflammation, including the healing of sinuses and the absence of a clinical effusion. All patients had repeat ESR and CRP in keeping with the standardized protocol at our institution [14]. We judged it important that the CRP had returned to normal (standardized cutoff point of CRP less than 10 mg/L) and that the ESR values continued to drop toward normal (standardized cutoff point less than 30 mm/hr). In patients in whom there was some question as to whether the infection had been controlled, we performed an aspiration biopsy 4 weeks after the antibiotics had been stopped. Patients were then readmitted for removal of the spacer and implantation of a definitive knee arthroplasty.
All 110 patients had two-stage revision arthroplasties using the PROSTALAC revision knee system. The mean interval between stages was 17 weeks (range, 5–123 weeks). All patients had a combination of vancomycin (1.5 g/40 g package of cement) and tobramycin (3.6 g/40 g package of cement) in the cement. Penicillin G was added to the cement in five patients based on the type and sensitivity profiles of the organisms cultured. Wounds were closed without suction drains to avoid the elution of antibiotics away from the periprosthetic region. A condylar-type revision implant was used in all patients except for four patients in whom a rotating hinge was required. Intravenous antibiotics were continued until the final intraoperative culture results from the second stage were obtained at 5 days. Of the 115 knees, 33 had a rectus snip at the second stage, five a V-Y quadriceps turndown, and one knee a tibial tubercle osteotomy.
The rehabilitation program was identical to the rehabilitation protocol used by our hospital for aseptic revision knee arthroplasty. After the first stage, patients were mobilized under the supervision of a physical therapist on the first postoperative day. Weightbearing was allowed as tolerated by the patient using a walker or crutches initially. Regular inpatient physical therapy for ROM was continued until discharge. All patients were requested to attend outpatient physical therapy for ROM exercises and quadriceps strengthening. Routine immobilization was not used, and no routine splints or knee immobilizers were used. Patients were allowed to discontinue the use of crutches or walkers at their discretion, and most patients attended the followup clinic at 6 weeks walking with the assistance of a single cane. All patients were followed by the infectious diseases consultant to monitor antibiotic levels and response to treatment. Close contact was maintained with the orthopaedic surgeon in case the infectious diseases consultant had any concerns. All patients were seen by the orthopaedic surgeon at 6 weeks for a repeat ESR and CRP measurement and for a clinical and radiographic evaluation. This was repeated 4 weeks later, and if a trend toward a decrease in the ESR and CRP was noted, the second stage was booked. If, however, there was any concern, the second stage was delayed with further monitoring of the ESR and the CRP. Routine aspiration was initially done at the 10-week mark after the first stage. However, as a result of the lack of positive cultures in any patients, this practice has since been discontinued because we did not believe it was of sufficient accuracy to guide us toward reimplantation or not.
After the second stage, each patient was reviewed at 6 weeks with repeat inflammatory markers (ESR and CRP) as well as a clinical examination, including the range of knee motion. A record of the patient outcome scores was also made. Further followup was arranged at 3 months, 6 months, and at 1 year and then yearly thereafter. Outcome scores were available for 48 of the 70 patients (69%) who were still alive. It is important to note that the ESR and CRP alone at 6 weeks cannot definitively diagnose persistence or control of infection. However, they serve as a baseline for further monitoring. At the initial stages, control of infection was diagnosed on clinical grounds.
We compared the preoperative and postoperative quality-of-life outcome scores, which included the WOMAC, Oxford, and SF-12, using the paired Student’s t test. To evaluate for factors that may be associated with reinfection, we performed logistic regression analyses. The variables for this analysis were patient comorbidity as measured by the Charnley score [7], patients’ gender, American Society of Anesthesiologists score [30], presence of a sinus, number of previous revisions for loosening or change of spacer and previous débridement, age, and body mass index.
Results
Fourteen of the 110 knees that had a two-stage revision with the PROSTALAC articulating spacer became reinfected giving an initial infection control rate of 87%. Four of these 14 patients had a recurrent infection within 1 year of the initial second-stage operation and the remaining eight patients had later recurrent infections from 13 months to 81 months after the second stage. Twelve of these 14 patients responded to revision surgery with a repeat two-stage procedure with a PROSTALAC spacer and became infection-free in the medium- to long-term (mean, 5.83 years; range, 1–11 years) for an eventual infection control rate of 98%, leaving only two permanent failures. Three of these 14 patients subsequently died after their repeat PROSTALAC revision at 37 months, 48 months, and 52 months, respectively, for reasons unrelated to their knee. For the remaining patients who died, we contacted their last known family physician and were able to confirm that they were all infection-free at the time of their death. Of the 14 patients who failed the initial two-stage revision, four had recurrence of the organisms, which had been isolated at the time of preoperative investigations at the first stage (Table 2). Interestingly, of the eight patients with a methicillin-resistant staphylococcal organism, the result was control of infection in six.
Table 2.
The organisms identified in the 14 patients with recurrent infections
| Patient number | Organism identified at the first stage | Reinfected organism | Time to reinfection (months) |
|---|---|---|---|
| 1 | Staphylococcous aureus | Same organism | 1 |
| 2 | S. aureus and Staphylococcus epidermidis | Candida and S. epidermidis | 3 |
| 3 | S. aureus (methicillin-resistant) | S. aureus (methicillin-sensitive) | 7 |
| 4 | S. aureus | S. epidermidis | 10 |
| 5 | Hemophilus influenzae | Streptococcus viridans | 11 |
| 6 | S. aureus | Same organism | 12 |
| 7 | S. epidermidis (methicillin-resistant) | None isolated | 13 |
| 8 | S. aureus | S. epidermidis (methicillin-resistant) | 15 |
| 9 | Enterobacter | S. epidermidis | 36 |
| 10 | S. aureus | Same organism | 50 |
| 11 | Group B Streptococcus | Same organism | 54 |
| 12 | Group B Streptococcus | Serratia marcescens | 58 |
| 13 | S. epidermidis | Streptococcus viridans | 61 |
| 14 | No growth | Group B Streptococcus | 81 |
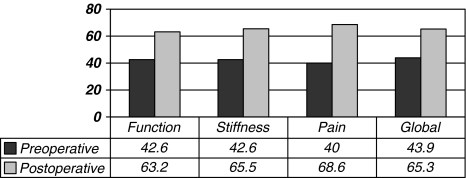
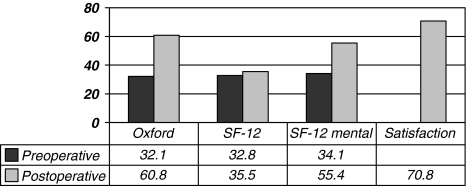
We observed an improvement in the mean postoperative WOMAC function, WOMAC pain, and WOMAC global scores (Table 3; Fig. 4). An improvement was also noted in the Oxford and the SF-12 (mental) scores and the satisfaction scores were recorded at last followup (Fig. 5). The mean postoperative UCLA score at final followup was 4.1. WOMAC function (p = 0.001), WOMAC pain (p = 0.02), and WOMAC global (p = 0.002) scores as well as the Oxford (p = 0.0003) and the SF-12 (mental) (p = 0.008) scores all improved at last followup (Table 3).
Table 3.
Mean preoperative and postoperative WOMAC, Oxford, and SF-12 scores along with their p values and 95% confidence intervals and the postoperative Satisfaction and UCLA scores for the PROSTALAC knee*
| Outcome score | Preoperative mean | Postoperative mean | p Value | Lower limit of 95% confidence interval | Upper limit of 95% confidence interval |
|---|---|---|---|---|---|
| WOMAC function | 42.6 | 63.2 | 0.001 | 12.9 | 30.9 |
| WOMAC stiffness | 42.6 | 65.5 | 0.062 | 1.5 | 36 |
| WOMAC pain | 40 | 68.6 | 0.024 | 6.3 | 39.4 |
| WOMAC global | 43.9 | 65.3 | 0.002 | 11.6 | 32 |
| Oxford | 32.1 | 60.8 | 0.0003 | 23.2 | 47 |
| SF-12 (physical) | 32.8 | 35.5 | 0.904 | −6.1 | 7 |
| SF-12 (mental) | 34.1 | 55.4 | 0.008 | 9.1 | 27.6 |
| Satisfaction score | 70.8 | ||||
| UCLA | 4.1 |
* The WOMAC and Oxford scores were normalized to a scale of 0 to 100 with 100 being a perfect score.
Fig. 4.
The graph demonstrates the mean preoperative and postoperative WOMAC scores for the PROSTALAC knee. The WOMAC scores were normalized to a scale of 0 to 100 with 100 being a perfect score.
Fig. 5.
The graph demonstrates the mean preoperative and postoperative Oxford, SF-12, and the postoperative satisfaction and UCLA scores. The Oxford scores were normalized to a 0 to 100 scale in which 100 is a perfect score.
The flexion deformity assessed preoperatively improved from a mean of 6.7° (range, −5° to 50°) to a mean of 3.74° (range, 0°–20°) postoperatively (p = 0.04); however, postoperative knee flexion of 93.2° (range, 30°–140°) and preoperative knee flexion of 86.2° (range, 15°–140°) were similar (p = 0.11). Logistic regression did not identify any variables that predicted success or failure.
Further revision surgery was required for reasons other than infection in 10 patients for a total of 13 revision operations giving a rate of reoperation of 11%. Three patients required revision for aseptic loosening of the tibial component at a mean of 3.8 years (range, 2–7.3 years). One patient required revision of the tibial component three times at yearly intervals for aseptic loosening, and on the final occasion, a trabecular metal cone on the tibial side was needed and the femoral component was exchanged for a constrained design. One patient required revision of the femoral component at 7.6 years. Three patients required revision of the tibial and femoral components for aseptic loosening at a mean of 6.2 years (range, 5–8 years). One patient had a liner exchange for polyethylene wear at 9.3 years. One patient required exchange to a thicker liner for instability at 8 months. One patient sustained a patella fracture after a fall and required a patellectomy and a subsequent liner exchange for a post fracture.
There were 19 (17%) additional surgical complications and 18 (16%) medical complications. Three patients sustained a fracture of their femoral PROSTALAC component, which were left alone, and two patients had problems with dislocation after the first stage. The first patient had a frank dislocation of the knee, which required a repeat first stage to remove the old components and cement in new ones and the second patient sustained a dislocation of the tibial component, which required an open reduction (Table 4).
Table 4.
Complications that occurred after the PROSTALAC knee arthroplasty
| Complication | Number of patients |
|---|---|
| Further revision surgery | 13 |
| Additional surgical complications | 5 |
| Wound problem after first stage | 3 |
| Fracture of the femoral PROSTALAC component requiring repeat first stage | 1 |
| Fracture of both PROSTALAC components noted at time of second stage | 1 |
| Intraoperative longitudinal split fracture of femur with implant removal at second stage requiring Luque wires | 1 |
| Late periprosthetic fracture after second stage | 1 |
| Knee dislocation after first stage requiring repeat first stage | 1 |
| Dislocation of tibial component after first stage requiring repeat first stage | 1 |
| Hematoma after first stage requiring débridement | 1 |
| Hematoma after second stage requiring débridement | 1 |
| Patella fracture after second stage | 1 |
| Subluxation of the extensor mechanism laterally after second stage (previous patellectomy) | 1 |
| Common peroneal nerve palsy after second stage requiring ankle–foot orthotic | 3 |
| Medical complications | 2 |
| Postoperative pneumonia | 2 |
| Acute pulmonary edema | 2 |
| Gastrointestinal bleed | 1 |
| Postoperative atrial fibrillation | 1 |
| Myocardial infarction after first stage | 1 |
| Deep vein thrombosis/pulmonary embolism | 1 |
| Chronic renal failure secondary to vancomycin | 1 |
| Acute renal failure | 1 |
| Antibiotic-induced interstitial nephritis | |
| Reaction to vancomycin/ampicillin requiring hospital admission for vomiting/dehydration | 1 |
| Multiorgan failure secondary to sepsis requiring intensive care unit admission | 1 |
| Urinary tract infection | 1 |
| Paralytic ileus after first stage |
Discussion
A two-stage revision TKA for infection without the use of a spacer can lead to stiffness as well as ligament contracture resulting in considerable problems when it comes to reimplantation [3, 29, 38]. Problems with static spacers [3, 36] prompted the development of antibiotic-loaded, articulating spacers to promote joint motion, maintenance of extensor mechanism length, and tissue planes and to facilitate the second stage. Of the two articulating-type spacers, one is made completely of antibiotic-impregnated cement, similar to our prototypes in 1987 (Fig. 6) to 1991 (Fig. 7A–B), and the second type is made of antibiotic-loaded cement with a low friction articulation. The first report of the development and clinical use of this concept for the two-stage management of infected TKAs was presented from our center in 1992 [9]. The aim of this study was to review all our patients who had a two-stage revision knee arthroplasty with the PROSTALAC knee spacer with a minimum followup of 5 years with the purpose of addressing the following questions: (1) What is the rate of infection control using the PROSTALAC two-stage exchange arthroplasty at followup from 5 to 12 years? (2) What is the clinical outcome based on quality-of-life outcome scores, including the WOMAC, Oxford, and SF-12, after reimplantation? (3) Is there any improvement in the flexion deformity or ROM when using the PROSTALAC spacer? (4) Are there any factors that predispose patients to reinfection? (5) What is the rate of reoperation for reasons other than recurrence of infection?
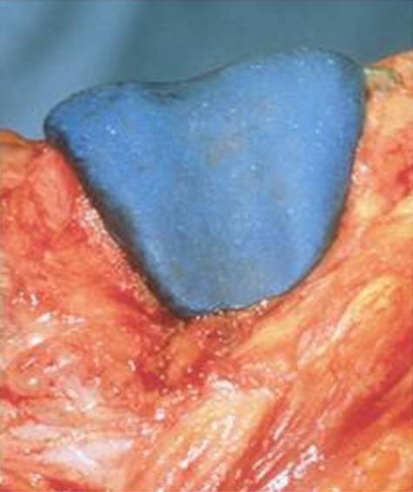
Fig. 6.
The 1987 handmade prototypical femoral component design is shown.
Fig. 7A–B.
(A) The 1991 style femoral mold and (B) the implanted femoral and tibial acrylic components without the low-friction interface, which was developed later, are shown.
However, we acknowledge certain limitations to our study. First, of the 70 patients who were alive at the time of final followup, only 48 completed the outcome score questionnaires and they may not have truly represented the entire cohort of patients. Second, we did not perform a radiographic review because most patients were functioning well and lived some distance from our tertiary center. Third, although we obtained the last recorded infection status of the 32 patients who died, from their last known family physicians, this information may not have been correct. Fourth, we had no control group using traditional spacers. Although the ideal study would be an adequately powered randomized study perhaps comparing static and articulating spacers, we introduced the articulating spacer to enhance function and used it exclusively. Finally, while reporting a substantial number of patients with two-stage arthroplasty, we were unable to identify any variables that influenced the risk of infection recurrence owing to the relatively small numbers of patients with recurrence of infection.
We found a recurrence of the original infection in 3.5% and a new infection in another 8% (Table 5). All but two of these patients responded to revision surgery with a repeat two-stage procedure and became infection-free in the medium- to long-term (mean, 5.83 years; range, 1–11 years) for a total infection control rate of 98%. These results follow the encouraging short-term results published by Haddad et al. [15] who investigated the treatment of 45 infected TKAs with the PROSTALAC system from our center. Ninety-one percent of patients reviewed had no evidence of infection recurrence at a mean followup of 48 months. Fehring et al. [11] went on to compare an articulating spacer with a static spacer as part of a two-stage revision technique. They reported a reinfection rate of 12% with the static spacer and 7% with an articulating spacer. Emerson et al. [10] reported on 26 patients who had a two-stage revision knee arthroplasty with a static spacer and 22 patients treated with an articulating spacer with a metal and plastic component. The reinfection rates were comparable at 36 months with 7.6% in the spacer block group and 9% for the patients with articulating spacers.
Table 5.
Summarizing the literature review of the results of different techniques of two-stage revision TKA for infection
| Study | Method of infection eradication | Reinfection rate | Range of movement |
|---|---|---|---|
| Rosenberg et al. [29] | No spacer | No infection recurrence in 26 infected TKAs at mean followup of 29 months | 21 knees able to flex more than 90° and three knees had an extensor lag of more than 20° |
| Windsor et al. [38] | No spacer | 1 reinfection in 38 TKAs at mean followup of 4 years | |
| Borden and Gearen [4] | Impregnated cement beads or spacer | 90% success rate in 21 TKAs with mean followup of 46 months | |
| Whiteside [34] | Antibiotic beads | 32 of 33 patients remained free of infection at 2 to 8 years after surgery; 4 required repeat débridement and cement beads | |
| Haddad et al. [15] | Articulating spacer (PROSTALAC) | 41 out of 45 patients had no evidence of infection at mean followup of 48 months | ROM maintained during interval between stages |
| Emerson et al. [10] | 26 patients had static spacer 22 patients had an articulating spacer |
2 persistent infections out of 26 2 persistent infections out of 22 patients Mean followup of 36 months |
Flexion postoperatively 93.7° Flexion postoperatively 107.8° |
| Gooding et al. [current issue] | Articulating spacer (PROSTALAC) | 14 out of 115 patients became reinfected with a minimum followup of 5 years (mean, 9 years) | Flexion deformity improved from a mean of 6.7° preoperatively to a mean of 3.74° postoperatively |
We observed an improvement in the postoperative WOMAC function, pain, and global scores as well as the Oxford, SF-12 (mental) scores, and the satisfaction scores at last followup. Similar improvements have also been reported in other studies. Meek et al. [24] reported improved WOMAC, Oxford-12, SF-12, and patient satisfaction questionnaires at a mean followup of 41 months, whereas Rosenberg et al. [29] reported 75% good or excellent results based on the Hospital for Special Surgery knee rating system with no spacer in 25 patients with a mean followup of 29 months. The difficulty in comparing the outcomes of different techniques is that individual studies use different outcome scores to assess their patients. For this reason, a randomized study would be useful so a direct comparison can be made.
Although we observed an improvement in fixed flexion from a mean of 6.7° preoperatively to a mean of 3.74° postoperatively, we did not detect an improvement in postoperative range of flexion. Haddad et al. [15] were able to conclude ROM as well as flexion deformities had improved after two-stage revision with an articulating spacer. Emerson et al. [10] also reported patients with articulating spacers had a better ROM at followup compared with those patients who had a spacer block.
Success cannot be measured only in terms of reinfection and functional outcome, but also must consider the durability of the reconstruction. Our study data showed a reoperation rate for reasons other than infection was 11%, similar to a previous report on the treatment of infected knee arthroplasties from this center [24]. In our experience, articulating spacers encourage early mobilization as well as the retention of tissue planes facilitating the implantation of a definitive implant at the second stage of a two-stage revision.
Acknowledgments
We thank Daphne Savoy, research coordinator, without whose valuable help this article would not have been possible, and Eric Sayre for performing the statistical analysis.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bengtson S, Knutson K. The infected knee arthroplasty. A 6-year follow-up of 357 cases. Acta Orthop Scand. 1991;62:301–311. doi: 10.3109/17453679108994458. [DOI] [PubMed] [Google Scholar]
- 2.Bengtson S, Knutson K, Lidgren L. Revision of infected knee arthroplasty. Acta Orthop Scand. 1986;57:489–494. doi: 10.3109/17453678609014776. [DOI] [PubMed] [Google Scholar]
- 3.Booth RE, Lotke PA. The results of spacer block technique in revision of infected total knee arthroplasty. Clin Orthop Relat Res. 1989;248:57–60. [PubMed] [Google Scholar]
- 4.Borden LS, Gearen PF. Infected total knee arthroplasty. A protocol for management. J Arthroplasty. 1987;2:27–36. doi: 10.1016/S0883-5403(87)80028-1. [DOI] [PubMed] [Google Scholar]
- 5.Brause BD. Infected total knee replacement: diagnostic, therapeutic, and prophylactic considerations. Orthop Clin North Am. 1982;13:245–249. [PubMed] [Google Scholar]
- 6.Calton TF, Fehring TK, Griffin WL. Bone loss associated with the use of spacer blocks in infected total knee arthroplasty. Clin Orthop Relat Res. 1997;345:148–154. doi: 10.1097/00003086-199712000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Charnley J. The long-term results of low-friction arthroplasty of the hip performed as a primary intervention. J Bone Joint Surg Br. 1972;54:61–76. [PubMed] [Google Scholar]
- 8.Deburge A. Guepar. Guepar hinge prosthesis: complications and results with two years’ follow-up. Clin Orthop Relat Res. 1976;120:47–53. [PubMed] [Google Scholar]
- 9.Duncan CP, Beauchamp CP, Masri BA, McGraw RW, Paris NJ, Breault MJ.The antibiotic loaded joint replacement system. A novel approach to the management of the infected knee replacement J Bone Joint Surg Am. 1992742961541625 [Google Scholar]
- 10.Emerson RH, Muncie M, Tarbox TR, Higgins LL. Comparison of a static with a mobile spacer in total knee infection. Clin Orthop Relat Res. 2002;404:132–138. doi: 10.1097/00003086-200211000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Fehring TK, Odum S, Calton TF, Mason JB. Articulating versus static spacers in revision total knee arthroplasty for sepsis. The Ranawat Award. Clin Orthop Relat Res. 2000;380:9–16. doi: 10.1097/00003086-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Gill GS, Joshi AB. Long-term results of cemented, posterior cruciate ligament-retaining total knee arthroplasty in osteoarthritis. Am J Knee Surg. 2001;14:209–214. [PubMed] [Google Scholar]
- 13.Goldman RT, Scuderi GR, Insall JN. 2-stage reimplantation for infected total knee replacement. Clin Orthop Relat Res. 1996;331:118–124. doi: 10.1097/00003086-199610000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Greidanus NV, Masri BA, Garbuz DS, Wilson SD, McAlinden MG, Xu M, Duncan CP. Use of erythrocyte sedimentation rate and C-reactive protein level to diagnose infection before revision total knee arthroplasty. A prospective evaluation. J Bone Joint Surg Am. 2007;89:1409–1416. doi: 10.2106/JBJS.D.02602. [DOI] [PubMed] [Google Scholar]
- 15.Haddad FS, Masri BA, Campbell D, McGraw RW, Beauchamp CP, Duncan CP. The PROSTALAC functional spacer in two-stage revision for infected knee replacements. Prosthesis of antibiotic-loaded acrylic cement. J Bone Joint Surg Br. 2000;82:807–812. doi: 10.1302/0301-620X.82B6.10486. [DOI] [PubMed] [Google Scholar]
- 16.Insall JN. Infection of total knee arthroplasty. Instr Course Lect. 1986;35:319–324. [PubMed] [Google Scholar]
- 17.Insall JN. Surgical technique and instrumentation in total knee arthroplasty. In: Insall JN, Windsor RE, Scott WN, Kelly MA, Aglietti P, eds. Surgery of the Knee. New York, NY: Churchill Livingstone; 1993:739–804.
- 18.Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65:1087–1098. [PubMed] [Google Scholar]
- 19.Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 2002;84:490. doi: 10.2106/00004623-200203000-00024. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs MA, Hungerford DS, Krackow KA, Lennox DW. Revision of septic total knee arthroplasty. Clin Orthop Relat Res. 1989;238:159–166. [PubMed] [Google Scholar]
- 21.Kelly MA, Clarke HD. Long-term results of posterior cruciate-substituting total knee arthroplasty. Clin Orthop Relat Res. 2002;404:51–57. doi: 10.1097/00003086-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Konig A, Kirschner S. Long-term results in total knee arthroplasty [in German] Orthopade. 2003;32:516–526. doi: 10.1007/s00132-003-0481-7. [DOI] [PubMed] [Google Scholar]
- 23.Meek RM, Dunlop D, Garbuz DS, McGraw RW, Greidanus NV, Masri BA. Patient satisfaction and functional status after aseptic versus septic revision total knee arthroplasty using the PROSTALAC articulating spacer. J Arthroplasty. 2004;19:874–879. doi: 10.1016/j.arth.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Meek RM, Masri BA, Dunlop D, Garbuz DS, Greidanus NV, McGraw R, Duncan CP. Patient satisfaction and functional status after treatment of infection at the site of a total knee arthroplasty with use of the PROSTALAC articulating spacer. J Bone Joint Surg Am. 2003;85:1888–1892. doi: 10.1302/0301-620X.85B8.14214. [DOI] [PubMed] [Google Scholar]
- 25.Ranawat CS, Flynn WF, Saddler S, Hansraj KK, Maynard MJ. Long-term results of the total condylar knee arthroplasty. A 15-year survivorship study. Clin Orthop Relat Res. 1993;286:94–102. [PubMed] [Google Scholar]
- 26.Rand JA, Chao EY, Stauffer RN. Kinematic rotating-hinge total knee arthroplasty. J Bone Joint Surg Am. 1987;69:489–497. [PubMed] [Google Scholar]
- 27.Rand JA, Fitzgerald RH. Diagnosis and management of the infected total knee arthroplasty. Orthop Clin North Am. 1989;20:201–210. [PubMed] [Google Scholar]
- 28.Rorabeck CH. Session IV: salvage of the infected total knee replacement. Infection: the problem. Clin Orthop Relat Res. 2002;404:113–115. doi: 10.1097/00003086-200211000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg AG, Haas B, Barden R, Marquez D, Landon GC, Galante JO. Salvage of infected total knee arthroplasty. Clin Orthop Relat Res. 1988;226:29–33. [PubMed] [Google Scholar]
- 30.Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2:281–284. doi: 10.1097/00000542-194105000-00004. [DOI] [Google Scholar]
- 31.Spangehl MJ, Hanssen AD. Management of the infected total knee replacement. Curr Opin Orthop. 2002;13:23–29. doi: 10.1097/00001433-200202000-00004. [DOI] [Google Scholar]
- 32.Teeny SM, Dorr L, Murata G, Conaty P. Treatment of infected total knee arthroplasty. Irrigation and débridement versus two-stage reimplantation. J Arthroplasty. 1990;5:35–39. doi: 10.1016/S0883-5403(06)80007-0. [DOI] [PubMed] [Google Scholar]
- 33.Walker RH, Schurman DJ. Management of infected total knee arthroplasties. Clin Orthop Relat Res. 1984;186:81–89. [PubMed] [Google Scholar]
- 34.Whiteside LA. Treatment of infected total knee arthroplasty. Clin Orthop Relat Res. 1994;299:169–172. [PubMed] [Google Scholar]
- 35.Wilde AH. Management of infected knee and hip prostheses. Curr Opin Rheumatol. 1994;6:172–176. doi: 10.1097/00002281-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Wilde AH, Ruth JT. Two-stage reimplantation in infected total knee arthroplasty. Clin Orthop Relat Res. 1988;236:23–35. [PubMed] [Google Scholar]
- 37.Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am. 1990;72:878–883. [PubMed] [Google Scholar]
- 38.Windsor RE, Insall JN, Urs WK, Miller DV, Brause BD. Two-stage reimplantation for the salvage of total knee arthroplasty complicated by infection. Further follow-up and refinement of indications. J Bone Joint Surg Am. 1990;72:272–278. [PubMed] [Google Scholar]
- 39.Woods GW, Lionberger DR, Tullos HS. Failed total knee arthroplasty. Revision and arthrodesis for infection and noninfectious complications. Clin Orthop Relat Res. 1983;173:184–190. [PubMed] [Google Scholar]